Abstract
Previous studies have shown that the selection of women who are at high breast cancer risk for treatment with chemoprevention agents leads to an enhanced benefit/risk ratio. However, further efforts to implement this strategy will require the development of new models to predict the breast cancer risk of particular individuals. Postmenopausal women with elevated plasma or serum estrogens are at increased risk for breast cancer. Therefore, the roles of various enzymes involved in the biosynthesis of estrogens in postmenopausal women have been reviewed in detail. In addition, the potential genotoxic and/or proliferative effects of the different estrogen metabolites as risk factors in the etiology of breast cancer have been examined. Unfortunately, much of the current bioanalytical methodology employed for the analysis of plasma and serum estrogens has proved to be problematic. Major advances in risk assessment would be possible if reliable methodology were available to quantify estradiol and its major metabolites in the plasma or serum of postmenopausal women. High performance liquid chromatography (HPLC) coupled with radioimmunoassay (RIA) currently provides the most sensitive and best validated immunoassay method for the analysis for estrone and estradiol in serum samples from postmenopausal women. However, inter-individual differences in specificity observed with many other immunoassays have caused significant problems when interpreting epidemiologic studies of breast cancer. It is almost impossible to overcome the inherent assay problems involved in using RIA-based methodology, particularly for multiple estrogens. For reliable measurements of multiple estrogens in plasma or serum, it will be necessary to employ stable isotope dilution methodology in combination with liquid chromatography-tandem mass spectrometry (LC-MS/MS) Extremely high sensitivity can be obtained with pre-ionized estrogen derivatives when employed in combination with a modern triple quadrupole mass spectrometer and nanoflow LC. Using [13C6]-estrone as the internal standard it has proved possible to analyze estrone as its pre-ionized Girard T (GT) derivative in sub-fg (low amol) amounts on column. This suggests that in the future it will be possible to routinely conduct LC-MS assays of multiple estrogen metabolites in serum and plasma at even lower concentrations than the current lower limit of quantitation of 0.4 pg/mL (1.6 pmol/L). The ease with which the pre-ionization derivatization strategy can be implemented will make it possible to readily introduce high sensitivity stable isotope dilution methodology in laboratories that are currently employing LC-MS/MS methodology. This will help conserve important plasma and serum samples as it will be possible to conduct high sensitivity analyses using low sample volumes.
Keywords: estrogens, catechol estrogens, radioimmunoassay, electrochemiluminescence immunoassay, stable isotope dilution, gas chromatography/mass spectrometry liquid chromatography/mass spectrometry
1. Introduction
17β-estradiol (estradiol) induces tumors in animal models and in humans and elevated estrogen levels in postmenopausal women are associated with increased breast cancer risk [1]. This is thought to arise from a dual mechanism in which estradiol can act either as a hormone to stimulate aberrant cell proliferation or as the precursor to the formation of genotoxic metabolites [2]. Estrogen biosynthesis which occurs in the breast tissue of postmenopausal women is fundamentally different from that which occurs in the ovaries of premenopausal women. Unlike the ovaries, breast tissue lacks the ability to synthesize androgen precursors. Hence, estrogen production is dependent upon the availability of circulating C-19 androgen precursors and local conversion to estrogens in target tissues such as the breast. The estrogens can then be released into the circulation, which provides biomarkers of tissue estrogen biosynthesis.
Investigative studies and new therapies have significantly improved the recurrence-free and overall survival rates in breast cancer patients [3]. However, the ability to prevent breast cancer in the first place is a much more desirable goal, particularly as the world population is aging and age is an important determinant of breast cancer risk [4,5]. This requires the selection of women who are at higher breast cancer risk for treatment with chemoprevention agents because previous studies have shown that this approach leads to an enhanced benefit/risk ratio [6,7]. Further implementation of this strategy will require the development of new models to predict breast cancer risk of particular individuals [8]. Postmenopausal women with elevated plasma or serum estrogens are at increased risk for breast cancer [4,9-13]. Unfortunately, much of the current bioanalytical methodology employed for the analysis of plasma or serum estrogens has proved to be problematic [14,15]. Major advances in risk assessment would be possible if more reliable methodology were readily available to quantify estradiol and its major metabolites in the plasma or serum of postmenopausal women [4]. These measurements could then be coupled with other risk factors such as mammographic density [16], bone density [17], BMI [18], and single-nucleotide polymorphisms associated with breast cancer [19] to provide an improved model of breast cancer risk [6]. In addition, the availability of sensitive and specific plasma or serum estrogen assays would make it possible to more reliably assess the effects of aromatase inhibitors in postmenopausal women [20]. The present review will focus on the enzymes involved in estrogen biosynthesis, the biological effects of different estrogen metabolites, and the analysis of total (free and non-covalently bound) forms of estradiol and its metabolites in plasma and serum.
2. Enzymology of estrogen biosynthesis in postmenopausal women
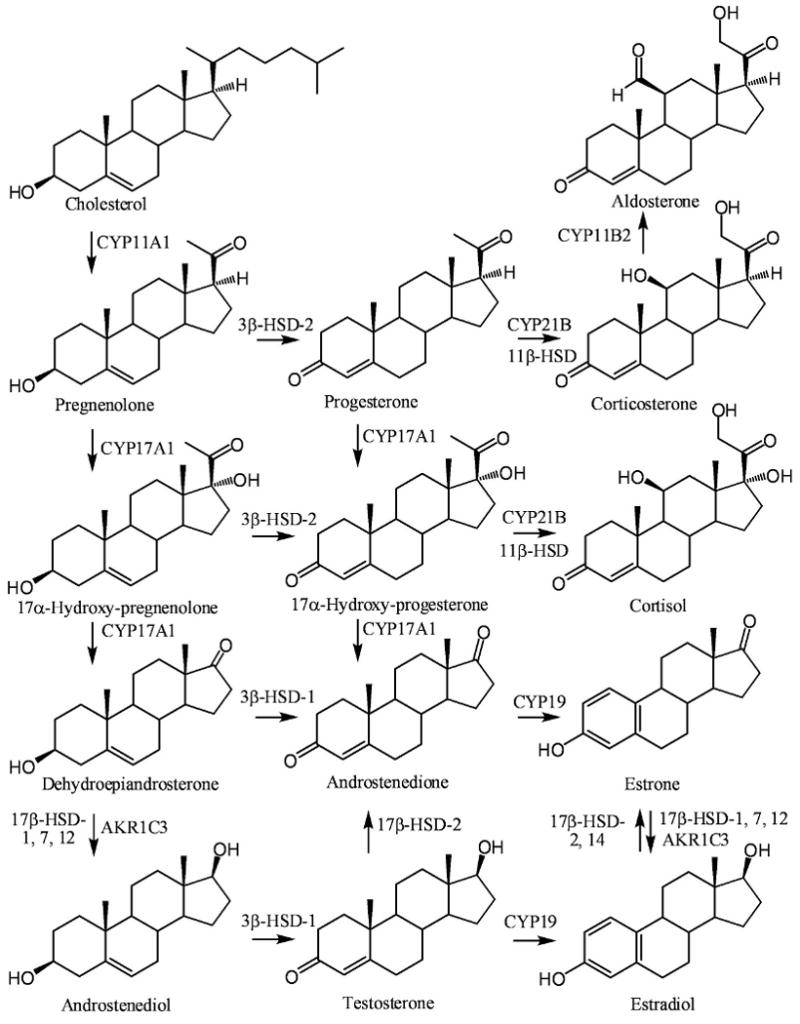
The adrenal cortex is the principal source of the C19 androgens, dehydroepiandrosterone (DHEA) and androstenedione (Figure 1). Cholesterol is first metabolized to pregnenolone by cytochrome P-450 (CYP) 11A1, the side-chain cleavage enzyme [21]. Pregnenolone is then metabolized to progesterone through the action of 3β-hydroxysteroid dehydrogenase (HSD) or to 17α-hydroxy-pregnenolone by CYP17A. 17α-hydroxy-pregnenolone is further metabolized to 17α-hydroxy-progesterone by 3β-HSD. 17α-hydroxy-progesterone can also arise from CYP17A1-mediated hydroxylation of progesterone. Progesterone serves as the precursor to the formation of corticosterone and aldosterone, whereas 17α-hydroxy-progesterone is the precursor to the formation of cortisol (Figure 1). DHEA arises from CYP17A1-medated metabolism of 17α-hydroxy-pregenolone. It is converted to androstenedione by 3β-HSD-2 [22]. Androstenedione can also arise from CYP17A1-mediated oxidation of 17α-hydroxy-progesterone [21].
Figure 1.
Biosynthesis of estrogens from circulating C-19 androgens arising from cholesterol metabolism in the adrenal cortex of menopausal women.
Circulating DHEA and androstenedione derived from the adrenal cortex in postmenopausal women can undergo further metabolism to androgens and estrogens in the breast (Figure 1). DHEA is metabolized to androstenediol by 17β-HSDs [21,23] and to androstenedione by 3β-HSD-1 [22]. Androstendiol is converted to testosterone by 3β-HSD-1 [22]. Testosterone can also arise from 17β-HSD-3-mediated reduction of androstenedione in testicular Leydig cells [24] and so this pathway is not significant in premenopausal women. 17β-HSD-1, 5, 7, and 12; the individual isoforms that can metabolize androstenediol to androstenedione, are expressed in epithelial cells of acini and/or ducts as well as in the stromal cells in the breast [25]. Human 17β-HSD-5 belongs to the aldo-keto reductase (AKR) superfamily [26] is identical to AKR1C3 [27]. It is widely expressed in human tissues including the prostate, endometrium, and mammary gland [28]. AKR1C3 as well as 17β-HSD-1, 7, and 12 are all expressed in breast tumor tissue [26,29]. Aromatase (CYP19), which converts androstenedione to estrone and testosterone to estradiol, is expressed in stromal and carcinoma or parenchymal components of breast cancer tissue [30]. Estrone is converted to estradiol in breast tissue by AKR1C3 together with 17β-HSD-1, 7, and 12 (Figures 1 and 2) [23,27,31,32]. In contrast, 17β-HSD-2, converts estradiol back to estrone using NAD+ as a co-factor [31], in a similar mechanism to that observed with hydroxyprostaglandin dehydrogenase [33]. It is expressed in normal epithelium of the breast and is thought to modulate the exposure of breast tissue to estradiol. In addition to its ability to metabolize estradiol, 17β-HSD-2 can convert testosterone back to androstenedione [34]. The more recently discovered 17β-HSD-14, is also involved in NAD+-mediated conversion of estrone to estradiol and so it could also be involved in increasing the exposure of breast tissue to estradiol [35].
Figure 2.
Enzymes involved in estradiol metabolism.
Estrogens synthesized in the breast from androgens in postmenopausal women are probably only biologically active at a local tissue level in a paracrine [21] or intracrine fashion [36]. Therefore, the total amount of estrogens synthesized in the breast tissue is quite low but the local concentrations are sufficient to exert significant biological activity. In postmenopausal women mesenchymal cells of the adipose tissue become an important source of estrogens [21]. As a result, the extent of estrogen biosynthesis in postmenopausal women is determined to a significant extent by the amount of adipose tissue that is present. This is of clinical importance because there is a decreased risk of osteoporosis in overweight postmenopausal women, which is thought to arise from increased estrogen biosynthesis [6]. For example, women with a body mass index (BMI) < 18.5 kg/m2 (underweight) nearly tripled the fracture risk compared with a BMI > 25 kg/m2 (overweight) [37]. In contrast, obesity is positively correlated with an increase in breast cancer risk, which is thought to arise from increased estrogen biosynthesis in the breast [6,38]. Obese subjects have an approximately 1.5-3.5-fold increased risk of developing breast cancer compared with normal-weight subjects, and between 15 and 45% of these cancers have been attributed to being overweight (BMI, 25.0-29.9 kg/m2) [39]. Interestingly, recent studies have shown that exercise-induced fat loss can lead to a decrease in serum levels of estradiol and estrone [40,41].
3. Estradiol metabolism
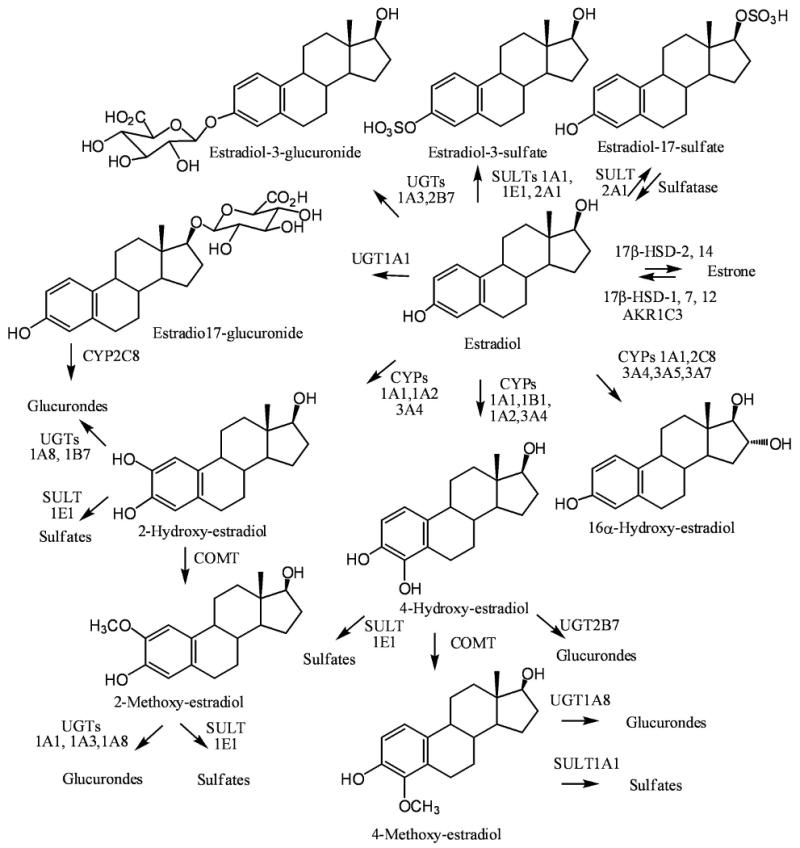
Estradiol is metabolized to a 3-glucuronide by UGT1A1 [42] and to a 17β-glucuronide by UGT1A3 and 2B7 (Figure 2) [43,44]. It is metabolized to estradiol-3-sulfate by SULT1A1 [45,46], SULT1E1 [46,47], and SULT2A1 [48]. Estradiol-17β-sulfate was also observed a minor metabolite in SULT2A1-mediated estradiol metabolism (Figure 2). Tissue steroid sulfatase can convert estradiol-3-sulfate back to estradiol [49]. Therefore, in menopausal women, circulating estradiol-3-sulfate can serve as a precursor to estradiol formation in breast tissue. Estradiol also undergoes extrahepatic oxidation by CYP1A1 and hepatic oxidation by CYP1A2 and CYP3A to give the catechol derivatives, 2-hydroxy-17β-estradiol (2-hydroxy-estradiol) and 4-hydroxy-17β-estradiol (4-hydroxy-estradiol) (Figure 2) [50]. Estradiol-17β-glucuronide is a substrate for hepatic CYP2C8-mediated oxidation to the corresponding 2-hydroxy-catechol metabolite [51]. There is also evidence that estradiol-17β-sulfate can be metabolized to 2- and 4-hydroxylated metabolites [52]. 2-hydroxy-estradiol undergoes COMT-mediated metabolism to 2-methoxy-3-hydroxy-17β-estradiol (2-methoxy-estradiol) and with lower catalytic efficiency in vitro it also forms 2-hydroxy-3-methoxy-17β-estradiol [53]. It also undergoes UGT1A8- and UGT1B7-mediated metabolism to glucuronide conjugates [44], and SULT1E1-mediated metabolism to sulfate conjugates [54]. Similarly, 2-methoxy-estradiol is converted to glucuronide conjugates by UGT1A1, 1A3, and 1A8 [44], and to sulfate conjugates by SULT1E1 [55].
CYP1B1, which is primarily expressed in extrahepatic tissues such as the breast and lung, converts estradiol to 4-hydroxy-estrodiiol with relatively high regioselectivity [56]. CYPs 1A1, 2C8, 3A4, 3A5, and 3A7 can also metabolize estradiol to 16α-hydroxy-estradiol [57]. 4-hydroxy-estradiol was found to mutagenic in the I-select cII assay in BB rat2 cells [58]. Under similar conditions, 2-hydroxy-estradiol was inactive [58]. The mutational spectrum obtained after treatment of the BB rat 2 cells with 4-hydroxy-estradiol contained a considerable proportion of mutations at A:T base pairs, suggesting that 2′-deoxadenosine was a major target of the reactive metabolite derived from the catechol estrogen. In addition, 4-hydroxy estradiol but not 2-hydroxy estradiol induced the expression of hypoxia-inducible factor 1α and vascular endothelial growth factor A in human ovarian carcinoma cells [59]. Furthermore, 4-hydroxy-estradiol can readily redox cycle with the corresponding semiquinones and quinones causing the generation of reactive oxygen species together with the formation of quinone-derived DNA adducts [60,61]. A potential route for quinone is detoxification is through the formation of glutathione (GSH)-adducts [62]. However, the quinone derivative from 4-hydroxy-estradiol was found to covalently modify and inactivate the glutathione-S-transferases involved in GSH-adduct formation, suggesting that alternative detoxification pathways are more important [63]. Detoxification of 4-hydroxy-estradiol occurs by COMT-mediated metabolism with high catalytic efficiency in vitro to give 3-hydroxy-4-methoxy-17β-estradiol (4-methoxy-estradiol) [53] as well as by UGT2B7-mediated metabolism to glucuronide conjugates [44,64] and SULT1E1-mediated metabolism to sulfate conjugates [47]. Similarly 4-methoxy-estradiol undergoes UGT1A8-mediated metabolism to a glucuronide conjugate [44] and SULT1A1-mediated conversion to a sulfate conjugate [55]. It has been suggested that CYP1B1-mediated 4-hydroxy-estradiol formation in breast tissue is involved in the initiation of carcinogenesis [65-69]. Conversely, COMT-mediated conversion of 2-hydroxy-estradiol to its 2-methoxy metabolite potently inhibits the proliferation of breast cancer cells in vitro [70,71]. Therefore, 2-methoxy-estrradiol could be a chemopreventive agent. Mechanistic studies indicate that the actions of 2-methoxy-estradiol are mediated through inhibition of the pro-angiogenic transcription factor hypoxia-inducible factor 1α, c-Jun NH2-terminal kinase signaling, and the generation of reactive oxygen species [72]. 2-methoxy-estradiol also efficiently induces mitotic arrest, apoptosis, and autophagic cell death in glioma cells in vitro, which has stimulated the search for new drugs to treat gliomas based on the structure of 2-methoxy-estradiol [73].
4. Estrone metabolism
Estrone is metabolized to a 3-glucuronide by UGT1A8 [44] and to a 3-sulfate at high concentrations by SULT1A1 and at low concentrations by SULT1E1 (Figure 3) [74]. Estrone also undergoes extrahepatic oxidation by CYP1A1 and hepatic oxidation by CYP1A2 and CYP3A in a similar manner to estradiol to give the catechol derivatives, 2-hydroxy-estrone and 4-hydroxy-estrone (Figure 3) [50]. In contrast, CYP1B1, metabolizes estrone to 4-hydroxy-estrone with relatively high regioselectivity [56]. 4-hydroxy-estrone stimulated the growth of MCF-7 breast cancer cells in vitro and had carcinogenic effects in animal models [71]. Significantly, 2-hydroxy-estrone was unable to induce tumors in the same animal models [71]. 2-hydroxy-estrone undergoes COMT-mediated metabolism to 2-methoxy-estrone and with low catalytic efficiency to 2-hydroxy-3-methoxy-estrone, whereas 4-hydroxy-estrone is primarily metabolized to 4-methoxy-estrone [53]. 2-hydroxy-estrone is converted to a 3-glucuronide conjugate by UGTA1A1, whereas and 4-hydroxy-estrone is converted to the 3-glucuronide conjugate by UGT1A8 [44]. 4-hydroxy-estrone is also converted to a 4-glucuronide conjugate by UGT1A9 [44] and there is evidence that glucuronide conjugation is mediated through the action of UGT2B7 [75].
Figure 3.

Enzymes involved in estrone metabolism
4-hydroxy-estrone can form depurinating DNA-adducts [2] and unlike 2-hydroxy-estrone, it possesses partial estrogenic activity [76]. 2-methoxy-estrone does not appear to have antitumor activity in its own right in contrast to the corresponding 2-methoxy-estradiol metabolite [70]. It has been suggested that reductive metabolism by 17β-HSD type 1 in the breast could convert 2-methoxy-estrone to the active 2-methoxy-estradiol [70]. However, oxidative metabolism of the resulting 2-methoxy-estradiol by 17β-HSD type 2 could convert it back to 2-methoxy-estrone (Figure 3) [77]. Therefore, the net anti-tumor activity would depend upon the relative amounts of reducing and oxidizing HSDs that are present in a particular target tissue.
In addition to CYP3A4-medatiated metabolism of estrone to the corresponding 2- and 4-hydroxy catechols [78], estrone also undergoes metabolism to 16α-hydroxy-estrone [57,79]. In addition, CYPs 1A1, 2C8, 3A5, and 3A7 can also metabolize estrone to a 16α-hydroxy-estrone, which could also potentially arise from spontaneous rearrangement of 3,16α-dihydroxy-17β-estradiol, (16α-hydroxy-estradiol) [80]. 16α-hydroxy-estrone has been identified as a circulating metabolite [50,81], which is present at higher concentrations in breast cancer tissue when compared with normal tissue [82]. In addition, 16α-hydroxy-estrone caused genotoxic damage and aberrant proliferation in mouse mammary epithelial cells [83]. 16-hydroxy-estrone was also shown to increase cyclin D1 protein levels in MCF7 breast cancer cells by almost fourfold compared with control un-treated cells [84]. This suggests that 16α-hydroxy-estrone is involved in tumor initiation thorough its genotoxic effects [85,86] and in tumor promotion and progression through its ability to induce cellular proliferation [80]. It has been proposed that a shift toward 2-hydroxy-estrone from the 16α-hydroxy-estrone metabolic pathway, as indexed by the 2-hydroxy-estrone to 16α-hydroxy-estrone ratio, will be inversely associated with breast cancer risk [87]. In fact, several recent epidemiological studies have found that women with a high ratio of serum 2-hydroxy-estrone metabolites to 16α-hydroxy-estrone metabolites are at a decreased risk for breast cancer [12,88].
5. Quantitative Analysis of estrogens in the plasma and serum of postmenopausal women
Estradiol and its metabolites are present in plasma and serum in the free unbound form, non-covalently bound to steroid binding proteins, and as glucuronide and sulfate conjugates. Concentrations of the free (unbound) forms of plasma and serum estrogens in postmenopausal women are in the fg/ml range, which puts them below the limit of quantitation (LOQ) of routine assays [89,90]. The LOQ is defined as the lowest concentration of an analyte in a sample that can be quantitatively determined with an acceptable precision and accuracy [91]. A minimum requirement is that replicate determinations (n=5) can be conducted with a precision of better that 20 % and an accuracy of between 80 % and 120 %. Therefore, estrogens are quantified as a combination of free and non-covalently bound (total) forms with typical serum estradiol concentrations of 2-21 pg/mL in postmenopausal women as determined using specific MS-based methodology [92]. The concentrations of free unbound forms are then determined by analyzing the amount of plasma steroid binding protein [93] and subtracting the amount of each individual estrogen calculated to non-covalently bind to this protein [94,95]. In contrast, glucuronide and sulfate estrogen conjugates re present in much higher concentrations can be readily quantified after hydrolysis of the plasma or serum with arylsulfatase/β-glucuronidase and methanolysis with acetyl chloride in methanol [96]. The minor amounts of non-conjugated forms can then be subtracted to determine the concentrations of conjugated estrogens that are present in the plasma or serum sample.
High-quality estrogen assays with high sensitivity, specificity, and reproducibility are essential for conducting sophisticated epidemiologic studies [97]. There are three major bioanalytical methods used currently, immunoassay [90], GC-MS/MS) [98], (LC)-MS/MS [92]. RIAs and electrochemiluminescence immunoassays (ECLIAs) are by far the easiest to implement and the most widely used. However, they are fraught with numerous problems it difficult to provide accurate concentrations for the low level samples in postmenopausal women [89,99-101]. For example, conjugated estrogens are present in plasma and serum at 2-3 orders of magnitude higher in concentration than the corresponding un-conjugated forms [102,103]. Therefore, even if cross-reactivity is in only in the 1 % range, they can contribute to the antigen-antibody interaction and provide falsely elevated values. In addition, un-conjugated steroids such as estriol that are present in plasma can lead to elevated values [104]. When prior chromatographic separations are performed to remove interfering cross-reacting substances, corrections for recovery can be made using radiolabeled analogs as internal standards [103]. Unfortunately, there is no way to readily determine whether the radioactive analogs have decomposed during the analytical procedure. In addition, the trace amounts of radioactivity that are used cannot act as carriers through the assay. Therefore, if selective binding to active sites on glassware or other surfaces occurs during extraction and chromatography, significant losses of the estrogen analytes can occur. Poor recoveries can significantly impact on assay precision and recovery. Furthermore, it is possible that there are endogenous substances present in an individual plasma or serum sample that can modulate the antibody/antigen interaction, which would lead to erroneous values. In spite of these potential problems, HPLC coupled RIA currently provides the most sensitive and best validated immunoassay method for the analysis for estrone and estradiol in serum samples obtained from postmenopausal women [105,106]. This contrasts with the inter-individual differences in specificity observed with many other immunoassays [100,101,104,107], which have caused significant problems when interpreting epidemiologic studies of breast cancer risk in menopausal women [15].
It is almost impossible to overcome the inherent assay problems involved in using RIA-based methodology, particularly for multiple estrogens. For reliable measurements of multiple estrogens in plasma or serum, it is necessary to employ stable isotope dilution methodology in combination with LC-MS/MS or GC-MS/MS. These technologies represent the “gold standard” for the analysis of multiple estrogens when they are used under rigorously validated conditions. Losses during the extraction and chromatographic analysis are inherently taken into account by the use of an internal standard for each estrogen analyte that has identical physical properties but differs only in mass. Stable isotope analogs also act as a carriers to prevent non-selective losses of trace analytes through binding to active surfaces during extraction and analyses [108]. Until recently, this ideal condition was not possible for estrogens because only deuterated analogs were available for use as internal standards. There is a small but significant separation of the deuterium analog internal standards and their corresponding endogenous protium forms during chromatography. Therefore, differential suppression or enhancement of ionization could still affect the quality of the analytical data. The recent availability of [13C6]-estrogen analogs from Cambridge Isotope Laboratories (Andover, MA) means that going forward it will be possible to use internal standards that have identical chromatographic retention times but a mass difference of 6-Da. The specificity of GC-MS/MS and LC-MS/MS methodology arises from three analytical parameters. The estrogen must have an identical relative retention time to the heavy isotope analog internal standard determined during assay validation, an identical parent ion on MS analysis and an identical product ion on MS/MS analysis. Immunoaffinity purification can improve potentially improve specificity still further as we found with the difficult amyloid β-peptides [108]. Unfortunately, the sensitivity of conventional immunoaffinty purification/MS-based procedures is inadequate for the analysis of plasma and serum samples from postmenopausal women [109].
Stable isotope dilution methodology coupled with GC-MS or LC-MS can in principle provide the optimal specificity for estrogen analysis because (as noted above) internal standards with identical physicochemical properties to the relevant analytes are carried through the entire analysis procedure. GC-MS/MS when used in the electron ionization mode does not have the sensitivity for the analysis of estrogens in postmenopausal serum and plasma samples. However, pentafluorobenzyl (PFB) and trimethylsilyl (TMS) derivatization of the 2- and 17-hydroxyl groups, respectively coupled with electron capture negative chemical ionization (ECNCI)/MS/MS provides outstanding sensitivity with a LOQ for serum estradiol of 0.6 pg/mL (2.3 pmol/L) [98] (Table 1). Elaboration of this methodology to multiple estrogens on a routine basis will be very challenging and limited to selected laboratories. LC-MS/MS methodology is not so challenging but unfortunately, endogenous estrogens are not effectively ionized using conventional electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) methodology. This has restricted the use of these ionization techniques to samples with higher concentrations of plasma and serum estrogens [92,101,109-111]. It is necessary enhance the ionization characteristics of estrogens by first converting them to suitable derivatives in order to conduct analyses in plasma or serum samples from postmenopausal women.
Table 1.
Limits of quantitation (LOQ) for analysis of serum and plasma estradiol and estrone by LC-MS/MS after derivatization to improve ionization efficiency. The LOQ is defined in section 5. Abbreviations: APCI atmospheric pressure chemical ionization; ECAPCI, electron capture atmospheric pressure chemical ionization; ECNCI, electron capture negative chemical ionization; ESI, electrospray ionization; LC, liquid chromatography; NMN, N-methyl-nicotinyl; P, picolinoyl; PFB, pentafluorobenzyl; PS, pyridyl-3-sulfonyl; TMS trimethylsilyl.
| Chromatography | Ionization | Derivative | LOQ (pg/mL) | LOQ (pmol/L) | Reference | ||
|---|---|---|---|---|---|---|---|
| Estradiol | Estrone | Estradiol | Estrone | ||||
| GC | ECNCI | PFB/TMS | 0.6 | ND | 2.3 | ND | 97 |
| LC | ECAPCI | PFB | 4.0 | 4.0 | 14.7 | 14.8 | 114 |
| LC | ESI | D | 8.0 | 8.0 | 29.4 | 29.6 | 115 |
| LC | ESI | P | 0.5 | 1.0 | 1.8 | 3.6 | 116 |
| LC | ESI | PS | 10.0 | ND | 36.7 | ND | 117 |
| LC | ESI | NMN | 0.4 | 0.4 | 1.6 | 1.3 | 120 |
ND = not determined
Three approaches to enhancing sensitivity of estrogen analysis through derivatization have been reported. The first approach, which we have pioneered, involves the preparation of an electron capturing PFB derivative of the estrogen 2-hydroxyl groups coupled with the use of electron capture atmospheric pressure chemical ionization (ECAPCI)/MS (Figure 4) [112]. The Higashi group has also explored the utility of ECAPCI/MS for estrogen analysis by using different electron capturing derivatives [113]. We showed that it was possible to quantify estrogens in the low pg/mL range in plasma using LC-ECAPCI/MS {Penning, 2010 1972/ id} (Table 1). The second approach uses conventional derivatization coupled with LC-ESI/MS. This approach is exemplified by studies of the Ziegler [115], Tai [111], and Kushnir [92] groups using the dansyl (D) derivative and studies by the Yamashita [116], and Spink [117] groups in which a picolinoyl (P), or pyridyl-3-sulfonyl (PS) derivatives were employed (Figure 4). The third approach involves the preparation of pre-ionized (quaternized) derivatives, so that ionization is not required in the ESI source of the mass spectrometer. This approach is exemplified by studies of the Chen [118], Higashi [119], and Adamec [120] groups in which N-methyl-2-pyridyl (NMP), 1-(2,4-dinitro-5-fluorphenyl)-4,4,-dimethylpiperazine (MPPZ), or N-methyl-nicotinyl (NMN) groups are attached to the 3-hydroxy moiety of the estrogen moiety (Figure 4).
Figure 4.
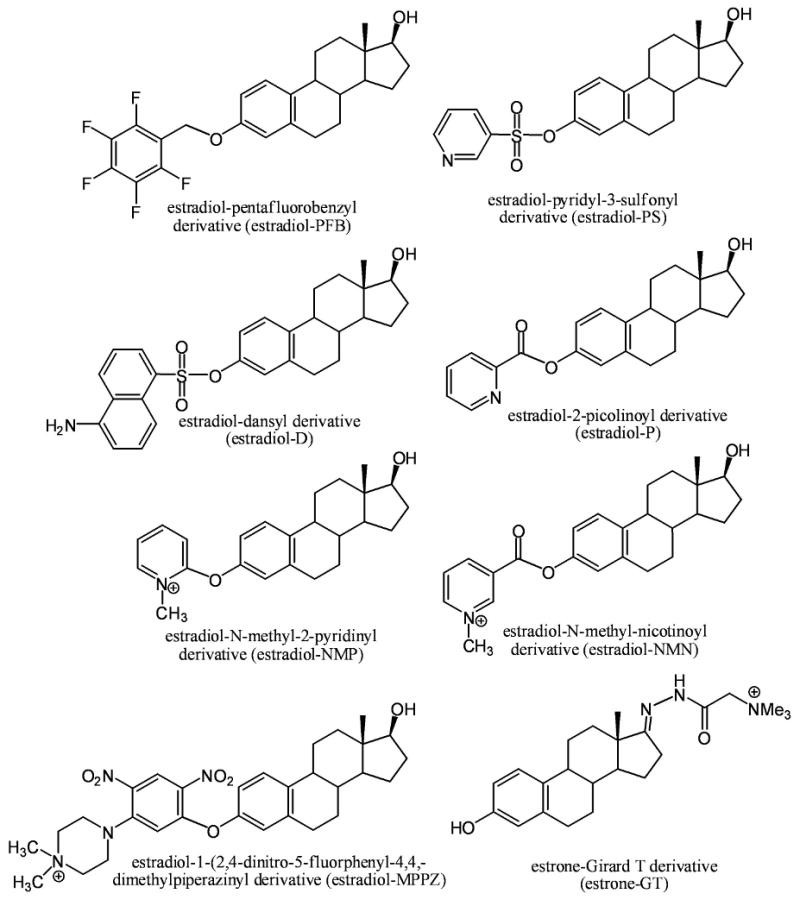
Derivatives used to enhance the ionization efficiency of estrogens in order to improve sensitivity for GC-MS/MS and LC-MS/MS analysis.
6. Future Directions
The three derivatization strategies described above make it possible to quantify plasma and serum estrogens with LOQs in the low pg/mL (pmol/L) range (Table 1) {Santen, 2007 1941 /id;Penning, 2010 1972 /id;Xu, 2005 1860 /id;Yamashita, 2007 1858 /id;Yang, 2008 1855 /id}. The availability of methodology based on these novel ionization techniques coupled with GC-MS/MS or LC-MS/MS will greatly facilitate future studies to rigorously establish the precise levels of individual estrogens that are present in the plasma of postmenopausal women.
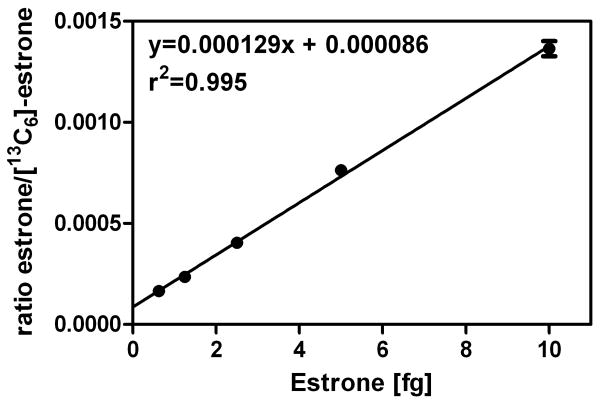
The ESI process requires ionization to occur in solution followed by desolvation of resulting protonated molecules in the source of the mass spectrometer. Therefore, it is difficult to achieve compete ionization of all analyte molecules. This contrasts with pre-ionized derivatives, which are already completely ionized (Figure 4). The Adamec group has employed the NMN pre-ionized derivative for the high sensitivity analysis of plasma and serum estrogens [120] (Table 1). Our laboratory has also explored this approach using the GT derivative, which has been employed previously to analyze keto steroids [122,123]. Extremely high sensitivity can be obtained with the pre-ionized estrone GT derivative (Figure 4) when employed in combination with a modern triple quadrupole mass spectrometer coupled with nanoflow LC. For example, using [13C6]-estrone as the internal standard, we have demonstrated linear standard curves in the range of 0.625 fg to 10.00 fg (2.2 amol to 37.0 amol) of estrone on column as its GT derivative (Figure 5). This suggests that in the future it will be possible to conduct LC-MS/MS assays on multiple estrogen metabolites in serum and plasma at an order of magnitude lower than the impressive sensitivity that can be obtained with currently available GC-ECNCI/MS, LC-ECAPCI/MS and LC-ESI/MS methodology (Table 1). The ease with which the pre-ionized derivatization strategy can be implemented will make it possible to readily introduce high sensitivity methodology in laboratories that are currently employing LC-MS/MS methodology. We anticipate that the use of pre-ionized estrogen derivatives will also help conserve important plasma and serum samples as it will be possible to conduct high sensitivity analyses using low sample volumes. This will make it possible to use existing banked plasma and serum samples without significantly depleting the amounts that are available. This will permit other studies to be conducted on the same samples in order to help understand the factors that cause an increase in breast cancer risk.
Figure 5.
Analysis of estrone in the range 0.625 fg to 10.00 fg (2.3 amol to 37.0 amol) on columns as the GT derivative using LC-multiple reaction monitoring (MRM)/MS. Chromatography was performed using a Halo C18 column (150 × 0.1 mm × id, 2.7 μm, 90 Å; Advanced Materials Technology, Wilmington, DE) using a linear gradient of water/acetonitrile at a flow rate of 1,000 nL/min. MS was conducted using a Thermo Analytical Vantage triple stage quadruple mass spectrometer. MRM/MS was conducted on the following ions m/z 384 (M+, estrone) → m/z 157 and m/z 390 (M+, [13C6]-estrone)→ m/z 157. Data points represent the means ± SEM (n=3).
It is possible that the ratio of 4-methoxy-estrogens to 2-methoxy-estrogens might provide an indirect measurement of the catechol estrogens 4-hydroxy-estradriol/2-hydroxy-estradiol and 2-hydroxy-estrone/4-hydroxy-estrone [124]. This is potentially important since the hydroxylated catechols are considered to be genotoxic estrogens while the 2-hydroxy catechols are metabolized to 2-methoxy-estrogens, which are considered to be anti-proliferative and protective against mammary carcinogenesis [12,13]. In contrast, 4-methoxy-estrogens do not appear to exert anti-proliferative effects. The ability to routinely conduct very high sensitivity analyses of 4-methoxy-estrogens to 2-methoxy-estrogens together with estrone, 16α-hydroxy-estrone, and estradiol will make it possible to develop and evaluate new and improved models of breast cancer risk [6,97]. The development of such models would impact significantly on the implementation of chemoprevention strategies for women newly identified to be in a high breast cancer risk category. Previous studies have shown that this would significantly improve breast cancer prevention [6,7]. Therefore, the ability to routinely analyze plasma and serum estrogens with very high sensitivity could potentially save a large number of women from this devastating disease [97].
Acknowledgments
I acknowledge the support of NIH grants UO1ES16004 and P30ES013508 and helpful discussions with Dr. Richard Santen of the University of Virginia and Dr. Trevor Penning of the University of Pennsylvania.
Abbreviations used
- 2-hydroxy-estradiol
2,3-dihydroxy-17β-estradiol
- 2-methoxy-estradiol
2-methoxy-3-hydroxy-17β-estradiol
- 4-methoxy-estradiol
3-hydroxy-4-methoxy-17β-estradiol
- 4-hydroxy-estradiol
3,4-dihydroxy-17β-estradiol
- 16α-hydroxy-estradiol
3,16α-dihydroxy-17β-estradiol
- estradiol
17β-estradiol
- AKR
aldo-keto reductase
- APCI
atmospheric pressure chemical ionization
- BMI
body mass index
- COMT
catechol O-methyl transferase
- CYP
cytochrome P-450
- D
dansyl
- DHEA
dehydroepiandrosterone
- ECLIA
electrochemiluminescence immunoassay
- ECAPCI
electron capture atmospheric pressure chemical ionization
- ECNCI
electron capture negative chemical ionization
- ESI
electrospray ionization
- GSH
glutathione
- GT
Girard T
- LC
liquid chromatography
- HSD
hydroxysteroid dehydrogenase
- LOQ
limit of quantitation
- MPPZ
1-(2,4-dinitro-5-fluorphenyl)-4,4,-dimethylpiperazine
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- NMN
N-methyl-nicotinyl
- NMP
N-methyl-2-pyridyl
- P
picolinoyl
- PFB
pentafluorobenzyl
- PS
pyridyl-3-sulfonyl
- RIA
radioimmunoassay
- SULT
sulfotransferase
- UGT
uridine diphosphate glucuronosyltransferases
- TMS
trimethylsilyl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 2.Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001;7:273–281. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 3.Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20:615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]
- 4.Santen RJ. Assessing individual risk for breast cancer: role of oestrogens and androgens. Breast Cancer Res. 2008;10:S10. doi: 10.1186/bcr2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ESHRE Capri Workshop Group. Hormones and breast cancer. Hum Reprod Update. 2004;10:281–293. doi: 10.1093/humupd/dmh025. [DOI] [PubMed] [Google Scholar]
- 6.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 7.Chan K, Morris GJ. Chemoprevention of breast cancer for women at high risk. Semin Oncol. 2006;33:642–646. doi: 10.1053/j.seminoncol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 9.Dorgan JF, Longcope C, Stephenson HE, Jr, Falk RT, Miller R, Franz C, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prevent. 1996;5:533–539. [PubMed] [Google Scholar]
- 10.Lamar CA, Dorgan JF, Longcope C, Stanczyk FZ, Falk RT, Stephenson HE., Jr Serum sex hormones and breast cancer risk factors in postmenopausal women. Cancer Epidemiol Biomarkers Prevent. 2003;12:380–383. [PubMed] [Google Scholar]
- 11.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 12.Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prevent. 2008;17:2029–2035. doi: 10.1158/1055-9965.EPI-08-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arslan AA, Shore RE, Afanasyeva Y, Koenig KL, Toniolo P, Zeleniuch-Jacquotte A. Circulating estrogen metabolites and risk for breast cancer in premenopausal women. Cancer Epidemiol Biomarkers Prevent. 2009;18:2273–2279. doi: 10.1158/1055-9965.EPI-09-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowsett M, Folkerd E. Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res. 2005;7:1–4. doi: 10.1186/bcr960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prevent. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 16.Boyd N, Martin L, Gunasekara A, Melnichouk O, Maudsley G, Peressotti C, et al. Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol Biomarkers Prevent. 2009;18:1754–1762. doi: 10.1158/1055-9965.EPI-09-0107. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Arendell L, Aickin M, Cauley J, Lewis CE, Chlebowski R. Hip bone density predicts breast cancer risk independently of Gail score: results from the Women's Health Initiative. Cancer. 2008;113:907–915. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gail MH. Value of adding single-nucleotide polymorphism genotypes to a breast cancer risk model. J Natl Cancer Inst. 2009;101:959–963. doi: 10.1093/jnci/djp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 21.Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Jones M, et al. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas JL, Duax WL, Addlagatta A, Kacsoh B, Brandt SE, Norris WB. Structure/function aspects of human 3beta-hydroxysteroid dehydrogenase. Mol Cell Endocrinol. 2004;215:73–82. doi: 10.1016/j.mce.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Adamski J, Jakob FJ. A guide to 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2001;171:1–4. doi: 10.1016/s0303-7207(00)00383-x. [DOI] [PubMed] [Google Scholar]
- 24.Andersson S. Molecular genetics of androgenic 17 beta-hydroxysteroid dehydrogenases. J Steroid Biochem Mol Biol. 1995;55:533–534. doi: 10.1016/0960-0760(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Luu-The V, Poisson-Pare D, Ouellet J, Li S, Labrie F, et al. Expression of enzymes involved in synthesis and metabolism of estradiol in human breast as studied by immunocytochemistry and in situ hybridization. Histol Histopathol. 2009;24:273–282. doi: 10.14670/HH-24.273. [DOI] [PubMed] [Google Scholar]
- 26.Plourde M, Ferland A, Soucy P, Hamdi Y, Tranchant M, Durocher F, et al. Analysis of 17beta-hydroxysteroid dehydrogenase types 5, 7, and 12 genetic sequence variants in breast cancer cases from French Canadian Families with high risk of breast and ovarian cancer. J Steroid Biochem Mol Biol. 2009;116:134–153. doi: 10.1016/j.jsbmb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Penning TM, Jin Y, Steckelbroeck S, Lanisnik RT, Lewis M. Structure-function of human 3 alpha-hydroxysteroid dehydrogenases: genes and proteins. Mol Cell Endocrinol. 2004;215:63–72. doi: 10.1016/j.mce.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song D, Liu G, Luu-The V, Zhao D, Wang L, Zhang H, et al. Expression of aromatase and 17beta-hydroxysteroid dehydrogenase types 1, 7 and 12 in breast cancer. An immunocytochemical study. J Steroid Biochem Mol Biol. 2006;101:136–144. doi: 10.1016/j.jsbmb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, et al. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007;67:3945–3954. doi: 10.1158/0008-5472.CAN-06-3105. [DOI] [PubMed] [Google Scholar]
- 31.Jansson A. 17Beta-hydroxysteroid dehydrogenase enzymes and breast cancer. J Steroid Biochem Mol Biol. 2009;114:64–67. doi: 10.1016/j.jsbmb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Breitling R, Krazeisen A, Moller G, Adamski J. 17beta-hydroxysteroid dehydrogenase type 7--an ancient 3-ketosteroid reductase of cholesterogenesis. Mol Cell Endocrinol. 2001;171:199–204. doi: 10.1016/s0303-7207(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 33.Wei C, Zhu P, Shah SJ, Blair IA. 15-Oxo-Eicosatetraenoic Acid, a Metabolite of Macrophage 15-Hydroxyprostaglandin Dehydrogenase that Inhibits Endothelial Cell Proliferation. Mol Pharm. 2009;76:516–529. doi: 10.1124/mol.109.057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vihko P, Isomaa V, Ghosh D. Structure and function of 17beta-hydroxysteroid dehydrogenase type 1 and type 2. Mol Cell Endocrinol. 2001;171:71–76. doi: 10.1016/s0303-7207(00)00389-0. [DOI] [PubMed] [Google Scholar]
- 35.Lukacik P, Keller B, Bunkoczi G, Kavanagh KL, Lee WH, Adamski J, et al. Structural and biochemical characterization of human orphan DHRS10 reveals a novel cytosolic enzyme with steroid dehydrogenase activity. Biochem J. 2007;402:419–427. doi: 10.1042/BJ20061319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol. 2008;108:181–185. doi: 10.1016/j.jsbmb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 37.LaFleur J, McAdam-Marx C, Kirkness C, Brixner DI. Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacother. 2008;42:375–386. doi: 10.1345/aph.1K203. [DOI] [PubMed] [Google Scholar]
- 38.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 39.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 40.van Gils CH, Peeters PH, Schoenmakers MC, Nijmeijer RM, Onland-Moret NC, van der Schouw YT, et al. Physical activity and endogenous sex hormone levels in postmenopausal women: a cross-sectional study in the Prospect-EPIC Cohort. Cancer Epidemiol Biomarkers Prevent. 2009;18:377–383. doi: 10.1158/1055-9965.EPI-08-0823. [DOI] [PubMed] [Google Scholar]
- 41.Monninkhof EM, Velthuis MJ, Peeters PH, Twisk JW, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol. 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 42.Fisher MB, Vandenbranden M, Findlay K, Burchell B, Thummel KE, Hall SD, et al. Tissue distribution and interindividual variation in human UDP-glucuronosyltransferase activity: relationship between UGT1A1 promoter genotype and variability in a liver bank. Pharmacogenetics. 2000;10:727–739. doi: 10.1097/00008571-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Gall WE, Zawada G, Mojarrabi B, Tephly TR, Green MD, Coffman BL, et al. Differential glucuronidation of bile acids, androgens and estrogens by human UGT1A3 and 2B7. J Steroid Biochem Mol Biol. 1999;70:101–108. doi: 10.1016/s0960-0760(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 44.Guillemette C, Belanger A, Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6:246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falany JL, Falany CN. Expression of cytosolic sulfotransferases in normal mammary epithelial cells and breast cancer cell lines. Cancer Res. 1996;56:1551–1555. [PubMed] [Google Scholar]
- 46.Lindsay J, Wang LL, Li Y, Zhou SF. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr Drug Metab. 2008;9:99–105. doi: 10.2174/138920008783571819. [DOI] [PubMed] [Google Scholar]
- 47.Adjei AA, Weinshilboum RM. Catecholestrogen sulfation: possible role in carcinogenesis. Biochem Biophys Res Commun. 2002;292:402–408. doi: 10.1006/bbrc.2002.6658. [DOI] [PubMed] [Google Scholar]
- 48.Wang LQ, James MO. Sulfotransferase 2A1 forms estradiol-17-sulfate and celecoxib switches the dominant product from estradiol-3-sulfate to estradiol-17-sulfate. J Steroid Biochem Mol Biol. 2005;96:367–374. doi: 10.1016/j.jsbmb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Nakata T, Takashima S, Shiotsu Y, Murakata C, Ishida H, Akinaga S, et al. Role of steroid sulfatase in local formation of estrogen in post-menopausal breast cancer patients. J Steroid Biochem Mol Biol. 2003;86:455–460. doi: 10.1016/s0960-0760(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Delaforge M, Pruvost A, Perrin L, Andre F. Cytochrome P450-mediated oxidation of glucuronide derivatives: example of estradiol-17beta-glucuronide oxidation to 2-hydroxy-estradiol-17beta-glucuronide by CYP 2C8. Drug Metab Dispos. 2005;33:466–473. doi: 10.1124/dmd.104.002097. [DOI] [PubMed] [Google Scholar]
- 52.Takanashi K, Watanabe K, Yoshizawa I. Evidence of conversion of estradiol 17-sulfate to its 2- and 4-hydroxylated catechols by human placental microsomes. Biol Pharm Bull. 1993;16:217–219. doi: 10.1248/bpb.16.217. [DOI] [PubMed] [Google Scholar]
- 53.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 54.Hui Y, Yasuda S, Liu MY, Wu YY, Liu MC. On the sulfation and methylation of catecholestrogens in human mammary epithelial cells and breast cancer cells. Biol Pharm Bull. 2008;31:769–773. doi: 10.1248/bpb.31.769. [DOI] [PubMed] [Google Scholar]
- 55.Spink BC, Katz BH, Hussain MM, Pang S, Connor SP, Aldous KM, et al. SULT1A1 catalyzes 2-methoxyestradiol sulfonation in MCF-7 breast cancer cells. Carcinogenesis. 2000;21:1947–1957. doi: 10.1093/carcin/21.11.1947. [DOI] [PubMed] [Google Scholar]
- 56.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 57.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Z, Kosinska W, Khmelnitsky M, Cavalieri EL, Rogan EG, Chakravarti D, et al. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem Res Toxicol. 2006;19:475–479. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 59.Gao N, Nester RA, Sarkar MA. 4-Hydroxy estradiol but not 2-hydroxy estradiol induces expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor A through phosphatidylinositol 3-kinase/Akt/FRAP pathway in OVCAR-3 and A2780-CP70 human ovarian carcinoma cells. Toxicol Appl Pharmacol. 2004;196:124–135. doi: 10.1016/j.taap.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 61.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blair IA. Endogenous glutathione adducts. Curr Drug Metab. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 63.Chandrasena RE, Edirisinghe PD, Bolton JL, Thatcher GR. Problematic detoxification of estrogen quinones by NAD(P)H-dependent quinone oxidoreductase and glutathione-S-transferase. Chem Res Toxicol. 2008;21:1324–1329. doi: 10.1021/tx8000797. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Z, Rios GR, King CD, Coffman BL, Green MD, Mojarrabi B, et al. Glucuronidation of catechol estrogens by expressed human UDP-glucuronosyltransferases (UGTs) 1A1, 1A3, and 2B7. Toxicol Sci. 1998;45:52–57. doi: 10.1006/toxs.1998.2494. [DOI] [PubMed] [Google Scholar]
- 65.Parl FF, Egan KM, Li C, Crooke PS. Estrogen exposure, metabolism, and enzyme variants in a model for breast cancer risk prediction. Cancer Inform. 2009;7:109–121. doi: 10.4137/cin.s2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Emburgh BO, Hu JJ, Levine EA, Mosley LJ, Perrier ND, Freimanis RI, et al. Polymorphisms in CYP1B1, GSTM1, GSTT1 and GSTP1, and susceptibility to breast cancer. Oncol Rep. 2008;19:1311–1321. [PMC free article] [PubMed] [Google Scholar]
- 67.Wen W, Ren Z, Shu XO, Cai Q, Ye C, Gao YT, et al. Expression of cytochrome P450 1B1 and catechol-O-methyltransferase in breast tissue and their associations with breast cancer risk. Cancer Epidemiol Biomarkers Prevent. 2007;16:917–920. doi: 10.1158/1055-9965.EPI-06-1032. [DOI] [PubMed] [Google Scholar]
- 68.Rylander-Rudqvist T, Wedren S, Granath F, Humphreys K, Ahlberg S, Weiderpass E, et al. Cytochrome P450 1B1 gene polymorphisms and postmenopausal breast cancer risk. Carcinogenesis. 2003;24:1533–1539. doi: 10.1093/carcin/bgg114. [DOI] [PubMed] [Google Scholar]
- 69.De V, I, Hankinson SE, Li L, Colditz GA, Hunter DJ. Association of CYP1B1 polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prevent. 2002;11:489–492. [PubMed] [Google Scholar]
- 70.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 71.Lippert TH, Seeger H, Mueck AO. The impact of endogenous estradiol metabolites on carcinogenesis. Steroids. 2000;65:357–369. doi: 10.1016/s0039-128x(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 72.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Kirches E, Warich-Kirches M. 2-methoxyestradiol as a potential cytostatic drug in gliomas? Anticancer Agents Med Chem. 2009;9:55–65. doi: 10.2174/187152009787047725. [DOI] [PubMed] [Google Scholar]
- 74.Pasqualini JR. Estrogen sulfotransferases in breast and endometrial cancers. Ann N Y Acad Sci. 2009;1155:88–98. doi: 10.1111/j.1749-6632.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- 75.Gestl SA, Green MD, Shearer DA, Frauenhoffer E, Tephly TR, Weisz J. Expression of UGT2B7, a UDP-glucuronosyltransferase implicated in the metabolism of 4-hydroxyestrone and all-trans retinoic acid, in normal human breast parenchyma and in invasive and in situ breast cancers. Am J Pathol. 2002;160:1467–1479. doi: 10.1016/S0002-9440(10)62572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westerlind KC, Gibson KJ, Evans GL, Turner RT. The catechol estrogen, 4-hydroxyestrone, has tissue-specific estrogen actions. J Endocrinol. 2000;167:281–287. doi: 10.1677/joe.0.1670281. [DOI] [PubMed] [Google Scholar]
- 77.Newman SP, Ireson CR, Tutill HJ, Day JM, Parsons MF, Leese MP, et al. The role of 17beta-hydroxysteroid dehydrogenases in modulating the activity of 2-methoxyestradiol in breast cancer cells. Cancer Res. 2006;66:324–330. doi: 10.1158/0008-5472.CAN-05-2391. [DOI] [PubMed] [Google Scholar]
- 78.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 79.Huang Z, Guengerich FP, Kaminsky LS. 16Alpha-hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis. 1998;19:867–872. doi: 10.1093/carcin/19.5.867. [DOI] [PubMed] [Google Scholar]
- 80.Nebert DW. Elevated estrogen 16 alpha-hydroxylase activity: is this a genotoxic or nongenotoxic biomarker in human breast cancer risk? J Natl Cancer Inst. 1993;85:1888–1891. doi: 10.1093/jnci/85.23.1888. [DOI] [PubMed] [Google Scholar]
- 81.Jernstrom H, Klug TL, Sepkovic DW, Bradlow HL, Narod SA. Predictors of the plasma ratio of 2-hydroxyestrone to 16alpha-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis. 2003;24:991–1005. doi: 10.1093/carcin/bgg047. [DOI] [PubMed] [Google Scholar]
- 82.Castagnetta LA, Granata OM, Traina A, Ravazzolo B, Amoroso M, Miele M, et al. Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin Cancer Res. 2002;8:3146–3155. [PubMed] [Google Scholar]
- 83.Telang NT, Suto A, Wong GY, Osborne MP, Bradlow HL. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84:634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 84.Lewis JS, Thomas TJ, Pestell RG, Albanese C, Gallo MA, Thomas T. Differential effects of 16alpha-hydroxyestrone and 2-methoxyestradiol on cyclin D1 involving the transcription factor ATF-2 in MCF-7 breast cancer cells. J Mol Endocrinol. 2005;34:91–105. doi: 10.1677/jme.1.01599. [DOI] [PubMed] [Google Scholar]
- 85.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 86.Liehr JG. Role of DNA adducts in hormonal carcinogenesis. Regul Toxicol Pharmacol. 2000;32:276–282. doi: 10.1006/rtph.2000.1432. [DOI] [PubMed] [Google Scholar]
- 87.Bradlow HL, Davis DL, Lin G, Sepkovic D, Tiwari R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect. 1995;103 7:147–150. doi: 10.1289/ehp.95103s7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Im A, Vogel VG, Ahrendt G, Lloyd S, Ragin C, Garte S, et al. Urinary estrogen metabolites in women at high risk for breast cancer. Carcinogenesis. 2009;30:1532–1535. doi: 10.1093/carcin/bgp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McShane LM, Dorgan JF, Greenhut S, Damato JJ. Reliability and validity of serum sex hormone measurements. Cancer Epidemiol Biomarkers Prevent. 1996;5:923–928. [PubMed] [Google Scholar]
- 90.Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68:1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS J. 2007;9:E109–E114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 93.Longcope C, Hui SL, Johnston CC., Jr Free estradiol, free testosterone, and sex hormone-binding globulin in perimenopausal women. J Clin Endocrinol Metab. 1987;64:513–518. doi: 10.1210/jcem-64-3-513. [DOI] [PubMed] [Google Scholar]
- 94.Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prevent. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 95.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prevent. 2003;12:1457–1461. [PubMed] [Google Scholar]
- 96.Hobe G, Schon R, Goncharov N, Katsiya G, Koryakin M, Gesson-Cholat I, et al. Some new aspects of 17alpha-estradiol metabolism in man. Steroids. 2002;67:883–893. doi: 10.1016/s0039-128x(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 97.Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, et al. Potential role of ultrasensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 2008;73:1318–1321. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Rinaldi S, Dechaud H, Toniolo P, Kaaks R. Reliability and validity of direct radioimmunoassays for measurement of postmenopausal serum androgens and estrogens. IARC Sci Publ. 2002;156:323–325. [PubMed] [Google Scholar]
- 100.Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 101.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prevent. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 102.Stanway SJ, Purohit A, Reed MJ. Measurement of estrone sulfate in postmenopausal women: comparison of direct RIA and GC-MS/MS methods for monitoring response to endocrine therapy in women with breast cancer. Anticancer Res. 2007;27:2765–2767. [PubMed] [Google Scholar]
- 103.Geisler J, Ekse D, Helle H, Duong NK, Lonning PE. An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J Steroid Biochem Mol Biol. 2008;109:90–95. doi: 10.1016/j.jsbmb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 104.Cao Z, Swift TA, West CA, Rosano TG, Rej R. Immunoassay of estradiol: unanticipated suppression by unconjugated estriol. Clin Chem. 2004;50:160–165. doi: 10.1373/clinchem.2003.023325. [DOI] [PubMed] [Google Scholar]
- 105.Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res. 2008;14:6330–6335. doi: 10.1158/1078-0432.CCR-07-5221. [DOI] [PubMed] [Google Scholar]
- 106.Lonning PE, Geisler J. Evaluation of plasma and tissue estrogen suppression with third generation aromatase inhibitors: of relevance to clinical understanding. J Steroid Biochem Mol Biol. 2009 doi: 10.1016/j.jsbmb.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Toniolo P, Lukanova A. The challenge of measuring circulating estradiol at low concentrations. Breast Cancer Res. 2005;7:45–47. doi: 10.1186/bcr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oe T, Ackermann BL, Inoue K, Berna MJ, Garner CO, Gelfanova V, et al. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer's disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3723–3735. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- 109.Ceglarek U, Kortz L, Leichtle A, Fiedler GM, Kratzsch J, Thiery J. Rapid quantification of steroid patterns in human serum by on-line solid phase extraction combined with liquid chromatography-triple quadrupole linear ion trap mass spectrometry. Clin Chim Acta. 2009;401:114–118. doi: 10.1016/j.cca.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 110.Hosogi J, Tanaka H, Fujita K, Kuwabara T, Ikegawa S, Kobayashi N, et al. LC-MS/MS coupled with immunoaffinity extraction for determination of estrone, 17beta-estradiol and estrone 3-sulfate in human plasma. J Chromatogr B: Analyt Technol Biomed Live Sci. 2009 doi: 10.1016/j.jchromb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Tai SS, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17beta in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77:6359–6363. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- 112.Singh G, Gutierrez A, Xu K, Blair IA. Liquid chromatography/electron capture atmospheric pressure chemical ionization/mass spectrometry: analysis of pentafluorobenzyl derivatives of biomolecules and drugs in the attomole range. Anal Chem. 2000;72:3007–3013. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 113.Higashi T, Takayama N, Nishio T, Taniguchi E, Shimada K. Procedure for increasing the detection responses of estrogens in LC-MS based on introduction of a nitrobenzene moiety followed by electron capture atmospheric pressure chemical ionization. Anal Bioanal Chem. 2006;386:658–665. doi: 10.1007/s00216-006-0371-z. [DOI] [PubMed] [Google Scholar]
- 114.Penning TM, Lee SH, Jin Y, Gutierrez A, Blair IA. Liquid-chromatography mass spectrometry (LC-MS) of steroid hormone metabolites and its applications. J Steroid Biochem Mol Biol. 2009 doi: 10.1016/j.jsbmb.2010.01.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 116.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72:819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Xu L, Spink DC. Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 2008;375:105–114. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin YH, Chen CY, Wang GS. Analysis of steroid estrogens in water using liquid chromatography/tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom. 2007;21:1973–1983. doi: 10.1002/rcm.3050. [DOI] [PubMed] [Google Scholar]
- 119.Nishio T, Higashi T, Funaishi A, Tanaka J, Shimada K. Development and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal. 2007;44:786–795. doi: 10.1016/j.jpba.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Yang WC, Regnier FE, Sliva D, Adamec J. Stable isotope-coded quaternization for comparative quantification of estrogen metabolites by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B: Analyt Technol Biomed Live Sci. 2008;870:233–240. doi: 10.1016/j.jchromb.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 122.Griffiths WJ, Liu S, Alvelius G, Sjovall J. Derivatisation for the characterisation of neutral oxosteroids by electrospray and matrix-assisted laser desorption/ionisation tandem mass spectrometry: the Girard P derivative. Rapid Commun Mass Spectrom. 2003;17:924–935. doi: 10.1002/rcm.1002. [DOI] [PubMed] [Google Scholar]
- 123.Johnson DW. Ketosteroid profiling using Girard T derivatives and electrospray ionization tandem mass spectrometry: direct plasma analysis of androstenedione, 17-hydroxyprogesterone and cortisol. Rapid Commun Mass Spectrom. 2005;19:193–200. doi: 10.1002/rcm.1771. [DOI] [PubMed] [Google Scholar]
- 124.Joubert A, Van ZH, Laurens J, Lottering ML. C2- and C4-position 17beta-estradiol metabolites and their relation to breast cancer. Biocell. 2009;33:137–140. [PubMed] [Google Scholar]