Abstract
This study investigated the role of post-ingestive signals in the satiation of thirst or salt appetite. Post-ingestive signals, defined as those arising from the passage of fluid into the duodenum and proximal jejunum, were manipulated by implanting rats with gastric fistulas. After recovery, rats were water deprived and the following day gastric fistulas were opened (sham-drinking) or closed (control). Deprivation-induced thirst significantly increased water intake with sham-drinking rats consuming four-fold more than controls after 120 min access. Subsequently, rats were given sodium deficient chow for 48 h and the next day were administered furosemide and urine was collected. Twenty-four hours later, gastric fistulas were manipulated and rats were given water and 0.5 M NaCl and intakes were measured. After 120 min of access, rats were sacrificed and plasma sodium (pNa) and plasma-renin-activity (PRA) were measured. Furosemide resulted in a loss of 2.2 mEq of sodium in urine and sham-drinking rats consumed significantly more water and 0.5 M NaCl when compared to controls. At 120 min sham-drinking rats consumed 7.5 mEq of sodium nearly twice that of controls but had significantly lower pNa and significantly increased PRA. Interestingly, the ratio of water to 0.5 M NaCl intake was similar in both groups, with each making a mixture of ≈ 0.25 M NaCl. The results suggest that post-ingestive signals are necessary for the satiation of thirst and salt appetite.
Keywords: salt appetite, sham-drinking, angiotensin II
Introduction
Water and sodium consumption are compensatory behavioral responses that increase blood volume and plasma sodium (pNa) when faced with such deficits. Though the peripheral signals that stimulate these behaviors and the central actions responsible for their expression have been studied extensively, the processes that mediate the satiation of thirst and salt appetite have received much less attention. In this regard, the research that has investigated the satiation of water and sodium consumption has used a variety of approaches that have produced conflicting results.
Drinking bouts elicited by water deprivation or intravenous infusion of hypertonic saline are terminated ≈ 8 min after initiation (1). At this time, the increased plasma osmolality that accompanies these treatments is alleviated, but 80% of the water that was ingested remains in the stomach and small intestine (1). The mechanisms underlying the cessation of stimulated water intake remain an area of investigation and oropharyngeal signals (2), visceral osmoreceptors (3), gastric distention (4) and gastrointestinal fill (1) have been examined as contributors. Similarly, the mechanisms underlying the satiation of salt intake driven by sodium deficit remain uncertain. For example, intragastric loading of NaCl failed to reduce subsequent salt intake resulting from adrenalectomy or intraperitoneal dialysis, suggesting that digestive or post-ingestive signals do not contribute to the satiation of sodium consumption (5, 6). These results were interpreted to mean that gustatory or oral-pharyngeal signals arising from the natural act of ingestion are critical for reducing the drive to consume sodium. Nonetheless, ensuing research demonstrated the contrary. Specifically, bypass of gustatory or oral-pharyngeal signals via direct gavage or nasopharyngeal infusion of NaCl into the stomach of rats treated with the natriuretic, furosemide, attenuated salt intake indicating that post-ingestive signals do contribute to the satiation of salt appetite (7, 8). The onset of gavage-induced satiation was much slower than that observed during the natural consumption of NaCl, which incorporates gustatory signals, suggesting that both gustatory and post-ingestive factors may contribute to the satiation of salt intake (8). Thus, the role that gustatory and post-ingestive signals play in sating the salt intake that follows sodium deficiency has not been explicitly discerned.
The goal of the present study was to evaluate the contribution of oral-pharyngeal and post-ingestive signals to the satiation of thirst and salt appetite. Post-ingestive signals, defined here as those arising from the passage of fluid and electrolytes into the duodenum and proximal jejunum, were manipulated by implanting rats with chronic dwelling gastric fistulas, and subsequently, thirst and salt appetite were elicited by water deprivation or furosemide administration, respectively. Together, the results suggest that post-ingestive signals are necessary for the satiation of the thirst or salt appetite that follows water deprivation and sodium depletion. However, the ratio of water to 0.5 M NaCl that is consumed by sodium- depleted rats is independent of post-ingestive feedback and intact gustatory or oral-pharyngeal signals are sufficient to generate this ratio.
Methods
Animals
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300-400 g at the beginning of the study were used. Rats arrived at least 2 wk before the onset of the experiment and were individually housed on a 12-h light, 12-h dark cycle (0600-1800 h) with ad libitum access to pelleted rat chow (LM-485; Harlan Teklad, Madison, WI) and water unless noted otherwise. All procedures were approved by the University of Cincinnati Internal Animal Care and Use Committee. Body weights were monitored throughout the course of the study.
Gastric fistula surgery
For all experiments, rats were anesthetized with ketamine HCl (100 mg/kg body wt, ip; Bristol Laboratories, Syracuse, NY) and acepromazine (1.37 mg/kg body wt, ip; Ayerst Laboratories, Inc., New York, NY). After the onset of anesthesia, rats were given buprenorphine (0.25 mg/kg, sc) and gentamycin (0.2 ml, im). Subsequently, rats were placed on their back and a ventral incision on the midline of the skin below the xiphoid was made, and a separate incision was made on the midline abdominal wall to visualize the stomach. The stomach was gently retracted onto sterile gauze, and a small incision was made on the ventral fore-stomach to introduce a stainless steel fistula. Purse string suture, using chronic gut was made around the incision, the gastric fistula was inserted, and anchored with 2 ties of suture made through the base of the fistula that rest inside the stomach, thereby firmly mounting the fistula to the stomach wall. Purse string suture was closed, a puncture wound was made through the skin just outside of fistula entry point, and the outer portion of fistula housing was passed through the skin puncture wound. Skin incisions were closed with prolene and Vetbond skin adhesive when necessary. A stainless steel washer was threaded over the outer housing of fistula to hold it in place. All rats were given 2 wk to recover prior to the start of experimental testing.
Deprivation-induced water intakes
Rats were implanted with gastric fistulas as described. After 2 wk recovery, rats were placed into hanging wire cages, which contained a longitudinal slit in the floor. To acclimate rats to the gastric fistula preparation, fistulas were opened and stomachs were rinsed with 5 ml of warmed isotonic saline. Gastric fistulas were coupled to plastic tubing that was inserted through the longitudinal slit in the cage bottom. The plastic tubing drained into a 50 ml conical tube, which collected gastric contents. Rats were acclimated this way for 2 h each day for 3 consecutive days. Forty-eight hours later (at 1100 h), rats were water deprived for 24 h. After the water deprivation period, gastric fistulas were opened and stomach contents were rinsed and drained with 5 ml of warmed isotonic saline. Next, gastric fistulas were closed (control; n = 7) or remained open (sham-drinking; n = 6) and fitted for drainage as described above. Subsequently, rats were given water in graduated cylinders and intakes were recorded at 15, 30, 60 and 120 min. After the final intakes were recorded, fistulas were closed and rats were returned to plastic shoebox cages.
Furosemide-induced water and salt intakes
Two weeks later, the same rats were placed into hanging wire cages and acclimated to the gastric fistula preparation for 2 h each day for 3 consecutive days. Forty-eight hours later, rats were injected with 0.35 ml of isotonic saline to establish basal water and salt intakes with the gastric fistulas open or closed. The following day, stomachs were rinsed with 5 ml of warmed water and gastric fistulas were closed (control; n = 6) or remained open (sham-drinking; n = 7) and fitted for drainage as described. Water and 0.5 M NaCl were given in graduated cylinders and intakes were recorded at 15, 30, 60 and 120 min. The subsequent morning, rats were placed on sodium deficient rat chow (Harlan Teklad, Madison, WI) and, 48 h later injected with furosemide (10 mg/kg s.c. in 2 equal injections spaced 1 h apart) and the 0.5 M NaCl solution was removed. Twenty-four hours later, urine was collected, gastric fistulas were opened and stomachs were rinsed with 5 ml of warmed water. Rats were then randomly assigned, regardless of previous sham-drinking experience, to have gastric fistulas closed (control; n = 6) or opened (sham-drinking; n = 7) and fitted for drainage as described. Subsequently, water and 0.5 M NaCl was given in graduated cylinders and intakes were recorded at 15, 30, 60 and 120 min. After the final intakes were recorded, rats were sacrificed and trunk blood samples were collected.
Analysis of urine and plasma sodium
Urine volume was recorded for each rat and the amount of sodium lost after furosemide was determined using a Dual Channel Flame Photometer (Cole-Parmer Instrument Company, Veron Hills, IL). Plasma sodium was determined using a Rapid Lab Blood Gas Analyzer (Bayer, Boston, MA).
Analysis of plasma-renin-activity
A separate cohort of rats was implanted with gastric fistulas and sodium depleted as previously described. Sham-drinking (n=4) and control rats (n=4) were given water and 0.5 M NaCl as in the previous experiment and after 120 min access were sacrificed and trunk bloods were taken for the determination of PRA. Plasma-renin-activity was measured by radioimmunoassay using a 125I kit from Diasorin (Vercelli, Italy) as previously described (9, 10, 11).
Statistical analysis
All data are expressed as means ± SEM. Data were analyzed using Statistica (StatSoft, Tulsa, OK). Deprivation-induced water intakes were assessed using 2-factor repeated measures ANOVA with condition (sham-drinking or control) and time as the factors. For experiments examining salt appetite, water and 0.5 M NaCl intakes were assessed with 3-factor repeated measures ANOVA with condition (sham-drinking or control), treatment (saline or furosemide) and time as the factors. The amount of sodium lost or consumed was assessed with a 1-factor ANOVA. Main effects or interactions (P<0.05) were assessed with a Student Newman Keuls test. The pNa, PRA and the molar concentration of the mixture of water and 0.5 M NaCl that was ingested were assessed with a t-test.
Results
Deprivation-induced water intake
Figure 1 shows deprivation-induced water consumption by control and sham-drinking rats during 120 min access to water. As expected, there were significant effects of time (F = 101.3, P < 0.001) and condition (F = 202, P < 0.001) on deprivation-induced water intake. There also was a significant interaction between time and condition (F=46.03, P < 0.001). Post-hoc analyses revealed that the water intake of control rats was significantly increased (P < 0.05) at 15 and 30 min; however, significant increases were not found thereafter, suggesting satiation. In contrast, the water intake of sham-drinking rats significantly increased (P < 0.05) after each time point with intakes at 120 min significantly greater than that at 60 min. Moreover, the intake of sham-drinking rats was significantly greater (P < 0.05) at 15, 30, 60 and 120 min when compared to that of controls. In fact, after 120 min access to water the intakes of sham-drinking rats was over 4-fold that of their real drinking counterparts.
Figure 1.
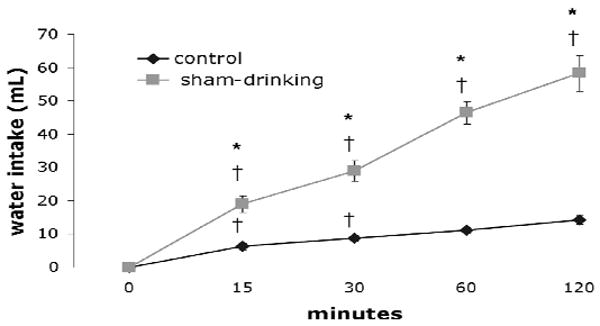
Cumulative intake of control and sham-drinking rats during 120 min access to water. At 15, 30, 60 and 120 min sham-drinking rats had greater intakes when compared to controls. Water intakes of controls ceased to significantly increase after 30 min whereas the intakes of sham-drinking rats increased over time. * = significantly greater than controls P < 0.05; † = significantly greater than the previous time point P < 0.05.
Furosemide-induced water and salt intakes
Figure 2 depicts the intakes of rats treated with isotonic saline or furosemide and then given access to water and 0.5 M NaCl in the control or sham-drinking condition. As expected, there was a significant effect of treatment on the intake of water (F = 40.8, P < 0.001) and 0.5 M NaCl (F = 60.3, P < 0.001) with rats treated with furosemide consuming more when compared to those given isotonic saline. It should be noted that control and sham-drinking rats consumed similar amounts after injection of isotonic saline (i.e. non-stimulated condition). There were also effects of time and condition on the water (F = 34.3, P < 0.001; F = 6.02, P < 0.05) and 0.5 M NaCl (F = 4.45, P < 0.05; F = 4.45, P < 0.05) intakes that followed furosemide. Finally, there was a significant interaction between furosemide and time on water and 0.5 M NaCl intake (F = 31.5, P < 0.001; F = 23.5, P < 0.001). Post-hoc analyses found that the water intake of control rats treated with furosemide was significantly (P < 0.05) increased at 30 min; however, like deprivation-induced intake, furosemide treated controls failed to significantly increase thereafter, suggesting satiation. In contrast, sham-drinking rats treated with furosemide significantly (P < 0.05) increased water intake over time with intakes at 120 min, significantly greater than that at 60 min. Examination of 0.5 M NaCl consumption elicited by furosemide found that control rats significantly (P < 0.05) increased sodium intake at 15 min, but significant increases were not found thereafter. The 0.5 M NaCl intakes of sham-drinking rats treated with furosemide significantly increased at each time point and the amount consumed at 120 min was significantly greater (P < 0.05) than that at 60 min. As predicted, sham-drinking rats consumed significantly more (P < 0.05) water at 30, 60 and 120 min when compared to that of controls. Similarly, sham-drinking rats consumed significantly (P < 0.05) more 0.5 M NaCl at 30, 60 and 120 min when compared to that of controls. Interestingly, despite different absolute water and sodium intakes after furosemide, both control and sham-drinking rats had nearly identical ratios of water to 0.5 M NaCl intake with each making a mixture of ≈ 0.25 M NaCl (see Figure 3).
Figure 2.
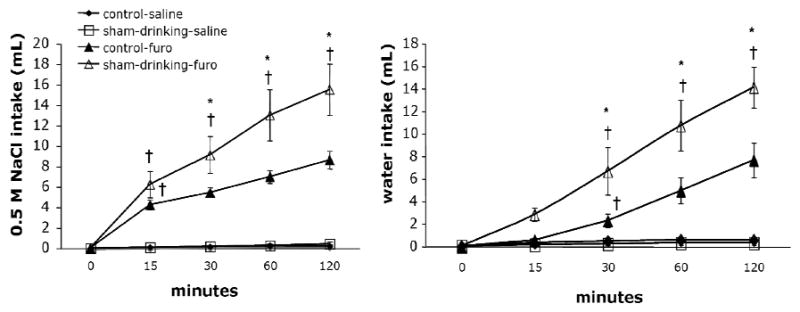
Cumulative intakes of control and sham-drinking rats during 120 min access to 0.5 M NaCl (left) and water (right). Control and sham-drinking rats consume similar amounts of 0.5 M NaCl and water after injection of saline. Administration of furosemide significantly increases 0.5 M NaCl and water intake. Sham-drinking rats consume significantly more 0.5 M NaCl and water at 30, 60 and 120 min when compared to controls. The intakes of sham-drinking significantly increased across time. * = significantly greater than closed P < 0.05; † = significantly greater than the previous time point P < 0.05.
Figure 3.
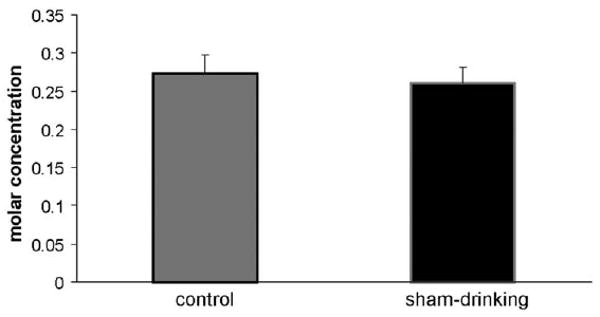
Ratio of 0.5 M NaCl to water intake (mol of Na+ / L of water). Rats treated with furosemide make similar mixtures of 0.5 M NaCl and water, regardless of condition (control vs sham-drinking).
Analysis of urine and plasma sodium
Figure 4 shows the total amount of sodium (mEq) lost and consumed in control and sham-drinking rats. There was no difference in the mEq of sodium lost by control and sham-drinking rats, and consequently, these groups have been combined. As can be seen, furosemide-treated rats lost ≈ 2.2 mEq of sodium in urine, which is similar (P = 0.10) to the amount of sodium consumed by control rats. However, the sodium intake of sham-drinking rats was significantly greater (P < 0.05) than the amount lost as a consequence of furosemide and the amount consumed by controls. Figure 5 shows the pNa of control and sham-drinking rats after 120 min access to water and 0.5 M NaCl. Despite consuming more water and sodium, sham-drinking rats have significantly lower (P < 0.05) pNa compared to controls.
Figure 4.
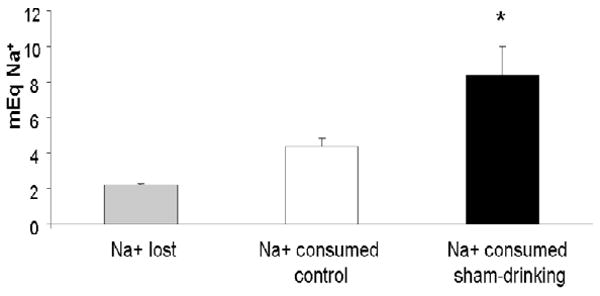
Amount of Na+ lost and consumed after furosemide and 120 min access to 0.5 M NaCl and water. As can be seen, furosemide-treatment caused ≈ 2.2 mEq of Na+ to be lost in urine, which is similar to the amount consumed by controls. In contrast, sham-drinking rats ingested ≈ 7.5 mEq of Na+ nearly twice the amount of that lost in urine or consumed by controls. * = significantly greater than Na+ lost in urine or consumed by controls rats P < 0.05.
Figure 5.
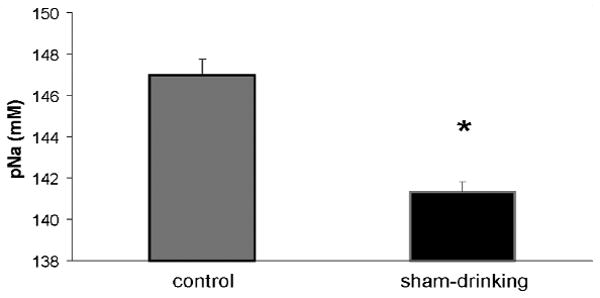
pNa of control and sham-drinking rats treated with furosemide and given 120 min access to 0.5 M NaCl and water. Despite consuming twice the amount of Na+, sham-drinking rats have significantly lower pNa when compared to their control counterparts. * = significantly less than closed P < 0.05.
Analysis of plasma-renin-activity
Figure 6 shows the PRA of rats treated with furosemide and then given 2 h access to water and 0.5 M NaCl in the control and sham-drinking conditions. Despite consuming more water and saline, the PRA of sham-drinking rats is significantly elevated (P < 0.05) when compared to that of controls.
Figure 6.
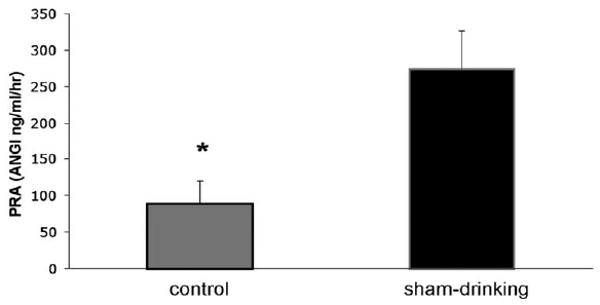
PRA of control and sham-drinking rats treated with furosemide and given 120 min access to 0.5 M NaCl and water. PRA of sham-drinking rats remains significantly elevated when compared to that of controls. * = significantly less than open P < 0.05.
Discussion
The goal of the current study was to evaluate the role of post-ingestive and oral-pharyngeal signals in the water and sodium intake that follow water deprivation or sodium depletion. Elimination of post-ingestive signals greatly increased the deprivation-induced water consumption of sham-drinking rats with intakes at 120 min over 4-fold greater than that of their real drinking counterparts. Furthermore, control rats ceased to increase water intake after 30 min, while the intakes of sham-drinking rats were significantly elevated across time. Similarly, furosemide-induced water and 0.5 M NaCl intake of sham-drinking rats was greatly augmented when compared to that of controls. Specifically, sham-drinking rats consumed nearly twice the amount of 0.5 M NaCl, but had significantly lower pNa and higher PRA when compared to that of controls. Moreover, the water or 0.5 M NaCl intake of control rats didn't significantly increase after 30 or 15 min, respectively; however, the intakes of sham-drinking rats significantly increased after each time point. Although control and sham-drinking rats had different absolute intakes, the ratio of water to sodium consumption was virtually identical with both groups making a mixture of ≈ 0.25 M NaCl. Collectively, these results suggest that post-ingestive signals are necessary for the satiation of water and sodium intake that follows water deprivation or sodium depletion. However, intact gustatory or oral-pharyngeal signals are sufficient to generate the ratio of water to sodium that is consumed after furosemide.
In the present study, water deprivation elicited a greater intake in sham-drinking rats when compared to controls. The deprivation-induced water intake of sham-drinking rats was robust and significantly increased at each time point, whereas most of the control intake occurred within the first 30 min. Collectively, these results suggest that the sham-drinking preparation eliminates a signal that inhibits deprivation-induced water intake.
During sham-drinking the ingested fluid is immediately drained from the stomach, thereby removing any gastric fill that would occur as a consequence of consumption. It is possible that sham-drinking rats consume more water after deprivation because gastric-distention has been eliminated and this potential inhibitory signal for water intake has been released. However, studies examining water intake while the ingested fluid is confined to the stomach argue against gastric distention as an inhibitory signal for water consumption. That is, water deprived rats consume more water when the ingested fluid is confined to the stomach by a pyloric cuff than when it is allowed to enter the duodenum, suggesting that the distention that occurs as a consequence of gastric filling does not contribute to the satiation of deprivation-induced water intake (4, 12, 13).
Water deprivation produces both extracellular and intracellular dehydration; however, the drinking that occurs after rats have been deprived of water, but not food, is believed to be stimulated by increased plasma osmolality (14, 15). This, in conjunction with reports that the increase in vascular volume that accompanies deprivation-induced drinking does not coincide with termination of consumption; indicate that restoration of extracellular volume is not a major factor in the satiation of deprivation-induced water intake (16). Rather, previous studies demonstrate that decreased plasma osmolality is tightly correlated with the cessation of deprivation-induced drinking (15, 16, 17). That is, animals stop drinking when plasma osmolality falls to pre-deprivation levels, an effect that is believed to be due, in part, to the rapid passage of electrolytes from the blood to the duodenum and proximal jejunum (15, 18, 19). The sham-drinking preparation prevents the entry of fluid into these portions of the small intestine, which prohibits the passage of electrolytes from the blood into the gut. Thus, it is possible that the augmented water intake of sham-drinking rats can be attributed to sustained elevation of plasma osmolality because the ingested fluid cannot access the small intestine and decrease plasma osmolality.
Administration of furosemide produces sodium depletion by inhibiting Na/K/Cl co-transporters in the loops of Henle, thereby promoting the excretion of sodium in urine. Subsequently, pNa is decreased and PRA is elevated, which in turn, increases circulating levels of angiotensin II and aldosterone (20). Angiotensin II and aldosterone act in the periphery to initiate vasoconstriction and sodium reabsorption, compensatory physiological responses to hyponaterima. These hormones also act synergistically in the brain to stimulate the arousal of salt appetite, a compensatory behavioral response to sodium deficit (21, 22). Though the arousal of salt appetite has been studied extensively (for review see 23), much less is known about the mechanism that sates depletion-induced sodium and water intake.
In the present study, the furosemide-induced water and 0.5 M NaCl intake of sham-drinking rats was significantly greater than that of controls. Specifically, sham-drinking rats consumed ≈ 7.5 mEq of sodium after furosemide, which is nearly double that of the control intake and is four-times the amount that was lost in urine. Moreover, control rats treated with furosemide did not significantly increase intake of 0.5 M NaCl after 15 min; however, the sham-drinking rats significantly increased consumption of 0.5 M NaCl across time with intakes at 120 min significantly greater than that at 60 min, strongly suggesting that the salt appetite of these animals was not sated. These results are consistent with those of previous research using this technique to investigate salt appetite (24, 25, 26) and strongly indicate that gustatory or oral-pharyngeal signals are not sufficient for the satiation of depletion-induced NaCl consumption.
Clearly, the passage of fluid and electrolytes beyond the stomach and into the duodenum and proximal jejunum is necessary for the satiation of salt appetite, but the neural and humoral signals that apprise the brain that sodium deficit has been alleviated remains unclear. In this regard, depletion-induced NaCl intake is rapidly decreased by infusion of saline into the hepatic-portal vein, which led to the hypothesis that the hepatic vagus nerve carries afferent signals pertaining to the sodium content of the hepatic portal vein and that such signals contribute to the satiation of salt appetite (27). However, research examining salt appetite after section of the hepatic branch of the vagus cast uncertainty on this hypothesis because surgical removal of this nerve did not affect NaCl intake induced by sodium depletion (28).
In our study, after 2 h access to 0.5 M NaCl and water, the pNa of sham-drinking rats was ≈ 142 mM and PRA, a stimulator of salt appetite, was significantly elevated compared to controls. Intravenous infusion of hypertonic saline rapidly increases plasma volume and osmolality and decreases PRA (29), thereby removing angiotensin II and aldosterone as excitatory signals for sodium intake. Because restoration of vascular volume does not predict satiation of fluid intake (16), it is reasonable to speculate that increased pNa inhibits the excitatory humoral signals for salt intake, and consequently, sates deficit induced NaCl consumption. In this regard, the concentration of the ingested fluid was ≈ 250 mM, which creates a concentration gradient that facilitates the passage of electrolytes from the duodenum and proximal jejunum into the blood. Such an exchange may rapidly increase plasma osmolality and attenuate PRA before the ingested fluid has entered the vasculature.
Finally, it is interesting that despite drinking drastically different total amounts of fluid after sodium depletion, sham-drinking and control rats consumed similar water to 0.5 M NaCl ratio. Specifically, sham-drinking and control groups both consumed water and 0.5 M NaCl in proportions that produced a mixture of ≈ 0.25 M NaCl, suggesting that the ratio of water to 0.5 M NaCl that is consumed after sodium depletion is not dependent on post-ingestive signals. Consequently, it is likely that gustatory or oral-pharyngeal signals are sufficient to mediate the amount of water to 0.5 M NaCl that is consumed after sodium deficit. Taste is the sensory modality most heavily implicated in the detection and consumption of sodium. Under normal conditions rodents consume NaCl solutions at a variety of concentrations with maximal consumption occurring at 0.15 M NaCl (30). Sodium depletion renders taste responses to sodium chloride less sensitive (Contreras 1977), which may explain the tendency for sodium-deficient rats to consume concentrated NaCl solutions that were previously rejected. Blunted taste sensitivity likely contributed to ratio of water to 0.5 M NaCl that was consumed by sodium depleted rats and may represent a behavioral mechanism that produces an osmotic gradient that promotes the passage of sodium from the duodenum and proximal jejunum into the blood.
In sum, the present results indicate that post-ingestive signals are required for the satiation of depletion-induced salt appetite. The humoral and neural mechanisms mediating the inhibition of the salt intake that follows sodium depletion remains to be elucidated. We propose that the passage of sodium beyond the stomach allows for the exchange of electrolytes from the duodenum and proximal jejunum to the blood, which rapidly increases plasma osmolality, thereby removing the excitatory humoral signals for sodium consumption.
Acknowledgments
We thank Susan Melhorn, Karen Scott and Anthony Azzara for their valuable advice and assistance. This work was supported by the National Institutes of Health DK79710 (EGK) and HL096830 (EGK), DK68273 (RRS) and DK66596 (RRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffmann ML, DenBleyker M, Smith JC, Stricker EM. Inhibition of thirst when dehydrated rats drink water or saline. Am J Physiolo Regul Integr Comp Phyisol. 2006;290:R1199–207. doi: 10.1152/ajpregu.00715.2005. [DOI] [PubMed] [Google Scholar]
- 2.Thrasher TN, Nistal-Herrera JF, Keil LC, Ramsay DJ. Satiety and inhibition of vasopressin secretion after drinking in dehydrated dogs. Am J Physiol. 1981;240:E394–401. doi: 10.1152/ajpendo.1981.240.4.E394. [DOI] [PubMed] [Google Scholar]
- 3.Baertschi AJ, Pence RA. Gut-brain signaling of water absorption inhibits vasopressin in rats. Am J Physiol. 1995;268:R236–47. doi: 10.1152/ajpregu.1995.268.1.R236. [DOI] [PubMed] [Google Scholar]
- 4.Davis JD, Sayler JL. Confining ingested fluid to the stomach increases water and decreases saline intake in the rat. Physiol Behav. 1997;61:127–130. doi: 10.1016/s0031-9384(96)00352-6. [DOI] [PubMed] [Google Scholar]
- 5.Nachman M, Valentino DA. Roles of taste and postingestional factors in the satiation of sodium appetite in rats. J Comp Physiol Psychol. 1966;62:280–283. doi: 10.1037/h0023667. [DOI] [PubMed] [Google Scholar]
- 6.Falk JL, Lipton JM. Temporal factors in the genesis of NaCl appetite by intraperitoneal dialysis. J Comp Physiol Psychol. 1976;63:247–251. doi: 10.1037/h0024356. [DOI] [PubMed] [Google Scholar]
- 7.Levy CJ, McCutcheon B. Importance of postingestional factors in the satiation of sodium appetite in rats. Physiol Behav. 1974;5:621–625. doi: 10.1016/0031-9384(74)90231-5. [DOI] [PubMed] [Google Scholar]
- 8.Wolf G, Schulkin J, Simson PE. Multiple factors in the satiation of salt appetite. Behav Neurosci. 1984;98:661–673. doi: 10.1037//0735-7044.98.4.661. [DOI] [PubMed] [Google Scholar]
- 9.Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;72:267–274. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 10.Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ. Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006;573:551–562. doi: 10.1113/jphysiol.2006.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall WG. A remote stomach clamp to evaluate oral and gastric controls of drinking in the rat. Physiol Behav. 1973;11:897–901. doi: 10.1016/0031-9384(73)90288-6. [DOI] [PubMed] [Google Scholar]
- 13.Hall WG, Blass EM. Orogastric determinants of drinking in rats: interaction between absorptive and peripheral controls. J Comp Physiol Psychol. 1977;91:365–373. doi: 10.1037/h0077325. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay DJ, Rolls BJ, Wood RJ. Body fluid changes which influence drinking in the water deprived rat. J Physiol. 1977;266:453–469. doi: 10.1113/jphysiol.1977.sp011777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houpt TR, Yang-Preyer H, Geyer J, Norris ML. A rapid feedback signal is not always necessary for termination of a drinking bout. Am J Physiol. 1999;276:R1156–R1163. doi: 10.1152/ajpregu.1999.276.4.R1156. [DOI] [PubMed] [Google Scholar]
- 16.Hatton GI, Bennett CT. Satiation of thirst and termination of drinking: roles of plasma osmolality and absorption. Physiol Behav. 1970;5:479–487. doi: 10.1016/0031-9384(70)90254-4. [DOI] [PubMed] [Google Scholar]
- 17.Novin D. The relation between electrical conductivity of brain tissue and thirst in the rat. J Comp Physiol Psychol. 1962;55:145–154. doi: 10.1037/h0044312. [DOI] [PubMed] [Google Scholar]
- 18.Visscher MB, Varco RH, Carr CW, Dean RB, Erickson D. Sodium ion movement between the intestinal lumen and the blood. Am J Physiol. 1944;141:488–505. [Google Scholar]
- 19.Houpt TR, Houpt KA, Swan AA. Duodenal osmoconcentration and food intake in pigs after ingestion of hypertonic nutrients. Am J Physiol. 1983;245:R181–R189. doi: 10.1152/ajpregu.1983.245.2.R181. [DOI] [PubMed] [Google Scholar]
- 20.Spielman WS, Davis JO. The renin-angiotensin system and aldosterone secretion during sodium depletion in the rat. Circ Res. 1974;35:615–624. doi: 10.1161/01.res.35.4.615. [DOI] [PubMed] [Google Scholar]
- 21.Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineralocorticoids. Behav Neurosci. 1983;97:746–758. doi: 10.1037//0735-7044.97.5.746. [DOI] [PubMed] [Google Scholar]
- 22.Sakai RR, Nicolaidis S, Epstein AN. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am J Physiol. 1986;251:R762–R768. doi: 10.1152/ajpregu.1986.251.4.R762. [DOI] [PubMed] [Google Scholar]
- 23.Krause EG, Sakai RR. Richter and sodium appetite: from adrenalectomy to molecular biology. Appetite. 2007;49:353–367. doi: 10.1016/j.appet.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tordoff MG, Schulkin J, Friedman MI. Hepatic contribution to satiation of salt appetite in rats. Am J Physiol. 1986;251:R1095–R1102. doi: 10.1152/ajpregu.1986.251.6.R1095. [DOI] [PubMed] [Google Scholar]
- 25.Frankmann SP, Sollars SI, Bernstein IL. Sodium appetite in the sham-drinking rat after chorda tympani nerve transection. Am J Physiol. 1996;271:R339–R346. doi: 10.1152/ajpregu.1996.271.2.R339. [DOI] [PubMed] [Google Scholar]
- 26.Roitman MF, Schafe GE, Thiele TE, Bernstein IL. Dopamine and sodium appetite: antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci. 1997;111:606–611. doi: 10.1037//0735-7044.111.3.606. [DOI] [PubMed] [Google Scholar]
- 27.Tordoff MG, Schulkin J, Friedman MI. Further evidence for hepatic control of salt intake in rats. Am J Physiol. 1987;253:R444–449. doi: 10.1152/ajpregu.1987.253.3.R444. [DOI] [PubMed] [Google Scholar]
- 28.Frankmann SP, Smith GP. Hepatic vagotomy does not disrupt the normal satiation of NaCl appetite. Physiol Behav. 1993;53:337–341. doi: 10.1016/0031-9384(93)90214-z. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T, Minai K, Matsui K, Mouri T, Sato T. Effect of various states of hydration on plasma ADH and renin in man. J Clin Endocrinol Metab. 1976;42:79–87. doi: 10.1210/jcem-42-1-79. [DOI] [PubMed] [Google Scholar]
- 30.Richter CP. L'instinct dans le comportement des animaux et de l'homme. Paris, France: 1956. Salt appetite of mammals: Its dependence on instinct and metabolism; pp. 577–632. [Google Scholar]
- 31.Contreras RJ. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977;121:373–8. doi: 10.1016/0006-8993(77)90162-7. [DOI] [PubMed] [Google Scholar]


