Abstract
Substance P (SP) is a proinflammatory mediator implicated in inflammatory bowel disease (IBD) and other inflammatory states. SP acts by stimulating the neurokinin-1 receptor (NK-1R) on T lymphocytes and other cell types, and regulates these cells in a complex interplay with multiple cytokines. The mechanisms of interaction among these inflammatory mediators are not yet fully understood. Here, we demonstrate that function of the NK-1R, a member of the G protein-coupled receptor (GPCR) superfamily, is modulated by TGF-β. The latter acts not on a GPCR but via serine-threonine kinase-class receptors. By flow confocal image analysis, we demonstrate that TGF-β delays SP-induced NK-1R internalization on mucosal T cells isolated from a mouse model of IBD and on granuloma T cells in murine schistosomiasis. Furthermore, luciferase reporter-gene assays revealed that NK-1R stimulation activates the nuclear factor of activated T cell- and activator protein-1-dependent signaling pathways, which are known triggers of effector T-cell cytokine production. TGF-β markedly increases SP-induced activation of these signaling cascades, suggesting that delayed NK-1R internalization results in enhanced signaling. Providing a link to amplified immune function, SP and TGF-β, when applied in combination, trigger a strong release of the proinflammatory cytokines IFN-γ and IL17 from intestinal inflammatory T cells, whereas either agonist alone shows no effect. These observations establish precedent that members of two distinct receptor superfamilies can interact via a previously unrecognized mechanism, and reveal a paradigm of GPCR transregulation that is relevant to IBD and possibly other disease processes.
Keywords: G protein–coupled receptor, substance P, transregulation, cytokine, inflammatory bowel disease
Substance P (SP) is a member of the tachykinin family of hormones and a well-established mediator of inflammation (1). SP, an 11-amino acid molecule, is the primary endogenous agonist of the neurokinin-1 receptor (NK-1R), a member of the G protein-coupled receptor (GPCR) superfamily (2). In the gut, these receptors are found on normal human intestinal lamina propria T cells, lymphoid follicles, vascular endothelium, epithelial cells, and myenteric plexus (3, 4), suggesting a role for NK-1Rs in gastrointestinal physiology. Furthermore, expression of NK-1Rs can be induced on subsets of T lymphocytes (5, 6) and macrophages (7, 8). These receptors are activated by SP, which is produced locally in the gut by sensory neurons and various cells of the immune system (i.e., sources of SP in other tissues as well) (9).
An important role of SP and NK-1Rs in immune responses was demonstrated in several mouse models of inflammation caused by various infectious agents (10–14), including the granuloma-inducing parasite, Schistosoma mansoni (15, 16). Inflammatory bowel disease (IBD) results from a dysregulated immune response to constituents of the normal intestinal flora (17). NK-1R has a role in IBD, as suggested by animal models of this condition where SP via NK-1R exacerbates the disease (18, 19). Investigation of the cellular mechanisms whereby SP exacerbates IBD has revealed that NK-1R stimulation induces intestinal lamina propria T cells and macrophages to secrete IFN-γ (20) and IL12 (8), respectively. The latter observations support the concept that activation of the NK-1R helps drive the helper T-cell 1 (Th1) component of the intestinal immune response.
Given the link between NK-1R signaling and immune regulation, it is of interest to understand how various immune modulators interact with and control NK-1R function, particularly in the context of IBD. For example, we showed that IL12, IL18, and TNF-α all induce T cells to express NK-1R, whereas IL10 prevents receptor expression (9, 21). The NK-1R is also regulated by its cognate ligand SP, which, after binding to this receptor triggers its internalization from the plasma membrane, thereby leading to desensitization (22). The latter mechanism of homologous receptor regulation is a common theme among members of the GPCR superfamily. In addition, down-regulation of this type of receptor can also be triggered via cross-talk with a different GPCR, a process known as heterologous desensitization (23).
Here, we establish precedent that modulation of GPCR trafficking and function can occur alternatively by GPCR transregulation by a member of another receptor family. This unique paradigm is exemplified by interaction of NK-1Rs with TGF-β, a potent immune modulator in IBD and other inflammatory states (24). Unlike NK-1Rs, the receptors for TGF-β are not GPCRs, but fall within a separate superfamily of serine-threonine kinase-type receptors (25), with distinct structure and no prior known link to GPCR trafficking. However, we found that TGF-β delays internalization of the SP/NK-1R complex, enabling SP to induce IFN-γ and IL17 production from intestinal T cells. Enhanced secretion of these cytokines suggests a TGF-β-dependent role of SP in the function of Th17, as well as Th1-effector T cells that help drive IBD.
Results
We previously demonstrated that resting naïve T cells express few NK-1Rs. However, NK-1Rs are highly induced in T lymphocytes at sites of inflammation, providing an important avenue of local immune regulation (9). To investigate modulation of NK-1Rs naturally expressed on disease-relevant activated T cells, we isolated such cells from two well-established murine inflammatory models of the disease. T lymphocytes were collected from either the intestinal lamina propria of C57BL/6 IL10−/− mice with NSAID-induced colitis (18), or hepatic granulomas that developed after infection of C57BL/6 wild-type mice with the parasite S. mansoni.
In T cells from either source, Alexa-labeled smSP [(Sar9,Met11)-substance P] was used as a fluorescent agonist ligand for the naturally expressed NK-1Rs, taking advantage of the high specificity of this SP analog for NK-1Rs vs. other neurokinin-receptor subtypes (26). To follow the subcellular localization of NK-1R/ligand complexes in trafficking studies, we configured a recently developed technology that enables time-resolved capturing of confocal images in a modified fluorescence-activated cell sorter (ImageStream). Automated analysis of these images by custom-defined criteria (detailed in Materials and Methods) enabled classifying the subcellular distribution of fluorescence within individual cells into two distinct patterns: (i) being evenly distributed across the cell surface or (ii) being condensed into bright intracellular clusters (corresponding to internalized molecules).
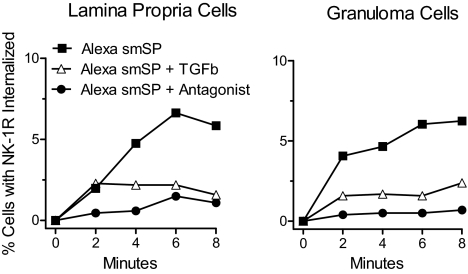
In both lamina propria and hepatic granuloma T cells, fluorescently labeled smSP internalized in a time-dependent manner, which reflects trafficking of the surface NK-1R/ligand complex (Fig. 1). Further supporting involvement of this specific receptor, internalization was prevented by SR 140333, a selective NK-1R antagonist (26). Interestingly, NK-1R internalization was greatly attenuated by TGF-β exposure (Fig. 1 and Fig. S1). The latter observation suggests a previously unknown interaction between NK-1Rs, which are typical members of the GPCR superfamily, and TGF-β receptors, which are dimeric serine-threonine kinase receptors. This interaction may be pathophysiologically relevant, given its applicability to T cells isolated from sites of inflammation.
Fig. 1.
Lamina propria T cells from colitic mice and granuloma T cells from mice infected with S. mansoni display slow NK-1R internalization following TGF-β (TGFb) exposure. T cells were isolated from the intestinal lamina propria of IL10−/− mice with colitis (Left) or from granulomas isolated from the liver of mice infected for 8 weeks with S. mansoni (Right). The T cells were preincubated with TGF-β or cultured alone for 18 h before exposure to the Alexa-labeled SP analog smSP (Alexa smSP, 10−9 M). This peptide binds only the NK1 tachykinin receptor with high-affinity. ImageStream analysis shows that TGF-β pre-exposure decreases smSP-induced NK-1R internalization. The smSP receptor-induced internalization was blocked when cells were cocultured with a highly specific NK-1R receptor antagonist (SR140333, 10−8 M). Data are representative results from multiple determinations. TGF-β effects on agonist-induced NK-1R internalization at early time points in several independent experiments are statistically compared in Fig. S1.
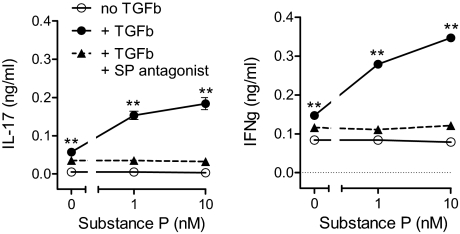
To explore the functional significance of reduced NK-1R internalization in the presence of TGF-β, we investigated the ability of SP to trigger cytokine secretion from lamina propria mononuclear cells (LPMCs) isolated from the murine IL10−/− model of colitis. Specifically, we monitored secretion of two putative drivers of intestinal inflammation, IFN-γ and IL17, which are considered markers of the Th1 and Th17 component of the disease process. Exposing lamina propria T cells to TGF-β was a prerequisite for enabling SP-induced secretion of both cytokines (Fig. 2), a function that was blocked by the NK-1R antagonist. In contrast, without TGF-β pre-exposure, SP had no appreciable effect on cytokine production. Thus, it appears that in the context of inflammation, TGF-β and NK-1R activation synergize to favor Th1 and Th17 expression.
Fig. 2.
After TGF-β (TGFb) exposure, SP induces LPMC to secrete IL17 and IFN-γ. LPMC isolated from IL10−/− mice were cultured 24 h with or without TGF-β (5 ng/mL). The cells were washed and then placed into fresh medium with or without SP, in the presence or absence of NK-1R antagonist (SR140333, 10−8 M). After another 24 h, IL17 and IFN-γ in the culture medium were measured by ELISAs. Data are the mean ± SEM of quadruplicate determinations, and are representative of three independent experiments. **, P < 0.01 vs. secretion in the absence of TGF-β.
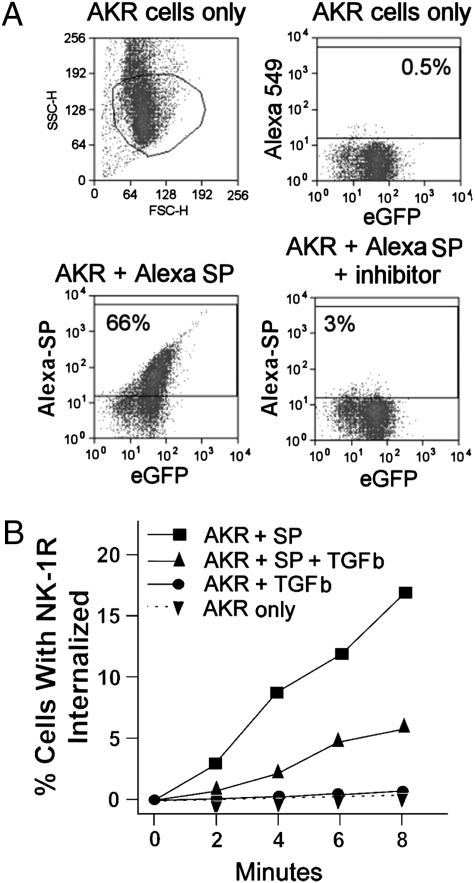
Although our findings with inflammatory T cells suggest that interactions between NK-1Rs and TGF-β occur at the level of receptor internalization, these studies could not exclude the possibility that TGF-β may enhance overall NK-1R expression. The latter scenario, if applicable, could result in an increased density of cell-surface receptors, even if there was no change in NK-1R trafficking. To address this ambiguity in a T cell-related yet better controllable system, complementary studies were conducted using the mouse AKR T-cell line. These cells do not naturally express endogenous NK-1R, but were stably transfected with eGFP-tagged recombinant NK-1R, which enables direct monitoring of receptor trafficking. Expression of the NK-1R eGFP construct is driven by a constitutively active CMV promoter, making expression essentially independent from transcriptional regulation as a potentially confounding variable. FACS analysis revealed that only eGFP-positive cells bound Alexa-labeled SP, suggesting specific complex formation between the fluorescently labeled ligand and the target receptor. In addition, ligand binding was almost completely blocked by the selective NK-1R antagonist/inhibitor SR140333, further confirming that Alexa-SP bound only at NK-1Rs (Fig. 3A).
Fig. 3.
TGF-β (TGFb) delays NK-1R internalization following SP engagement. AKR T cells were stably transfected to express an NK-1R-eGFP fusion protein, and were analyzed by either flow cytometry (A) or ImageStream analysis (B). (A) Flow cytometry shows that AKR cells express eGFP (Right Upper) and that Alexa-labeled SP (Alexa) bound to the majority of these cells (Left Lower). This binding was specific for NK-1R engagement, because a highly specific NK-1R inhibitor (SR140333) blocked Alexa-SP binding (Right Lower). (B) ImageStream analysis reveals that exposing AKR cells to SP at 10−9 M induces NK1R-eGFP internalization. This is reflected by a time-dependent increase in the percentage of cells with fluorescent SP/NK-1R clusters (% cells with NK-1R internalized). Cells preincubated for 18 h with TGF-β show much slower internalization of NK-1R following SP engagement. AKR T cells with or without prior TGF--β exposure and no SP contact display little, if any, NK-1R-eGFP internalization over the observation period. TGF-β effects on agonist-induced NK-1R internalization at early time points in several independent experiments are statistically compared in Fig. S1.
Reminiscent of our initial observations with native T cells, we found that SP (1 nM) induced time-dependent receptor internalization in AKR lymphocytes, and that TGF-β markedly inhibited this process (Fig. 3B, compare squares and upward triangles). In addition, the ability to follow the eGFP-labeled recombinant receptor directly enabled us to demonstrate that there was no spontaneous receptor internalization in the absence of SP, either with or without addition of TGF-β (circles and downward triangles, monitored by eGFP fluorescence to detect NK-1R distribution). Further analysis of these data showed that AKR cells exposed to TGF-β displayed comparable intensity and cell surface distribution of NK-1R-eGFP when compared with AKR cells receiving no such exposure (Fig. S2). These findings suggest that TGF-β does not increase the membrane density of NK-1Rs via altered overall expression, but enhances receptor retention at the cell surface by delaying SP-induced internalization.
To corroborate applicability of this conclusion to endogenously relevant cells, the distribution of Alexa-SP binding to mouse IBD lamina propria T cells was also compared with and without exposure to TGF-β (Fig. S2). Consistent with our observation with the AKR cell line, overnight treatment with TGF-β does not alter the binding of Alexa smSP to lamina propria T cells, providing indirect evidence that the membrane expression of cognate NK-1Rs is not altered. In line with this conclusion, we also observed that the abundance of NK-1R mRNA transcripts is not affected by exposure of either AKR or lamina propria T cells to TGF-β (Fig. S3).
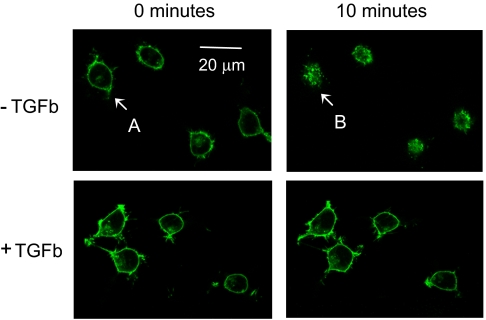
T cell secretion of IL17 and IFN-γ is critically dependent on several proinflammatory transcription factors, including the nuclear factor of activated T cell (NFAT) and activating protein-1 (AP-1) (27). We therefore explored if SP/NK-1R engagement activated these signaling pathways and if TGF-β exposure affected this function. Corresponding studies were performed in HEK293 cells. In contrast to native T cells and T-cell lines, HEK293 cells can be efficiently cotransfected with multiple genes and thus provide a suitable tool for quantitative analysis of NFAT and AP-1 activation by reporter-gene assays. Similar to the AKR line, HEK293 cells have no endogenous NK-1Rs, but do express serine-threonine kinase receptors for TGF-β (see microarray analysis linked to ref. 28). Reminiscent of T cells, HEK cells demonstrate surface expression of receptors when stably transfected with a recombinant NK-1R eGFP construct. Furthermore, these receptors internalize in response to SP (Fig. 4, upper panels), and this process is inhibited by TGF-β exposure (Fig. 4, lower panels). The initial cell surface expression of NK-1Rs, and their SP-induced redistribution into the intracellular space, is shown in more detail in Fig. S4. This figure also provides a semiquantitative assessment of NK-1R internalization in the absence vs. presence of TGF-β, illustrating again the ability of the latter to profoundly inhibit this process.
Fig. 4.
TGF-β (TGFb) markedly delays agonist-induced NK-1R internalization. HEK293 cells stably expressing NK-1R-eGFP were incubated overnight on microscopy coverslips, either in the absence or presence of 5 ng/mL TGF-β. After mounting to the confocal microscopy stage, SP (10 nM) was added at time “0 minutes,” and fluorescent images were taken every 30 s. Within 10 min, SP induces receptor internalization (Upper pair of panels), which is in large part prevented by TGF-β (Lower pair of panels). One cell that is highlighted by arrows (“A” before and “B” after SP exposure) is shown at higher magnification in Fig. S4).
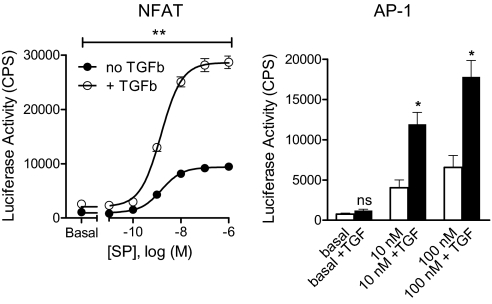
SP stimulation of NK-1R led to concentration-dependent activation of an NFAT-responsive promoter, as reflected by increased luciferase activity of the corresponding construct (Fig. 5, Left). Pretreatment with TGF-β, although not triggering NFAT signaling per se, markedly enhanced the degree to which SP could stimulate this pathway. In contrast to the change in maximal response, the half maximal effective concentration of SP (EC50) was comparable with or without TGF-β exposure (1.54 vs. 1.49 nM; pEC50 = 8.82 ± 0.10 vs. 8.83 ± 0.06, no significant difference). These observations are in line with our hypothesis that TGF-β synergizes with SP to increase NK-1R function by a mechanism that is based on delaying receptor internalization without affecting agonist potency (reflected by similar SP EC50 values, see above). Further analysis revealed that treatment with low TGF-β concentrations of about 1 ng/mL is sufficient for near maximal enhancement of SP-stimulated NFAT signaling in HEK 293 cells (Fig. S5) or inhibition of SP-induced NK-1R internalization in AKR cells (Fig. S6). However, both of these effects are not immediate but become apparent only after overnight exposure of cells to TGF-β (Figs. S5 and S6).
Fig. 5.
TGF-β (TGFb) enhances NK-1R-induced activation of NFAT and AP-1 promoters. HEK293 cells were transiently transfected with cDNAs encoding (i) NK-1R-eGFP, (ii) either NFAT-luciferase (Left) or AP-1-luciferase (Right), and (iii) a lac-Z control gene for data normalization. The cells were grown overnight either in the absence or presence of 5 ng/mL TGF-β, and were then washed and stimulated for 4 h with the indicated concentrations of SP. Data represent the mean ± SEM of three experiments. *, P < 0.05; **, P < 0.01; comparison of SP-induced activity in the absence vs. presence of TGF-β.
TGF-β enhancement of SP-induced function was also observed when activation of AP-1-luciferase as a different reporter gene was monitored (Fig. 5, Right). Thus, TGF-β works in concert with SP to increase two independent functional readouts (NFAT and AP-1-dependent transcription) that are part of separate NK-1R induced signaling cascades. This points to a site of amplification far upstream in the process, consistent with the concept that enhancement of SP-induced function by TGF-β may occur via inhibition of NK-1R internalization and desensitization.
Discussion
The major finding of this study is that TGF-β inhibits NK-1R internalization in cell lines and in T cells at sites of inflammation. The delay in receptor internalization amplifies SP-induced signaling down critical proinflammatory pathways, NFAT- and AP-1-dependent gene transcription, that are important for induction of IL17 and IFN-γ secretion (29). These cytokines, in turn, are known drivers of chronic inflammation. Our studies provide direct evidence for the potential of NK-1Rs to simultaneously stimulate both NFAT- and AP-1-regulated genes, a dual link that has not been previously demonstrated. This finding is of considerable interest because it suggests a plausible signaling mechanism behind the proinflammatory effects of SP in IBD.
Given that both SP and TGF-β are typically present in inflamed tissue, interplay between these two mediators is likely to contribute to the overall immune response in vivo. Our observations underscore the importance of considering such cooperation between two seemingly distinct inflammatory mediators that bind structurally dissimilar receptors yet, by virtue of their interaction, could have a major impact on T cell function.
In particular, this consideration sheds new light on the multifaceted immunomodulatory role of TGF-β. This cytokine per se does not directly stimulate IL17 production and is, in fact, a known inhibitor of IFN-γ synthesis (30). However, IL6 in conjunction with TGF-β, can induce Th17 T-cell differentiation and IL17 secretion (31). We now show that the biological spectrum of TGF-β function is in part dependent on SP, which via NK-1R activation conveys strong proinflammatory properties to this cytokine. This noteworthy synergism is reflected by TGF-β amplification of both SP-induced IL17 and IFN-γ secretion.
The molecular mechanisms whereby TGF-β delays NK1R internalization and leads to enhanced function remain to be established. Our current data suggest that this effect is not a result of increased overall NK1R expression, and requires several hours of TGF-β exposure to become effective. With this limited insight, one may speculate that TGF-β could affect the cellular machinery that controls receptor phosphorylation and internalization (e.g., by reducing the expression and availability of cognate G-protein receptor kinases and β-arrestins) (32). At the same time, given the complexity of GPCR regulation, there are also other candidate mechanisms (e.g., changes in β-arrestin ubiquitination) that may underlie our observations and need to be considered (33). Efforts are currently underway to further dissect the cellular events that link TGF-β with NK1R function.
Such follow-up studies appear particularly warranted, because our findings reveal an unexpected cross-talk between members of two unrelated major classes of membrane receptors, the serine-threonine kinase receptor and GPCR families. We are aware of only one other report showing that a structurally unrelated receptor, in this case the EGF receptor, could alter GPCR trafficking (34). The authors noted that activation of the EGF receptor, a tyrosine kinase-class receptor, led to translocation of G protein-receptor kinase 2 to the plasma membrane of HEK293 cells, thereby facilitating agonist-induced internalization of transfected delta-opioid receptors (which are prototypcial GPCRs). This observation contrasts with our findings, where an opposite effect (delay of NK-1R internalization) occurred after TGF-β exposure. However, taken together the two observations suggest an emerging nontraditional paradigm, one in which GPCRs can be transregulated at the plasma membrane level by structurally different types of receptors (e.g., by members of the serine-threonine or tyrosine-kinase receptor families). This kind of interaction, distinct from but complementing the established processes of GPCR regulation, may contribute to the interplay between different classes of mediators that cooperatively regulate normal physiology and disease.
Materials and Methods
Mice and Infection.
The study used C57BL/6 wild-type mice from Jackson Laboratory and C57BL/6 IL10−/− mice bred at Tufts University. Wild-type mice were s.c. inoculated with 35 cercariae of the Puerto Rican strain of the parasite S. mansoni (35). All studies had been reviewed and approved by the Tufts University & Tufts Medical Center Institutional Animal Care and Use Committee. At 8 weeks of infection, these mice were killed to obtain granulomas from the liver.
Induction of Colitis in IL10−/− Mice.
IL10−/− mice (about 5 weeks old) received piroxicam (Sigma) mixed in their food (NIH-31M) over a period of 2 weeks. The animals were fed with mixtures of 40 mg of drug per 250 g of food during week 1, and 60 mg of drug per 250 g of food during week 2. Mice were subsequently placed on normal rodent chow without piroxicam. LPMC were isolated and studied 7 days after the end of piroxicam treatment.
Isolation and Dispersal of LPMC.
Studies used LPMC from IL10−/− mice with colitis. Gut LPMC were isolated as described below. Intestinal tissue (terminal ileum) was washed extensively with Roswell Park Memorial Institute 1640 medium (RPMI), and all visible Peyer's patches were removed with a scissors. The intestine was opened longitudinally, cut into 5-mm pieces, and then incubated in 0.5 mM EDTA in calcium and magnesium-free HBSS (HBSS) for 20 min at 37 °C with shaking to release intraepithelial lymphocytes and epithelial cells. This was repeated after thorough washing. The tissue was then incubated for 20 min at 37 °C in 20 mL RPMI containing 5% FCS (FCS), 25 mM Hepes buffer, 2 mM L-glutamine, 100 U/mL penicillin, 5 mg/mL gentamycin, 100 mg/mL streptomycin (all GIBCO) and 1 mg/mL collagenase (Sigma #co130). At the end of the incubation, the tissue was subjected to further mechanical disruption using a 1-mL syringe. To remove debris, the LPMC preparations were washed through damp gauze layered in a funnel with RPMI. Then, LPMC were sieved through a prewet 2-cm nylon wool column gently packed into a 10-mL syringe. After washing, up to 2 × 107 cells were layered onto a column of Percoll with a 30:70% gradient. Cells were spun at 2,200 × g at room temperature for 20 min. The LPMC collected from the 30:70 Percoll interface were washed and maintained on ice until used. Cell viability was about 90% as determined by Eosin Y exclusion.
Preparation of Granuloma Cells.
Livers of mice killed after 8 weeks of infection were homogenized for 30 s at low speed in a Waring blender. Granulomas were collected by 1 × g sedimentation and washed three times in RPMI. To prepare a single-cell suspension, the intact granulomas were incubated in a shaking water bath at 37 °C for 30 min in RPMI containing 0.5% collagenase (type 1 from Clostridium histolyticum, Sigma Chemical Co.). The softened granulomas were then disrupted further by repeated suction and expulsion through a 1-mL syringe. The dispersed granuloma cell suspensions were passed through sterile gauze to exclude nondispersed fragments. The cells were collected by centrifugation, washed three times in RPMI, and counted. Cell viability was determined by Eosin Y exclusion.
Isolation of T Cells.
T cells (Thy 1.2+) were isolated from dispersed LPMCs or granulomas cells using paramagnetic beads (DYNAL, Invitrogen Corp) as suggested by the manufacturer. Flow analysis was used to ensure adequate enrichment of isolated cell subsets.
Generation of Stably Transfected Cell Lines.
The mouse lymphoma AKR T cell line was from ATCC and propagated in RPMI with 10% FCS, 25 mM Hepes buffer, 2 mM L-glutamine, 5 × 10−5 M β-mercaptoethanol, 1 mM sodium pyruvate, 100 U/mL penicillin, 5 mg/mL gentamycin, and 100 mg/mL streptomycin (complete medium; all components were from Invitrogen). HEK 293 cells (QB-1 clone) were from Q-Biogene/MP Biomedicals and kept in DMEM medium (Gibco), supplemented with 10% FBS, 100 U/mL penicillin G, and 100 μg/mL streptomycin. The cells were routinely maintained at 37 °C in a humidified environment containing 5% CO2. We introduced into these cells a construct in which rat NK-1R cDNA was subcloned into vector pEGFP-N3, thus encoding the receptor with a C-terminal eGFP tag (provided by Nigel Bunnett, University of California at San Francisco). Transfections were performed using Targetfect-Raw (Targeting Systems) for AKR cells and LipofectAmine (Invitrogen) for HEK 293 cells. Clonal, stably transfected AKR or HEK 293 lines were selected from single cell dilutions in 400 μg/mL Geneticin (Invitrogen).
Measurement of Receptor Internalization in AKR, LPMC, or Granuloma Cells.
Stably transfected AKR cells or freshly isolated LPMC or granuloma cells were cultured in complete medium at 107 cells/mL in 25-cm2 tissue-culture flasks. The cells were kept overnight with or without addition of TGF-β (5 ng/mL, R&D Systems). After harvesting with RPMI, the cells were washed twice and resuspended at 2 × 107 cells/mL in complete medium. Under these conditions, trypan blue exclusion assays suggested that TGF-β does not affect cell viability. Respective percentages of viable cells in the absence vs. presence of TGF-β were as follows: AKR cells, 94.0 ± 1.6% vs. 95.5 ± 2.0%; LPMC, 84.5 ± 1.2% vs. 86.0 ± 0.8%; granuloma cells, 92.0 ± 1.3% vs. 90.1 ± 1.2% (mean ± SEM of three representative experiments).
Just before monitoring receptor internalization, LPMC or granuloma cells were exposed to Alexa-smSP (10−9 M), with or without the SP receptor antagonist SR 140333 (10−8 M, Sanofi Recherche), for analysis of receptor internalization. AKR cells that expressed NK-1R-eGFP were handled similarly, however were exposed to unmodified SP (10−9 M).
The ImageStream system (Amnis) enables quantitative monitoring of the spatial distribution of fluorescent NK-1R/SP complexes within a large number of individual cells. Alexa (attached to smSP) and eGFP (attached to the NK-1R) were excited at wavelengths of 658 and 488 nm, respectively. Confocal images of individual cells taken in continuous suspension flow were automatically captured and analyzed. The device was calibrated to accept cells of the appropriate size (corresponding to either singlets or doublets) and shape (approaching a circle) that fell within a predefined window of fluorescence intensity. The cells were loaded into the ImageStream and data collected 2, 4, 6, and 8 min later. Data from 3,500 to 5,000 cells were recorded for each time interval.
We defined a mask that was applied to each captured-cell image to quantify the level of intracellular clustering of fluorescent NK-1R/SP complexes. Appearance of clusters was defined by detection of punctate fluorescence (being limited in area) with bright topical intensity (showing high peak/mean fluorescence ratio). Technical details on the instrument parameter setup are available upon request. The percentage of cells with detectable NK-1R/SP clusters was recorded at different time points as “% cells with NK-1R internalized.”
Measurement of Cytokine Secretion.
For IFN-γ and IL17 measurements, isolated LPMCs (107 cells/mL) were cultured at 37 °C overnight in 10 mL complete medium. The cells were kept in 25-cm2 flasks, either with or without TGF-β (5 ng/mL). The next day, the cells were harvested, washed, and plated in 96-well microtiter plates (Corning) at 5 × 105 cells per well, either with or without addition of SP (10−9 M). Where indicated, SP antagonist SR140333 (10−8 M) was added to the wells 20 min before SP. The cells were kept at 37 °C for 24 h, and supernatants were then harvested for cytokine measurement.
IL17 and IFN-γ were quantified by selective sandwich ELISAs. IFN-γ was captured with mAB HB170 (ATCC) and detected with a rabbit polyclonal anti-IFN-γ antiserum (Mary Wilson, University of Iowa, IA) followed by application of biotinylated goat anti-rabbit mAb (Accurate Chemical and Scientific Corp). IL17 was captured by mAb MAB721 and detected with a biotin-labeled mAb (BAF421, R&D Systems, Inc.). The ELISAs were developed after exposure to streptavidin-peroxidase conjugate, using 2,2'-azino-di(3-ethylbenzthiazoline-6-sulfonate) (ZYMED Laboratories Inc. Invitrogen) or 3,3′,5,5′-tetramethylbenzidine solutions (Thermo Fisher Scientific) as chromogenic substrates in the IFN-γ and IL17 assays, respectively. The assay sensitivity for either cytokine was 30 pg/mL.
Affinity Detection of Receptor by Flow Cytometry.
AKR cells were washed twice and adjusted to 107 cells/mL in FACS buffer (HBSS containing 20 mM Hepes, 10% FCS, and 0.02% sodium azide). The cell suspensions were then dispensed into microcentrifuge tubes, each containing 106 cells in 100 μL FACS buffer, and stained with saturating amounts of conjugated mAb anti-Thy 1.2-FITC (eBioscience, Inc.) for 30 min at 4 °C. Before adding labeled mAb, each tube received 1 μg anti-FcγR antibody (Rat anti-mouse CD16/32) to block nonspecific binding of conjugated antibodies to Fc receptors. Following staining, the cells were washed twice and resuspended for analysis on a Becton Dickinson FACS 440 flow cytometer (BD Biosciences).
For detection of NK-1Rs, we used smSP (Sigma-Aldrich) labeled with Alexa Fluor 647 (10−9 M) (Invitrogen, Molecular Probes). SmSP only binds the NK-1R, and at the used concentration has no detectable cross-reactivity with other tachykinin receptor subtypes. NK-1R specificity of fluorescent ligand binding was further ensured by its sensitivity to SR 140333 (10−8 M), the receptor selective antagonist.
Confocal Microscopy.
Stably transfected HEK cells were seeded at 1,000 per well in an eight-chambered coverslip slide (Nuc brand, Fisher) and cultured to 50% confluency. Next, 5 ng/mL TGF-β or water solvent was added to the wells 24 h before confocal microscopy. After overnight incubation, the medium was replaced with DME/25 mM Hepes, pH 7.4, to enable handling outside a 5% CO2 atmosphere. The cells were then analyzed using a Leica TCS SP2 AOBS inverted confocal microscope with a directly coupled Coherent Mira 900 femtosecond laser. SP (10 nM) was added directly to the chamber with the selected field visible on the confocal microscope. Images with laser excitation at 488 nM were captured every 30 s after adding SP. If NK-1R antagonist SR 140333 was used, it was added to the chamber 30 min before SP.
Transient HEK Cell Transfection and Luciferase Reporter-Gene Assay.
HEK 293 cells were plated at a density of 4,000 cells per well onto clear-bottom, white 96-well plates and grown for 2 days to ∼80% confluency. The cells were then transiently transfected overnight using Lipofectamine reagent (Invitrogen) with a combination of cDNAs encoding (i) NK-1 receptor (or empty expression vector, 5 ng per well), (ii) a luciferase reporter gene, and (iii) lac Z as a control (5 ng per well). The luciferase reporter constructs included either an NFAT response element or an AP-1 response element (Stratagene) as promoters, and were transfected at 20 and 40 ng per well, respectively. TGF-β (5 ng/mL) or solvent (water) was added after the cells had been exposed for 4 h to the transfection mix. Twenty-four hours later, the cells were incubated with increasing concentrations of SP (Sigma) for another 4 h. The medium was then gently aspirated, and the cells were lysed by addition of Steadylite reagent (PerkinElmer). Luciferase activity was quantified in a TopCount NXT HTS instrument (PerkinElmer). A β-galactosidase assay was then performed after adding the enzyme substrate, 2-Nitrophenyl β-D-galactopyranoside. Following incubation at 37 °C for 30 to 60 min, substrate cleavage was quantified by measurement of optical density at 420 nM using a SpectraMax microplate reader (Molecular Devices). Corresponding values were used to normalize the luciferase activity data.
Statistical Analysis.
Student's t test was used to compare the means of two populations for a significant difference. To test for changes over the course of treatment, the linear mixed model analysis for repeated measures was used. Pairwise comparisons of each follow-up period from baseline was performed with Dunnett's test.
Supplementary Material
Acknowledgments
This work was supported by generous gifts from the Ed Schneider, Ken Gilman, and Harold Friedman families and by Grants DK38327 and DK058755 from the National Institutes of Health and Grant 7-05-RA-08 from the American Diabetes Association.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905877107/DCSupplemental.
References
- 1.O'Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 2.Almeida TA, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 3.Smith VC, Sagot MA, Couraud JY, Buchan AM. Localization of the neurokinin 1 (NK-1) receptor in the human antrum and duodenum. Neurosci Lett. 1998;253:49–52. doi: 10.1016/s0304-3940(98)00618-1. [DOI] [PubMed] [Google Scholar]
- 4.Goode T, et al. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: molecular quantitation and localization. J Immunol. 1998;161:2232–2240. [PubMed] [Google Scholar]
- 5.Cook GA, et al. Molecular evidence that granuloma T lymphocytes in murine schistosomiasis mansoni express an authentic substance P (NK-1) receptor. J Immunol. 1994;152:1830–1835. [PubMed] [Google Scholar]
- 6.Goode T, et al. Differential expression of neurokinin-1 receptor by human mucosal and peripheral lymphoid cells. Clin Diagn Lab Immunol. 2000;7:371–376. doi: 10.1128/cdli.7.3.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 8.Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- 9.Weinstock JV. The role of substance P, hemokinin and their receptor in governing mucosal inflammation and granulomatous responses. Front Biosci. 2004;9:1936–1943. doi: 10.2741/1375. [DOI] [PubMed] [Google Scholar]
- 10.Kincy-Cain T, Bost KL. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J Immunol. 1996;157:255–264. [PubMed] [Google Scholar]
- 11.Castagliuolo I, et al. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest. 1998;101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agro A, Stanisz AM. Inhibition of murine intestinal inflammation by anti-substance P antibody. Reg Immunol. 1993;5:120–126. [PubMed] [Google Scholar]
- 13.Kataeva G, Agro A, Stanisz AM. Substance-P-mediated intestinal inflammation: inhibitory effects of CP 96,345 and SMS 201-995. Neuroimmunomodulation. 1994;1:350–356. doi: 10.1159/000097187. [DOI] [PubMed] [Google Scholar]
- 14.Verdrengh M, Tarkowski A. The impact of substance P signalling on the development of experimental Staphylococcal sepsis and arthritis. Scand J Immunol. 2008;67:253–259. doi: 10.1111/j.1365-3083.2007.02065.x. [DOI] [PubMed] [Google Scholar]
- 15.Blum AM, Metwali A, Mathew RC, Elliott D, Weinstock JV. Substance P and somatostatin can modulate the amount of IgG2a secreted in response to schistosome egg antigens in murine schistosomiasis mansoni. J Immunol. 1993;151:6994–7004. [PubMed] [Google Scholar]
- 16.Blum AM, et al. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. J Immunol. 1999;162:6080–6085. [PubMed] [Google Scholar]
- 17.Sellon RK, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg DJ, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista S, Tramontana M, Maggi CA. Spatial and temporal expression of tachykinins and NK1- and NK2-receptor gene during TNB induced colitis in rats. Neuropeptides. 2008;42:663–670. doi: 10.1016/j.npep.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Blum AM, Metwali A, Elliott DE, Weinstock JV. T cell substance P receptor governs antigen-elicited IFN-γ production. Am J Physiol Gastrointest Liver Physiol. 2003;284:G197–G204. doi: 10.1152/ajpgi.00271.2002. [DOI] [PubMed] [Google Scholar]
- 21.Blum A, Setiawan T, Hang L, Stoyanoff K, Weinstock JV. Interleukin-12 (IL-12) and IL-23 induction of substance p synthesis in murine T cells and macrophages is subject to IL-10 and transforming growth factor beta regulation. Infect Immun. 2008;76:3651–3656. doi: 10.1128/IAI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garland AM, Grady EF, Payan DG, Vigna SR, Bunnett NW. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J. 1994;303:177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 25.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signaling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 28.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 29.Sundrud MS, Rao A. New twists of T cell fate: control of T cell activation and tolerance by TGF-beta and NFAT. Curr Opin Immunol. 2007;19:287–293. doi: 10.1016/j.coi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Young HA. Unraveling the pros and cons of interferon-gamma gene regulation. Immunity. 2006;24:506–507. doi: 10.1016/j.immuni.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 33.Shenoy SK, et al. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Long H, Wu Z, Jiang X, Ma L. EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol Biol Cell. 2008;19:2973–2983. doi: 10.1091/mbc.E07-10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott DE. Methods used to study immunoregulation of schistosome egg granulomas. Methods. 1996;9:255–267. doi: 10.1006/meth.1996.0032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.