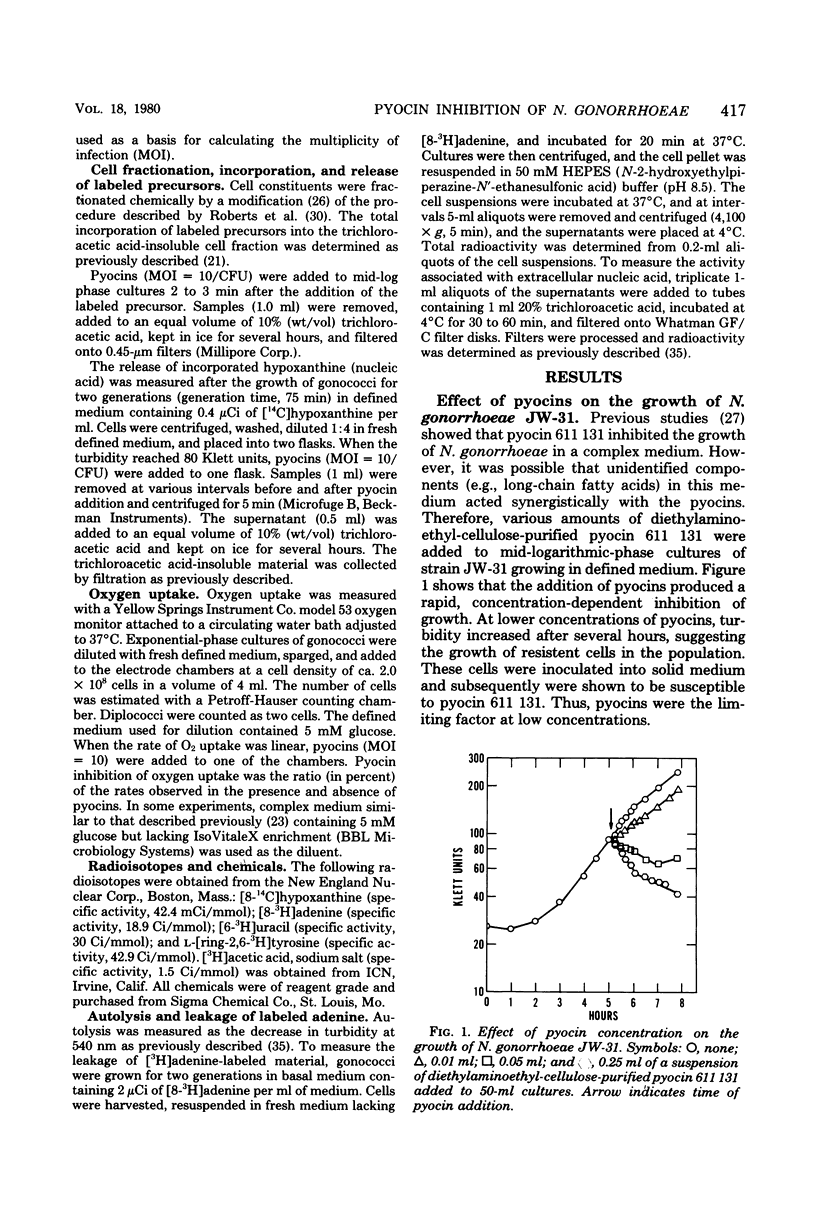
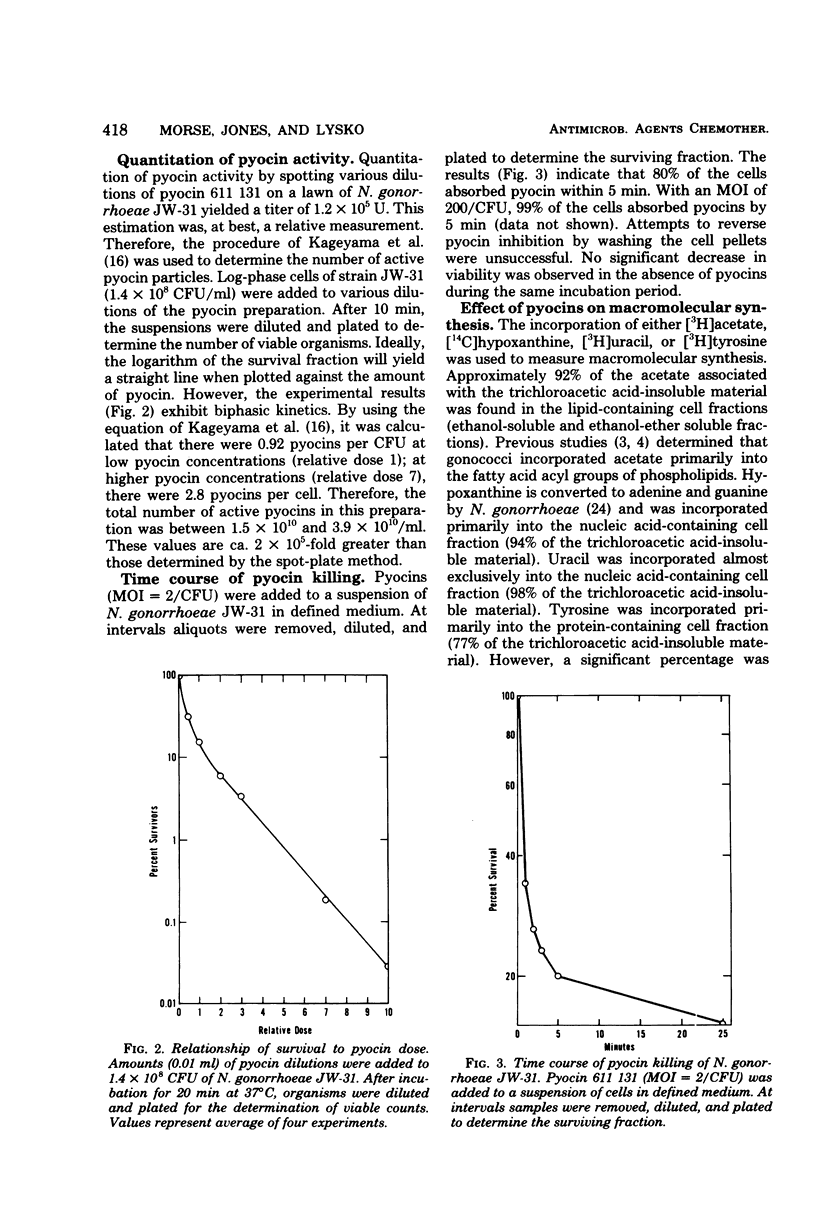
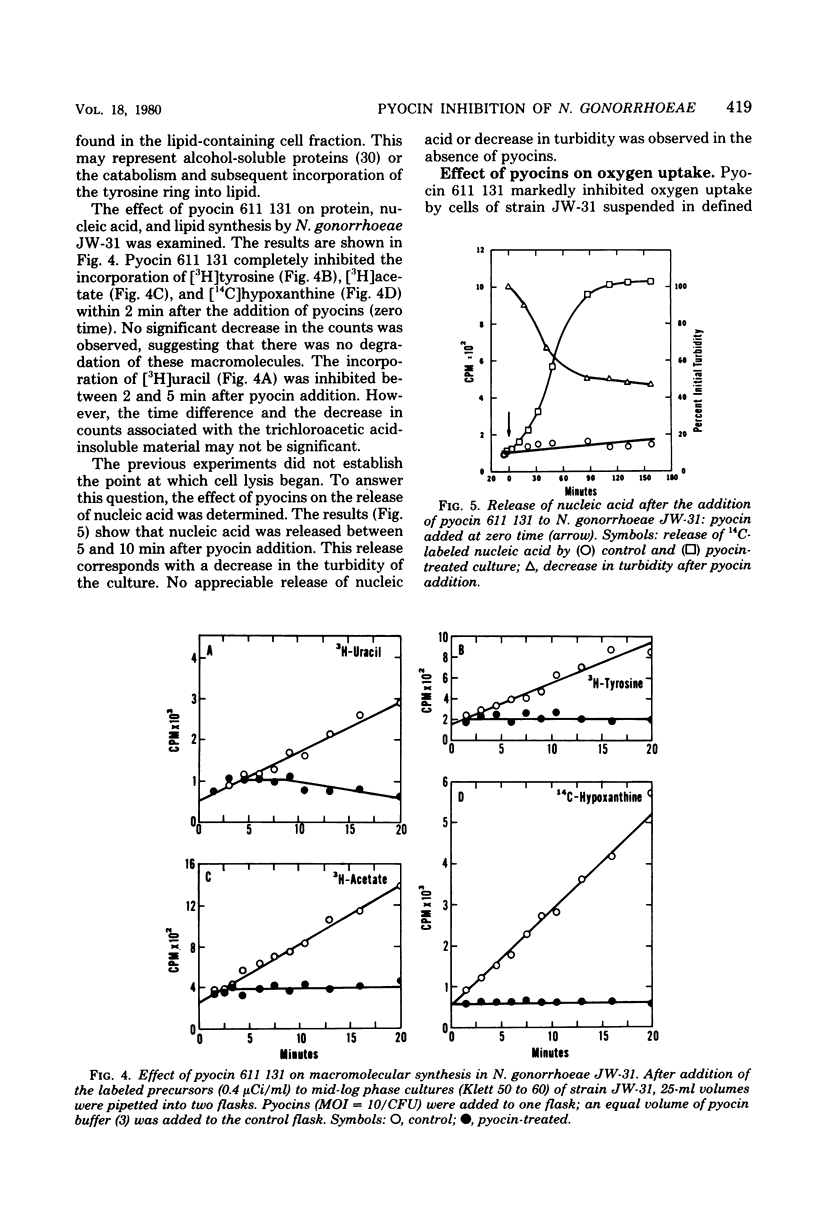
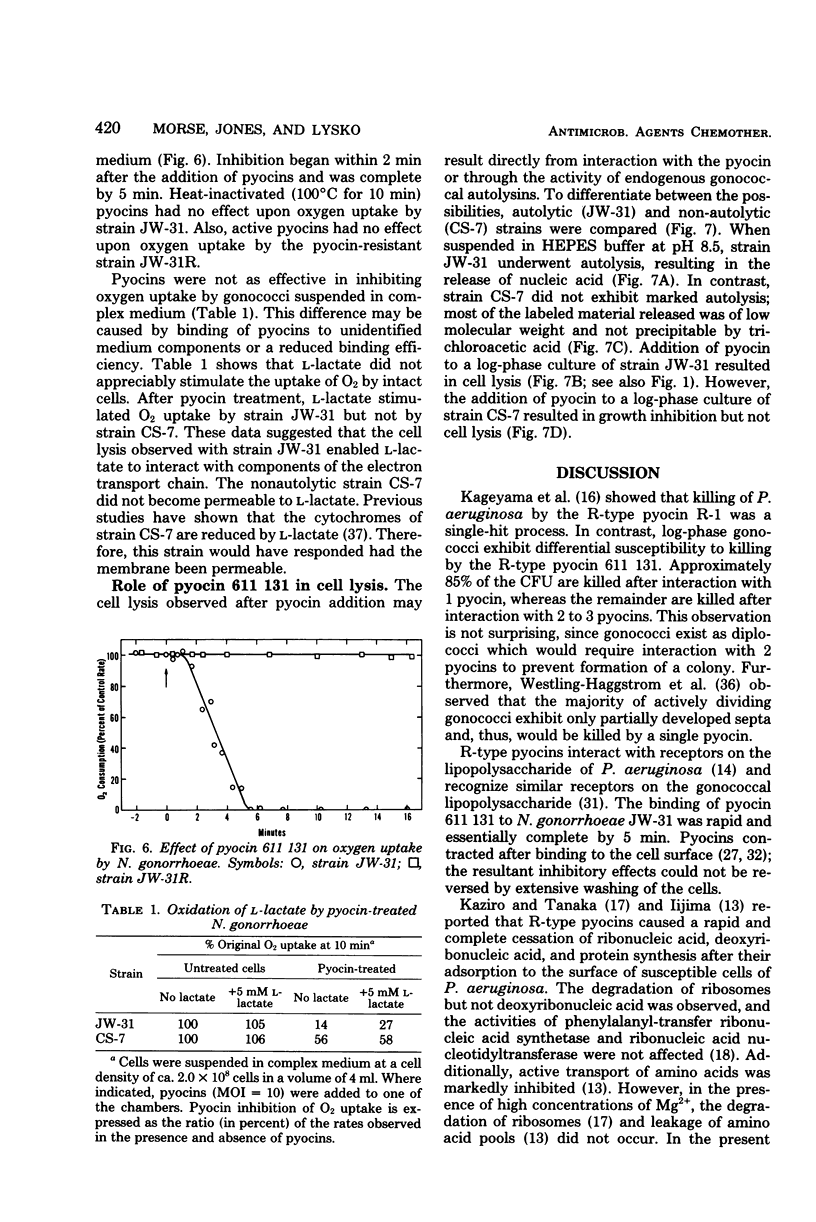
Abstract
Purified R-type pyocins (611 131) from Pseudomonas aeruginosa PA103 exhibited bactericidal activity against Neisseria gonorrhoeae. Killing of gonococci was a single-hit process requiring as few as 1 pyocin per colony-forming unit. Deoxyriboinucleic acid, ribonucleic acid, protein, and lipid syntheses were rapidly and completely inhibited. Oxygen uptake was also inhibited, but occurred after the inhibition of macromolecular synthesis. The cell lysis which occurred after pyocin inhibition of gonococcal growth was the result of endogenous gonococcal autolysin activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell C. C., Young H., Anderson I. Sensitivity of Neisseria gonorrhoeae to partially purified R-type pyocines and a possible approach to epidemiological typing. J Med Microbiol. 1979 Aug;12(3):321–335. doi: 10.1099/00222615-12-3-321. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapuoti A. F., Wegener W. S., Morse S. A. Cell envelope of Neisseria gonorrhoeae: phospholipase activity and its relationship to autolysis. Infect Immun. 1978 May;20(2):418–420. doi: 10.1128/iai.20.2.418-420.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapuoti A. F., Wegener W. S., Morse S. A. Phospholipid metabolism in Neisseria gonorrhoeae: phospholipid hydrolysis in nongrowing cells. Lipids. 1979 Aug;14(8):718–726. doi: 10.1007/BF02533897. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H. Biological activity of bacteriophage ghosts and "take-over" of host functions by bacteriophage. Bacteriol Rev. 1970 Sep;34(3):344–363. doi: 10.1128/br.34.3.344-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Geizer E. Antibacterial substances produced by different bacteria inhibiting the growth of Neisseria gonorrhoea (preliminary report). J Hyg Epidemiol Microbiol Immunol. 1968;12(2):241–243. [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974 Jan;80(1):1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Baechler C. A., Berk R. S. Morphological studies on relaxed and contracted forms of purified pyocin particles. J Bacteriol. 1969 Jun;98(3):1378–1389. doi: 10.1128/jb.98.3.1378-1389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Shionoya H. Relationship between pyocine and temperate phage of Pseudomonas aeruginosa. 3. Serological relationship between pyocines and temperate phages. Jpn J Exp Med. 1967 Oct;37(5):395–421. [PubMed] [Google Scholar]
- Iijima M. Mode of action of pyocin R1. J Biochem. 1978 Feb;83(2):395–402. doi: 10.1093/oxfordjournals.jbchem.a131926. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Egami F. Receptor substance for pyocin R. I. Partial purification and chemical properties. J Biochem. 1969 Apr;65(4):603–609. doi: 10.1093/oxfordjournals.jbchem.a129053. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M., IKEDA K., EGAMI F. STUDIES OF A PYOCIN. III. BIOLOGICAL PROPERTIES OF THE PYOCIN. J Biochem. 1964 Jan;55:59–64. doi: 10.1093/oxfordjournals.jbchem.a127841. [DOI] [PubMed] [Google Scholar]
- Kageyama M. Effect of pyocin R1 on the glucose metabolism of sensitive cells of Pseudomonas aeruginosa. J Biochem. 1978 Dec;84(6):1373–1379. doi: 10.1093/oxfordjournals.jbchem.a132259. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Tanaka M. Studies on the mode of action of pyocin. I. Inhibition of macromolecular synthesis in sensitive cells. J Biochem. 1965 May;57(5):689–695. [PubMed] [Google Scholar]
- Kaziro Y., Tanaka M. Studies on the mode of action of pyocin. II. Inactivation of ribosomes. J Biochem. 1965 Oct;58(4):357–363. doi: 10.1093/oxfordjournals.jbchem.a128212. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Kageyama M. Biochemical properties of a new flexuous bacteriocin, pyocin F1, produced by Pseudomonas aeruginosa. J Biochem. 1979 Jan;85(1):7–19. doi: 10.1093/oxfordjournals.jbchem.a132332. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Adaptation of the Minitek system for the rapid identification of Neisseria gonorrhoeae. J Clin Microbiol. 1976 Jan;3(1):8–13. doi: 10.1128/jcm.3.1.8-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Mah R. A., Dobrogosz W. J. Regulation of staphylococcal enterotoxin B. J Bacteriol. 1969 Apr;98(1):4–9. doi: 10.1128/jb.98.1.4-9.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. The biology of the gonococcus. CRC Crit Rev Microbiol. 1978;7(2):93–189. doi: 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa I., Maruo B., Kageyama M. Preferential inhibition of lipid synthesis by the bacteriocin pyocin S2. J Biochem. 1975 Jul;78(1):213–223. [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidberry H. D., Sadoff J. C. Pyocin sensitivity of Neisseria gonorrhoeae and its feasibility as an epidemiological tool. Infect Immun. 1977 Feb;15(2):628–637. doi: 10.1128/iai.15.2.628-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Kageyama M. A fluorescent probe response to the interaction of pyocin R1 with sensitive cells. J Biochem. 1977 Feb;81(2):333–341. doi: 10.1093/oxfordjournals.jbchem.a131463. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. 1977 Oct;18(1):210–219. doi: 10.1128/iai.18.1.210-219.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Elmros T., Normark S., Winblad B. Growth pattern and cell division in Neisseria gonorrhoeae. J Bacteriol. 1977 Jan;129(1):333–342. doi: 10.1128/jb.129.1.333-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



