Abstract
Morphine is one of the most prescribed and effective drugs used for the treatment of acute and chronic pain conditions. In addition to its central effects, morphine can also produce peripheral analgesia. However, the mechanisms underlying this peripheral action of morphine have not yet been fully elucidated. Here, we show that the peripheral antinociceptive effect of morphine is lost in neuronal nitric-oxide synthase null mice and that morphine induces the production of nitric oxide in primary nociceptive neurons. The activation of the nitric-oxide pathway by morphine was dependent on an initial stimulation of PI3Kγ/AKT protein kinase B (AKT) and culminated in increased activation of KATP channels. In the latter, this intracellular signaling pathway might cause a hyperpolarization of nociceptive neurons, and it is fundamental for the direct blockade of inflammatory pain by morphine. This understanding offers new targets for analgesic drug development.
Keywords: hyperalgesia, inflammatory pain, nitric oxide, opioids, nociception
Morphine is one of the most prescribed and effective drugs used for treatment of postoperatory and acute severe pain. Nevertheless, its use is frequently limited by undesirable side effects including respiratory depression, tolerance, and addiction. The discovery that morphine can also produce peripheral analgesia in the setting of inflammatory pain opened the possibility of developing peripheral restricted opioids devoid of central side effects (1).
Morphine peripheral analgesia was discovered by its direct effect on already established inflammatory hypernociception induced by prostaglandin E2 (PGE2) injected in rat hind paws (1). Therefore, in contrast to aspirin-like drugs whose analgesic mechanism depends on prevention of nociceptor sensitization by inhibiting synthesis of prostaglandins, opioids are able to directly block ongoing nociceptor sensitization. However, the molecular mechanisms triggered by morphine to promote this action have not been fully elucidated. The present study reports on a series of experiments using behavioral, biochemical, and electrophysiological approaches to address this issue. The following major findings are reported herein: (i) the activation of peripheral opioid receptors in primary nociceptive neurons by morphine triggers a cascade of intracellular signaling events initiated by PI3Kγ/Protein kinase B (AKT); (ii) this is accompanied by activation of neuronal nitric oxide synthase (nNOS) and nitric oxide (NO) production, which (iii) induces an increase in KATP channel currents; and (iv) it causes a hyperpolarization of nociceptive neurons.
Results and Discussion
Based on the evidence that cAMP was the key intracellular second messenger involved in PGE2-induced nociceptor sensitization (2) and that opioid-receptor activation in vitro was coupled to adenylyl-cyclase inhibition (3), it was initially suggested that these drugs counteracted inflammatory hypernociception directly through inhibition of PGE2-induced adenylyl-cyclase activation (1, 4). Subsequent in vitro studies, which confirmed the ability of opioids to inhibit adenylyl-cyclase activity in dorsal root ganglion (DRG) neurons, reinforced the assumption that the peripheral action of morphine was caused by inhibition of adenylyl-cyclase activity (5, 6). Nevertheless, it was striking that morphine still displayed peripheral activity in rats when given 2 hours after intraplantar injection of PGE2, a time when hypernociception was significant but adenylyl-cyclase activity seemed to be irrelevant for the maintenance of the process (Fig. S1 A and B). In addition, hypernociception, induced downstream of adenylyl cyclase by a cAMP analog, was inhibited by morphine (Fig. S1C). Thus, it is clear that mechanisms other than inhibition of adenylyl cyclase account for the ability of peripheral morphine to counteract PGE2-induced hypernociception. It is noteworthy that inflammatory hypernociception is a consequence of primary sensory neuron sensitization, which can be mimicked by PGE2 or sympathetic amines administration in the paws of rats and mice (2, 7). Moreover, there is convincing evidence showing that hypernociception induced by mediators of inflammation, including cytokines and complex stimuli such as complete Freund`s adjuvant (CFA), is ultimately mediated by the direct neuronal acting mediators of hypernociception, such as PGE2 and adrenergic agonists (8). Therefore, in the present study, PGE2 and epinephrine hypernociception were the models used instead of more general inflammatory models to ensure that the mechanisms evaluated were restricted to primary nociceptive neurons. Nevertheless, to add greater relevance to our findings, we also tested our hypotheses in CFA inflammation-induced hypernociception.
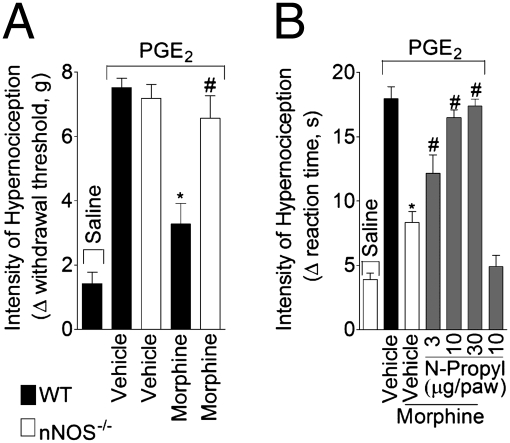
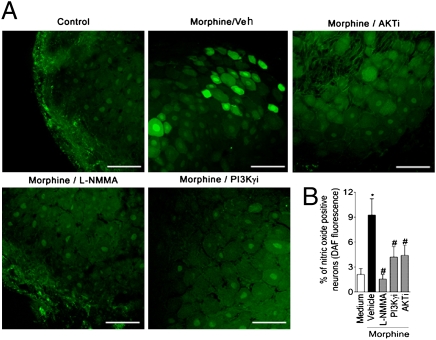
Indirect evidence has implicated the participation of NO derived from nNOS in the peripheral antinociceptive actions of opioid agonists (9, 10). To strengthen this suggestion, we first examined whether or not morphine produced peripheral antinociception in nNOS null mice. Whereas morphine blocked PGE2-induced mechanical hypernociception in wild-type mice, this was not observed in nNOS null mice (Fig. 1A). The effective and local (peripheral effect) dose of morphine was based on the dose–response curve of morphine on PGE2-induced mechanical hypernociception in wild-type mice (Fig. S2 A and B). In rats, a selective inhibitor of nNOS (N-propyl-L-arginine) also reversed the peripheral antinociceptive action of morphine on PGE2- and CFA-induced hypernociception (Fig. 1B and Fig. S2C). In line with these findings, S-nitroso-N-acetylpenicillamine (SNAP; a NO donor), injected locally in paws of mice, was an effective inhibitor of PGE2-induced hypernociception (Fig. S2D). To reinforce this in vivo observation, the ability of morphine to induce the production of NO in primary sensory neurons was examined using the NO-fluorescent indicator DAF. Ex vivo incubation of DRG neurons with morphine induced an increase in the number of neurons positive for DAF fluorescence (Fig. 2 A and B). The fact that L-NG-monomethyl Arginine (L-NMMA; a nonselective NOS inhibitor) treatment reduced the number of positive neurons indicated that the fluorescent signal, in fact, represented NO production (Fig. 2 A and B).
Fig. 1.
Morphine activates the nNOS/NO antinociceptive pathway. (A) Mechanical hypernociception was induced by the injection of PGE2 (30 ng/paw) in the paws of mice. After 30 min, morphine (10 μg/paw) was administrated to wild-type or nNOS-deficient mice (nNOS−/−). Hypernociception was evaluated 1 hour after morphine injection using the electronic von Frey test (n = 6). *P < 0.05 compared with wild type. (B) Mechanical hypernociception in rats was induced by intraplantar injection of PGE2 (100 ng/paw). The antinociceptive effect of a local injection of morphine (6 μg/paw 2 hour after PGE2 injection) on PGE2-induced hypernociception was prevented by treatment with a selective inhibitor of nNOS (N-propyl-L-arginine; 3–30 μg/paw 30 min before morphine injection; n = 7). *, P < 0.05 compared with vehicle treatment. #, P < 0.05 compared with morphine treatment.
Fig. 2.
Morphine stimulated NO production in primary sensitive neurons (the role of PI3Kγ/AKT). (A) Representative images of DRG slices after ex vivo incubation with morphine (10 μM) in the presence or absence of L-NMMA (10 μM), AS605240 (PI3KγI; 100 nM), and AKT inhibitor (100 nM). DAF-FM fluorescence (green) indicates NO production. (Scale bars, 100 μm.) (B) Quantitative analysis of percentage of DRG neurons that increased their DAF-FM fluorescence intensity. *P < 0.05 compared with medium treatment. #P < 0.05 compared with morphine treatment.
Although these results suggest that NO is an analgesic mediator in the periphery, it is noteworthy that the role of NO in the nociceptive system is controversial. In fact, depending on the nociceptive system level (central or periphery) and the amount of NO, it could have either antinociceptive or pronociceptive actions. For instance, in the spinal cord, low and high concentrations of NO cause antinociception and nociception, respectively (11). Furthermore, even in the periphery, the stimulation of the NOS/NO pathway in the epidermis and dermis causes mechanical hyper- and antinociception, respectively (12, 13). One simple explanation for this last contradiction may lay in the differential effect that NO has in intradermal and s.c. nociceptors. This may result from the fact that each tissue might be or is predominantly innervated by different subsets of primary nociceptive neurons.
How does morphine stimulate the production of NO by primary nociceptive neurons? Apart from the well-recognized activation of NO synthases by calcium/calmodulin, alternative mechanisms of activation have been proposed in the last decade. One of these mechanisms involves the phosphorylation of these enzymes by the PI3K/AKT signaling pathway (14, 15). Among the PI3K enzymes family, PI3Kγ is activated by the βγ subunits of Gi protein-coupled receptors, including the opioid receptors (16). Hence, we examined whether or not PI3Kγ/AKT signaling was the initial step involved in the stimulation of the NO pathway by morphine. Morphine-induced increases in NO production by DRG neurons were blocked by incubating cells with a selective PI3Kγ inhibitor (AS605240) or an AKT selective inhibitor (AKT inhibitor IV; Fig. 2 A and B).
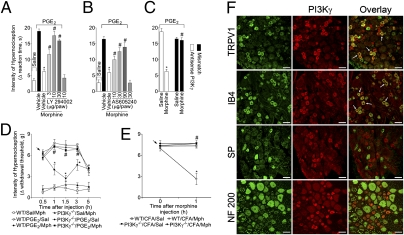
The hypothesis that PI3Kγ/AKT mediates the induction of the NO antinociceptive pathway by morphine implies that this pathway might be involved in the peripheral antinociceptive action of morphine. In agreement, local pretreatment of the rat paw with wortmannin or LY294002, two nonselective inhibitors of PI3Ks, or with the selective inhibitor of PI3Kγ, AS605240, all inhibited the peripheral antinociceptive effect of morphine on PGE2-induced mechanical hypernociception in a dose-dependent manner (Fig. 3 A and B and Fig. S3). The injection of these inhibitors alone in the rat paw did not alter nociceptive threshold (Fig. 3 A and B, last bars, and Fig. S3). It has been shown that intrathecal injection of oligodeoxynucleotide (ODN) antisense inhibits protein expression in peripheral sensory neurons, suggesting that this approach is appropriate to selectively investigate the role of proteins expressed by primary nociceptive neurons (17). We promoted the knockdown of PI3Kγ expression in nociceptive neurons by giving PI3Kγ-specific ODN antisense intrathecally in rats. The treatment prevented the peripheral antinociceptive effect of morphine (Fig. 3C) but did not affect PGE2-induced hypernociception (Fig. 3C). Western blot analyses of DRG from ODN antisense-treated rats showed a reduction in PI3Kγ expression and confirmed the efficacy of the ODN antisense treatment (Fig. S4A).
Fig. 3.
PI3Kγ expression in primary nociceptive neurons and its requirement for the peripheral antinociceptive action of morphine. (A–C) Mechanical hypernociception in rats was induced by ipsilateral injection of PGE2 (100 ng/paw). The antinociceptive effect of morphine (6 μg/paw 2 hours after PGE2 injection) on PGE2-induced hypernociception was prevented by treatment with an inhibitor of PI3Ks (30 min before morphine injection) or (A) LY294002 (3–30 μg/paw; n = 6) or with selective inhibition of PI3Kγ by (B) AS605240 (10–90 μg/paw; n = 7) or (C) ODN antisense (AS) against PI3Kγ (50 μg/it intrathecal/day for 4 days; n = 5). *, P < 0.05 compared with vehicle treatment. #, P < 0.05 compared with morphine or mismatch (MS) treatment. (D) In mice, mechanical hypernociception was induced by the injection of PGE2 (30 ng/paw). After 30 min, morphine (indicated arrow; 10 μg/paw) was injected in the wild-type (WT) or PI3Kγ−/− mice (n = 10). (E) Mice received an intraplantar injection of CFA (10 μL/paw). After 4 hours, morphine (indicated arrow; 20 μg/paw) or saline (Sal) was injected in the WT or PI3Kγ−/− mice. *P < 0.05 compared with vehicle treatment. #, P < 0.05 compared with wild type treated with morphine. (F) Confocal images of typical examples of anti-PI3Kγ immunoreactivity in subpopulations of rat DRG neurons labeled using binding to IB4 or using antibodies to TRPV1, substance P (SP), and neurofilament (NF) 200. Arrows indicate double-labeled neurons. (Scale bars, 50 μm.)
The contribution of PI3Kγ to the peripheral antinociceptive action of morphine was analyzed further in PI3Kγ null mice. PI3Kγ mRNA was expressed in the DRG of wild-type mice but not in DRG from PI3Kγ null mice (Fig. S4B). The mechanical and thermal hypernociception induced by PGE2 or the mechanical hypernociception induced by CFA were not different between wild-type and PI3Kγ−/− mice (Fig. 3 D and E and Fig. S5A). In agreement with the data obtained with PI3K inhibitors, morphine again had no antinociceptive effect in PI3Kγ null mice on PGE2-induced mechanical and thermal hypernociception (Fig. 3D and Fig. S5A). The PI3Kγ-dependent mechanism could be extended to a more natural model of inflammatory pain, because morphine did not present peripheral antinociceptive effect in PI3Kγ null mice subjected to CFA-induced hypernociception (Fig. 3E). However, the intraplantar injection of SNAP produced antinociceptive effect in PI3Kγ null mice as well as in wild-type mice (Fig. S5B), suggesting that PI3Kγ activation is upstream of the NO pathway.
Supporting these findings, immunostaining of rat DRG sections revealed that PI3Kγ was constitutively and selectively expressed by small-size cell diameter, presumably by nociceptive neurons (Fig. 3F). Although PI3Kγ mRNA has previously been detected in rat DRG (18), this shows that PI3Kγ is selectively expressed by nociceptive neurons at the protein level. Confocal analysis of dual immunofluorescence experiments revealed that >95% (± 1%) of PI3Kγ-expressing neurons were isolectin-B4 (IB4)-labeled nonpeptidergic nociceptors (Fig. 3F), and they were also Transient receptor potential cation channel, subfamily V, member 1 (TRPV1)-expressing cells (68 ± 3%) (Fig. 3F). In contrast, <10% (9 ± 1.5%) of PI3Kγ-expressing neurons were substance P-expressing peptidergic nociceptors (Fig. 3F), and none were large-diameter neurofilament 200 (NF200) immunoreactive neurons (Fig. 3F). Taken together, these results show that PI3Kγ is constitutively expressed in a significant proportion of primary nociceptive neurons, giving support to its participation in morphine peripheral analgesia. In functional terms, the expression of PI3Kγ in TRPV1 corroborates the idea that capsaicin-sensitive neurons mediate increased morphine antinociception during rat-paw inflammation and also that activation of opioid receptors inhibits TRPV1-mediated total whole-cell currents in a high number of primary nociceptive neurons (6, 19).
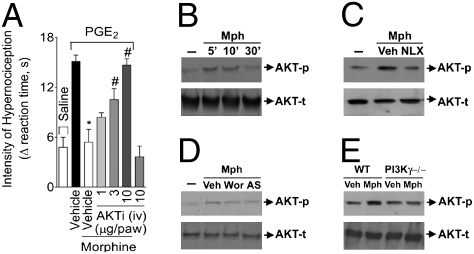
Because PI3Kγ may signal through AKT kinase (20), we investigated whether or not AKT was involved in the peripheral effect of morphine. Local pretreatment of rat hind paws with a selective AKT inhibitor (AKT inhibitor IV) reversed, in a dose-dependent manner, the peripheral antinociceptive effect of morphine (Fig. 4A). The AKT inhibitor did not alter the nociceptive threshold when injected alone in the rat paw (Fig. 4A, last bar). Supporting these in vivo observations, the incubation of DRG neurons with morphine induced a rapid (5–10 min) and transient activation of AKT, revealed by the increase in its phosphorylated form (Fig. 4B). This effect was dependent on opioid-receptor activation, because it was blocked by naloxone (Fig. 4C). Furthermore, the activation of AKT by morphine in DRG-cultured neurons was also dependent on PI3Kγ, because it was prevented by treatment of the cells with wortmannin and AS605240, nonselective and selective PI3Kγ inhibitors, respectively (Fig. 4D). In addition, morphine-induced AKT activation was significantly reduced in DRG-cultured neurons from PI3Kγ null mice compared with neurons from wild-type mice (Fig. 4E). The contribution of the PI3Kγ/AKT/NO pathway to morphine peripheral effect was also observed on epinephrine-induced mechanical hypernociception in rats (Fig. S6A) and mice (Fig. S6B). Although the results presented clearly show an antinociceptive role for the PI3Kγ/AKT signaling pathway, there is also data suggesting a pronociceptive action for this intracellular pathway (21). For instance, thermal and mechanical hypernociception induced by NGF and capsaicin seem to be dependent on the PI3K/AKT pathway (21). These discrepancies are probably caused by the different PI3K isoforms involved in each model. In fact, NGF activates a tyrosine kinase receptor, which is then coupled to a different isoform of PI3K.
Fig. 4.
Participation of AKT in the peripheral antinociceptive effect of morphine. (A) The antinociceptive effect of morphine on PGE2-induced hypernociception was prevented by the treatment of rat paw with AKT selective inhibitor IV (AKTi; n = 10). *, P < 0.05 compared with vehicle treatment. #, P < 0.05 compared with morphine treatment. (B) In vitro stimulation of DRG primary culture neurons from rats with morphine (10 μM) increased the phosphorylation of AKT analyzed by Western blot. (C) Naloxone (NLX- 1 μM) preincubation (10 min) prevented AKT phosphorylation induced by morphine. (D) Preincubation with wortmannin (100 nM) and AS605240 (100 nM) also reduced morphine-induced AKT phosphorylation. (E) Incubation of DRG-cultured neurons from WT mice with morphine also increased AKT phosphorylation but not in neurons from PI3K−/− mice.
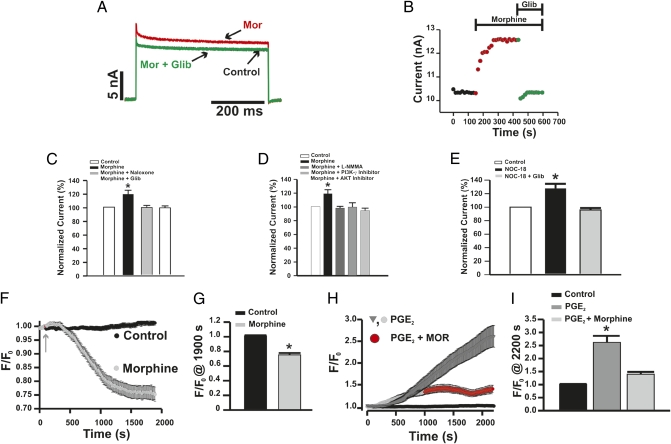
Indirect evidence has also suggested that KATP channels might play a role in the peripheral (22) and central antinociceptive (23) effects of morphine. However, there is no direct evidence that morphine modulates this type of potassium channel in primary nociceptive neurons. In this context, we performed patch-clamp recordings of primary nociceptive neurons in an attempt to analyze whether or not the activation of the PI3Kγ/AKT/NO antinociceptive pathway by morphine resulted in the increase of KATP currents. Figure 5 A and B shows a representative example of whole-cell K+ current and analyses in small-diameter neurons (<30 μm), revealing that there is a population of these neurons (∼40%) in which morphine induced an increase (∼20%) in total K+ current (Fig. 5 A and B). Moreover, the increase in K+ conductance was selectively prevented by glibenclamide, suggesting that it was caused by modulation of KATP channels (Fig. 5 A and B). Furthermore, the increase in activation of KATP channels induced by morphine was also prevented by treating cells with naloxone (Fig. 5C), L-NMMA (Fig. 5D), or selective inhibitors of both PI3Kγ (Fig. 5D) and AKT (Fig. 5D). It is noteworthy that, in these conditions, these inhibitors alone did not alter the baseline K+ conductance. Supporting our hypothesis, NO-donor perfusion also increased total K+ current in a population of neurons in a glibenclamide-sensitive manner (Fig. 5E). These results corroborate the following findings: (i) the up-regulation of KATP by NO was recently shown in primary sensitive neurons (24); (ii) in vivo NO donor-reduced, PGE2-induced hypernociception was prevented by treatment with glibenclamide (25); and (iii) intratecal treatment of rats with ODN antisenses against Kir6.2 and SUR1, two different subunits of KATP that are expressed by DRG neurons (26), prevented morphine and NO-donor antinociceptive effects (Fig. S7 A and B). A role for KATP modulation in the final step of the morphine peripheral antinociceptive pathway is also supported by data showing that activation of KATP channels reverses the sensitization of primary nociceptive neurons caused by PGE2 and that this effect is associated with a hyperpolarization of resting membrane potential and consequently, a reduction in neuronal excitability (26). Furthermore, the incubation of primary nociceptive neurons from the rat trigeminal ganglion with an opioid agonist was shown to produce hyperpolarization of resting membrane potential (27). Here, using a fluorescent indicator of membrane potential, potentiometric fluorescence dye bis-oxonol [DiBAC4(3)], it was observed that morphine produced a hyperpolarization of the membrane potential of DRG neurons (Fig. 5 F and G). Furthermore, the incubation of neurons with PGE2 causes a depolarization of the membrane potential, which was limited by morphine addition (Fig. 5 H and I). Altogether, the present results suggest that morphine, through activation of a PI3Kγ/AKT/NO/KATP channels pathway, blocks ongoing inflammatory hypernociception by containing changes in membrane potential of nociceptive neurons. This mechanism could not be immediately extended to neuropathic pain, because the peripheral analgesic effectiveness of morphine is greatly abrogated during neuropathic pain states (28) (Fig. S8 A and B). Indeed, after peripheral nerve lesions, there is a consistent reduction in opioid-receptor expression in primary nociceptive neurons (28). Recently, it was shown that during neuropathic states, there is a reduction of KATP currents in rat primary sensitive neurons, probably by a defect in up-modulation of these currents by NO and the Ca2+/Calmodulin/CAMKII pathway (24, 29). These findings could be associated with the enhancement of pain sensitivity in neuropathy. Furthermore, based on our present results, the reduction in KATP currents could also account for the reduced analgesic effectiveness of morphine in neuropathic pain.
Fig. 5.
Morphine increases KATP channel currents in primary nociceptive neurons (the role of the PI3Kγ/AKT/NO pathway). (A and B) Under voltage-clamp conditions, incubation of DRG neurons with morphine (10 μM, red line) elicited sustained increases in total whole-cell K+ currents that were not observed in the presence of glibenclamide (10 μM, green line). (C and D) The effect of morphine was not observed when the cells were incubated with naloxone (1 μM), L-NMMA (10 μM), PI3Kγ selective inhibitor (AS605240; 100 nM), and AKT inhibitor (100 nM). (E) In the same conditions, DRG neurons were incubated with NO donor (NOC-18; 10 μM) in the absence or presence of glibenclamide (10 μM). (F) Morphine causes a hyperpolarization of primary nociceptive neurons. Basal fluorescence intensity [DiBAC4(3)] was monitored during 5 min (only the last 100 s are present) followed by the incubation with morphine (10 μM) for 20 min. (G) Analyses of the maximal changes taken at 1,900 s in the membrane potential caused by morphine (control n = 32; morphine n = 9) are shown. (H and I) Morphine attenuates depolarization of nociceptive neurons caused by PGE2. Basal fluorescence of neurons was measured followed by incubation with PGE2 (1 μM; n = 26). After 15 min, morphine (10 μM) was added, and the fluorescence was monitored for 20 min (n = 24). *, P < 0.05 compared with medium treatment. #, P < 0.05 compared with morphine or DETA-NONOate (NOC-18) NOC-18 treatment.
The peripheral antinociceptive action of morphine depends, at least in part, on μ-opioid–receptor activation (30). Therefore, in the last part of this study, we examined whether or not the selective activation of μ-opioid receptors would also induce peripheral antinociception by stimulation of PI3Kγ/AKT. As observed for PI3Kγ expression, rat DRG neurons that express μ-opioid receptors were also positive for TRPV1 (65 ± 2%) (Fig. S9A) or labeling with IB4 (40 ± 3%; Fig. S9A). The peripheral antinociceptive effect of the selective μ-opioid receptor agonist [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO) DAMGO was prevented by treatment with either wortmannin, AS605240, or a selective AKT inhibitor (Fig. S9B). Furthermore, incubation of rat DRG-cultured neurons with DAMGO induced an increase in the activation of AKT, which was prevented by either naloxone or AS605240 (Fig. S9C). The mechanism by which DAMGO induced peripheral antinociception was also evaluated in mice. It was observed that, as in rats, DAMGO, when given locally, produced a dose-dependent antinociceptive effect in the paws of mice (Fig. S10A). The dose of 1 μg DAMGO per paw induced local antinociception without any systemic effect (Fig. S10B). In accordance, the peripheral antinociceptive effect of DAMGO was not observed in PI3Kγ null mice (Fig. S10C). Although the coexpression of PI3Kγ and μ-opioids receptors in primary sensitive neurons was not analyzed directly by immunofluorescence, the pharmacological and biochemical data strongly suggest that these two molecular entities are expressed in the same nociceptive neurons. At this point, we cannot discount the possibility that besides μ-opioid receptors, other opioid receptors might be involved in the peripheral analgesic effect of morphine. Indeed, there is experimental evidence that activation of δ- and κ-opioid receptors produce peripheral analgesia, which is also dependent on NO production (31, 32).
Conclusion
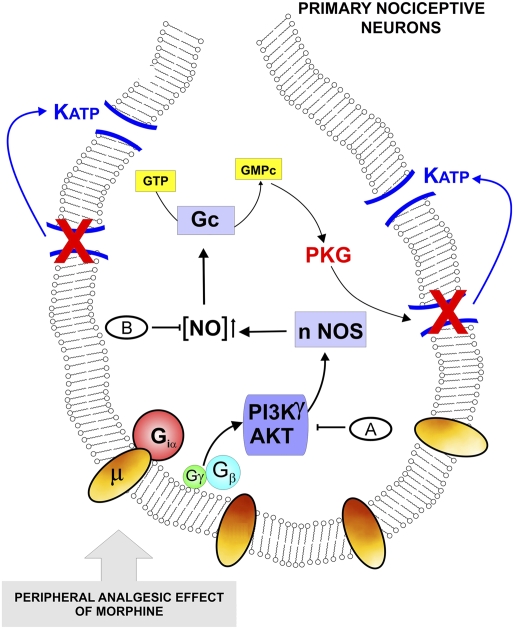
In summary, our results clearly show that the activation of a PI3Kγ/AKT signaling pathway is central to the ability of morphine to directly block inflammatory hypernociception. Morphine seems to act directly on primary nociceptive neurons to activate PI3Kγ/AKT and consequently, stimulate the nNOS/NO/KATP channel antinociceptive pathway (Fig. 6). In the case of the latter, this pathway might cause a hyperpolarization of nociceptive neurons, counteracting their enhanced excitability during the inflammatory process. In the schematic representation of the present hypothesis, we also indicate the possible mechanism of NO modulating KATP currents indirectly through the activation of cGMP/PKG signaling (33). The development of drugs that mimic the action of morphine on this pathway may represent strategies for the treatment of inflammatory pain.
Fig. 6.
Schematic representation of the molecular basis of morphine peripheral analgesia. The activation of opioid receptors in primary nociceptive neurons by morphine triggers the activation of the PI3Kγ/AKT pathway that in turn might cause the stimulation of nNOS and an increase in NO production. In last instance, NO, indirectly through stimulation of cGMP/PKG, causes the up-regulation of KATP currents and promotes the hyperpolarization of primary nociceptive neurons. The results described in the figure indicate the following treatments: (A) wortmannin, AS605240, LY294002, and AKT inhibitor IV, and (B) N-propyl-L-arginine. Morphine did not showed peripheral antinociceptive effect in (B) nNOS−/− and (A) PI3Kγ−/− mice.
Methods
Please see SI Methods for full methods.
Animals.
The experiments were performed in Wistar male rats, C57BL/6 male mice, PI3Kγ-deficient mice (PI3Kγ−/−), and neuronal NO synthase-deficient mice.
Nociceptive Tests.
Constant-pressure rat-paw test.
Mechanical hyperalgesia was tested in rats (180–200 g) as previously described (34). In this method, a constant pressure of 20 mmHg (measured using a sphygmomanometer) is applied by a syringe piston moved by compressed air to a 15-mm2 area on the dorsal surface of the hind paw, and it is discontinued when the rat presents a typical freezing reaction.
Electronic pressure-meter test.
Mechanical hypernociception was tested in mice (20–30 g) and rats as previously reported (35, 36). In a quiet room, mice or rats were placed in acrylic cages with wire-grid floors. The test consisted of evoking a hind-paw flexion reflex with a hand-held force transducer (electronic aesthesiometer; IITC Life Science) adapted with a 0.5- (mice) or 0.7-mm2 (rats) polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hind paw with a gradual increase in pressure.
Primary DRG-Neuron Culture.
Rats or mice were killed by decapitation under anesthesia. DRG were collected and processed as previously described (37). Cells were dissociated and plated in glass-bottomed Petri dishes (for confocal microscopy), six-well plastic plates (for Western blot analysis), or plastic coverslips coated with Matrigel (BD; for patch-clamp analysis).
Western Blot Analysis.
After indicated stimulation, DRG cells were homogenized in a lysis buffer containing a mixture of protease and phosphatase inhibitors (Sigma). Protein samples were separated on SDS/PAGE gel and transferred to nitrocellulose membranes followed by incubation with specific antibodies (21).
DRG Immunohistochemistry.
Animals were terminally anesthetized with urethane and perfused through the ascending aorta with saline followed by 4% paraformaldehyde. After the perfusion, DRG were removed and postfixed, and then, they replaced overnight with 20% sacarose. All of the DRG were embedded in optimum cutting temperature (OCT) OCT, and DRG sections were cut in a cryostat and processed for immunofluorescence. All of the sections were blocked and incubated with a mixture of primary antibodies and finally, by a mixture of conjugated secondary antibodies (Molecular Probes) for 1 hour at room temperature.
Measurement of NO Production by DRG Neurons with 4,5-Diaminofluorescein Diacetate.
NO production by DRG neurons was evaluated as previously described (38) with modifications. Male Wistar rats were killed by decapitation under anesthesia. Lumbar DRG were collected and transferred to a sterile HBSS containing Hepes 10 mM. The DRG were incubated in plastic dishes containing HBSS/Hepes in the presence of 10 μM 4,5-diaminofluorescein diacetate (DAF-FM diacetate; Molecular Probe) for 1 hour at 37 °C. After loading with DAF, the DRG were transferred into DAF-free medium and exposed to the agonists/inhibitors. DRG were cut in slices and then applied to cover slips in Fluormont diluted in PBS (2:1). Sections were examined using a confocal laser-scanning microscope (Leica SP5) using the 488-nm excitation wavelength.
Oligodeoxynucleotides Targeting of PI3Kγ and KATP Channels.
Antisenses ODNs were used to induce a knockdown of PI3Kγ and KATP channel expression in rat DRG neurons. On the day after the ODN treatments, the peripheral effect of morphine (6 μg/paw) or NO donor (SNAP; 200 μg/paw) (25) was evaluated with PGE2-induced hypernociception followed by the removal of DRG (L4-L-6) of the ipsilateral side of PGE2 injection. PI3Kγ, Kir6.2, or SUR1 expressions in DRG from antisense and mismatch were evaluated using Western blot analyses.
Electrophysiology.
A whole-cell, patch-clamp technique was used using an HEKA EPC9 amplifier, ITC 1600 interface, and pulse-pulsefit software (HEKA) (39).
Assay for Changes in Membrane Potential.
The effect of morphine and PGE2 on the membrane potential of DRG-culture neurons was evaluated using DiBAC4(3) (Molecular Probes). DRG-cultured neurons were incubated with normal Tyrode solution containing 5 μM DiBAC4(3). DiBAC4(3) is a bis-barbituric acid oxolol compound that partitions into the membrane as a function of membrane potential. Fluorescence intensity was monitored at 10-s intervals with a confocal microscope (LSM510-Zeiss; Zeiss) using the excitation and emission wavelengths of 470 and 525 nm, respectively (40).
Data Analyses and Statistics.
All results are presented as means ± SEM. The experiments were repeated at least two times. Two-way ANOVA was used to compare the groups and doses at all times (curves) when the hypernociceptive responses were measured at different times after the stimulus injection. The factors analyzed were treatments, time, and time-by-treatment interaction. When there was a significant time-by-treatment interaction, one-way ANOVA followed by Bonferroni’s t test were performed for each time. Alternatively, when the hypernociceptive responses were measured one time after the stimulus injection, the differences between responses were evaluated by one-way ANOVA followed by Bonferroni’s t test. P < 0.05 was considered as significant.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical assistance of Sergio R. Rosa, Fabiola Mestriner, Eleni Tamburus, and Vani Correa. We also thank Dr. Marcelo Napimoga for the help in ODNs construction. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Pesquisa. T.M.C is a recipient of a fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914733107/DCSupplemental.
References
- 1.Ferreira SH, Nakamura M. II—Prostaglandin hyperalgesia: The peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins. 1979;18:191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira SH, Nakamura M. I—Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979;18:179–190. doi: 10.1016/0090-6980(79)90103-5. [DOI] [PubMed] [Google Scholar]
- 3.Collier HO, Roy AC. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974;248:24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- 4.Levine JD, Taiwo YO. Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience. 1989;32:571–575. doi: 10.1016/0306-4522(89)90279-0. [DOI] [PubMed] [Google Scholar]
- 5.Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endres-Becker J, et al. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol. 2007;71:12–18. doi: 10.1124/mol.106.026740. [DOI] [PubMed] [Google Scholar]
- 7.Khasar SG, McCarter GJ, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 8.Verri WA, Jr, et al. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991;201:121–122. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- 10.Leánez S, Hervera A, Pol O. Peripheral antinociceptive effects of mu- and delta-opioid receptor agonists in NOS2 and NOS1 knockout mice during chronic inflammatory pain. Eur J Pharmacol. 2009;602:41–49. doi: 10.1016/j.ejphar.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001;897:9–19. doi: 10.1016/s0006-8993(01)01995-3. [DOI] [PubMed] [Google Scholar]
- 12.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivancos GG, Parada CA, Ferreira SH. Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br J Pharmacol. 2003;138:1351–1357. doi: 10.1038/sj.bjp.0705181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens L, et al. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 17.Bilsky EJ, Wang T, Lai J, Porreca F. Selective blockade of peripheral delta opioid agonist induced antinociception by intrathecal administration of delta receptor antisense oligodeoxynucleotide. Neurosci Lett. 1996;220:155–158. doi: 10.1016/s0304-3940(96)13262-6. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett SE, Reynolds AJ, Tan T, Heydon K, Hendry IA. Differential mRNA expression and subcellular locations of PI3-kinase isoforms in sympathetic and sensory neurons. J Neurosci Res. 1999;56:44–53. doi: 10.1002/(SICI)1097-4547(19990401)56:1<44::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Barthó L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- 20.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues AR, Duarte ID. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K(+) channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocaña M, Del Pozo E, Barrios M, Robles LI, Baeyens JM. An ATP-dependent potassium channel blocker antagonizes morphine analgesia. Eur J Pharmacol. 1990;186:377–378. doi: 10.1016/0014-2999(90)90466-j. [DOI] [PubMed] [Google Scholar]
- 24.Kawano T, et al. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol Pain. 2009;5:12. doi: 10.1186/1744-8069-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares AC, Leite R, Tatsuo MA, Duarte ID. Activation of ATP-sensitive K(+) channels: Mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur J Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 26.Chi XX, Jiang X, Nicol GD. ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons. Brain Res. 2007;1145:28–40. doi: 10.1016/j.brainres.2007.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda M, et al. Opioidergic modulation of excitability of rat trigeminal root ganglion neuron projections to the superficial layer of cervical dorsal horn. Neuroscience. 2004;125:995–1008. doi: 10.1016/j.neuroscience.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- 29.Kawano T, et al. Suppressed Ca2+/CaM/CaMKII-dependent K(ATP) channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc Natl Acad Sci USA. 2009;106:8725–8730. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han SR, et al. Intramuscular administration of morphine reduces mustard-oil-induced craniofacial-muscle pain behavior in lightly anesthetized rats. Eur J Pain. 2008;12:361–370. doi: 10.1016/j.ejpain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Funez MI, et al. Teleantagonism: A pharmacodynamic property of the primary nociceptive neuron. Proc Natl Acad Sci USA. 2008;105:19038–19043. doi: 10.1073/pnas.0807922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacheco DF, et al. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci. 2005;78:54–60. doi: 10.1016/j.lfs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira SH, Lorenzetti BB, Corrêa FM. Central and peripheral antialgesic action of aspirin-like drugs. Eur J Pharmacol. 1978;53:39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- 35.Cunha TM, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- 36.Vivancos GG, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37:391–399. doi: 10.1590/s0100-879x2004000300017. [DOI] [PubMed] [Google Scholar]
- 37.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 38.Haberberger RV, Henrich M, Lips KS, Kummer W. Nicotinic receptor alpha 7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem Cell Biol. 2003;120:173–181. doi: 10.1007/s00418-003-0550-3. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira FA, Galan DT, Ribeiro AM, Santos Cruz J. Thiamine deficiency during pregnancy leads to cerebellar neuronal death in rat offspring: Role of voltage-dependent K+ channels. Brain Res. 2007;1134:79–86. doi: 10.1016/j.brainres.2006.11.064. [DOI] [PubMed] [Google Scholar]
- 40.Dall’Asta V, et al. Membrane potential changes visualized in complete growth media through confocal laser scanning microscopy of bis-oxonol-loaded cells. Exp Cell Res. 1997;231:260–268. doi: 10.1006/excr.1996.3469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.