Abstract
There is increasing recognition that the optical and antioxidant properties of the xanthophyll carotenoids lutein and zeaxanthin play an important role in maintaining the health and function of the human macula. In this review article, we assess the value of non-invasive quantification of macular pigment levels and distributions to identify individuals potentially at risk for visual disability or catastrophic vision loss from age-related macular degeneration, and we consider the strengths and weaknesses of the diverse measurement methods currently available.
Introduction
The prevalence of age-related eye disease is increasing steadily due to an aging population. It is predicted that cases of early age-related macular degeneration (AMD) will increase approximately 96% from 9.1 million in 2010 to 17.8 million in 2050 (Rein, et al., 2009). In the United States, AMD is the estimated cause of 54.4% of visual impairment and 22.9% of blindness in the Caucasian population. Among Hispanic and African-Americans, these percentages are smaller but significant (Congdon, et al., 2004). Its irreversible, devastating impact upon vision and the threat it poses to the quality of everyday life has prompted intense research efforts to find ways to either prevent or delay its progression.
Although no current FDA-approved AMD treatment is likely to completely restore vision lost to macular degeneration, some nutrients and drugs may be able to preserve or improve remaining vision. For exudative (wet) AMD, treatments are aimed at stopping abnormal blood vessel growth primarily by way of injecting compounds directly into the vitreous that inhibit vascular endothelial growth factor (VEGF). Approximately one-third of wet AMD patients can expect significant improvement of vision while another one-third can expect stabilization of their vision. The remainder of patients, however, will continue to lose vision. Treatment of dry AMD’s advanced stage, which is known as geographic atrophy, is less amenable to treatment, and no interventions beyond antioxidant vitamins and minerals have had any demonstrable effect. Thus, there is still considerable interest in interventions that can be used at AMD’s earliest stages or before it manifests at all in order to prevent progression to its advanced stages.
Given the enormous human and economic impact, it is imperative to consider proven means of reducing risk of AMD in the aging population. Within the last two decades, there has been growing recognition that dietary constituents can have an important role in maintaining eye health. Increasing the consumption of these nutrients may be a safe, easy, and effective measure to improve visual function and to possibly decrease the risk of some eye diseases such as AMD. Two such nutrients, lutein and zeaxanthin, were identified in the macula in 1985, but their function in the eye is just now being elucidated (Landrum & Bone, 2001; Schalch, 1999; Snodderly, 1995; Wald, 1945). This review article provides an overview of how and why these dietary constituents have a positive impact on macular health with particular emphasis on the potential value of measurement of their concentrations and distributions as early predictors of AMD risk.
Macular Pigment
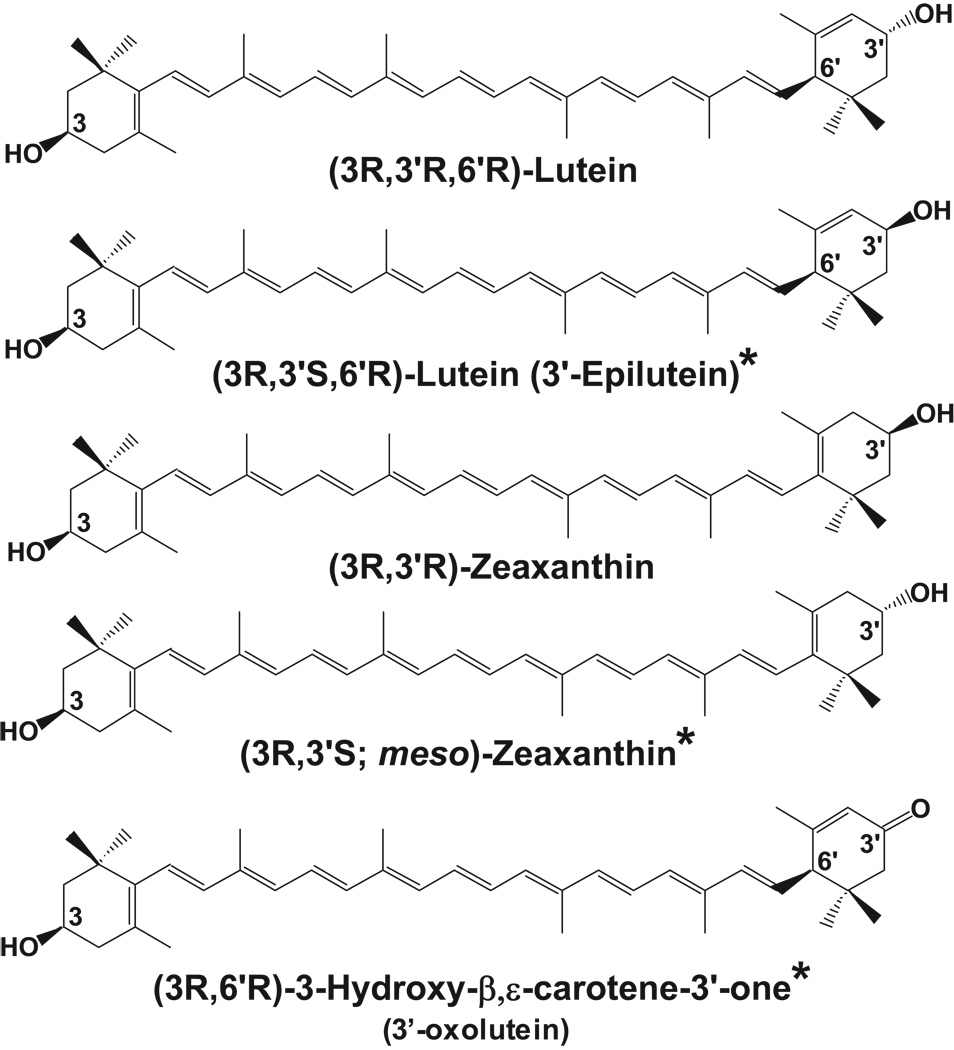
Lutein and zeaxanthin are structural isomers that belong to a class of molecules called carotenoids. Carotenoids are pigments synthesized by plants for coloration and absorption of light energy. They are divided into two classes: xanthophylls and carotenes. Lutein and zeaxanthin are xanthophylls biochemically distinct from other carotenoids due to the presence of hydroxyl groups located at each end of these molecules. This functionality allows xanthophylls to be oriented in lipid membranes exposed to aqueous environments in a unique and possibly protective way (Gruszecki & Sielewiesiuk, 1990). Of the 600+ carotenoids found in nature, forty to fifty are consumed in the typical diet, and only 14 have been detected in serum (Khachik, et al., 1992a; Khachik, et al., 1992b). Of these 14, only lutein and zeaxanthin and their metabolites (Figure 1) are located in the macula where they are found at the highest concentrations anywhere in the human body, suggesting an important functional role for these molecules in the eye (Handelman, et al., 1988).
Figure 1.
Xanthophyll carotenoids found in the human retina and macula. The asterisks denote metabolites of dietary lutein and zeaxanthin.
The characteristic yellow coloration of the macula is due to the presence of macular pigment comprised of lutein and zeaxanthin (Beatty, et al., 1999; Beatty, et al., 2004). Macular pigment is deposited preferentially in the fovea in the Henle fiber layer which consists of the foveal cones’ axons, and in the parafovea, macular pigment is also located in the inner plexiform layers of the retina (Snodderly, Auran & Delori, 1984a; Trieschmann, et al., 2008). The concentration of macular pigment peaks in the center of the macula, the foveola, and decreases 100-fold within a few millimeters of eccentricity. Lutein is more prevalent in the peripheral retina as the ratio of lutein to zeaxanthin changes from approximately 1:2.4 in the central retina to 2:1 in the peripheral region (Bone, et al., 1988; Bone, et al., 1997). Variation with eccentricity of this ratio corresponds to the rod-cone ratio, suggesting a preferential accumulation in these structures, although immunohistochemical localization of the lutein and zeaxanthin binding proteins in primate retina show equal staining of both rods and cones (Bhosale, et al., 2009; Bone et al., 1988; Handelman, et al., 1991). The only other carotenoid present in substantial amounts in the macula is meso-zeaxanthin (Bone, et al., 1993; Landrum & Bone, 2001). Meso-zeaxanthin is a stereoisomer of zeaxanthin that is not generally present in the diet nor detected in the serum (Bone et al., 1997). It has been demonstrated recently in monkeys and Japanese quails that meso-zeaxanthin is a metabolic product of lutein (Bhosale, et al., 2007a; Johnson, et al., 2005). Interestingly, in infants under the age of two, the ratio of lutein to zeaxanthin in the central macula is higher than in adults, suggesting that the lutein distribution is altered in order to adapt to retinal maturation and environmental exposure (Bone et al., 1988).
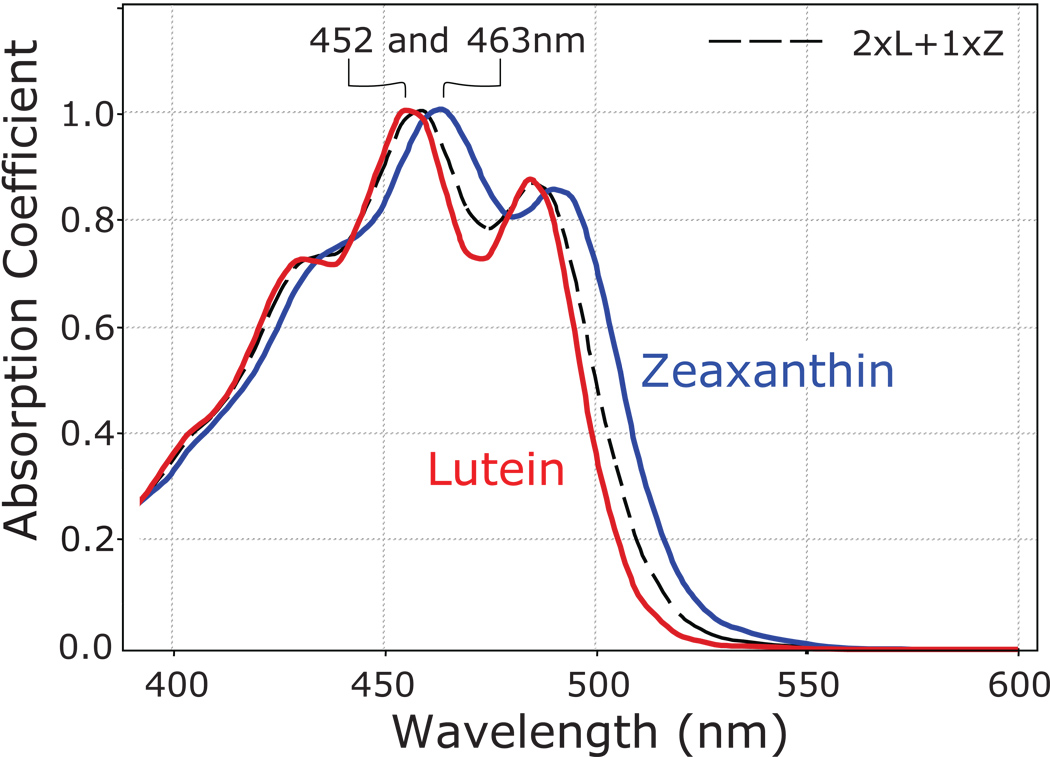
Macular pigment attenuates short wavelengths of visible light, as it maximally absorbs at a wavelength of 460 nm (Figure 2) (Snodderly et al., 1984a; Snodderly, et al., 1984b); these blue wavelengths have been shown to be more dangerous than longer wavelengths of visible light since they are more energetic and seem to be more efficient at generating reactive oxygen species from endogenous photosensitizers such as lipofuscin. Additionally, lutein and zeaxanthin are versatile antioxidants which neutralize reactive oxygen species, in both the low pO2 inner retina and the high pO2 environment of the photoreceptor-RPE complex. This oxygen-rich outer retina in particular is a highly vulnerable region for oxidative damage due to high concentrations of polyunsaturated fatty acids that are susceptible to photo-oxidation and exposure to high-energy blue light (De La Paz & Anderson, 1992). Although lutein and zeaxanthin levels are rather low in the photoreceptor outer segments, a prime target for oxidative damage in the retina due to its high levels of polyunsaturated acids, their binding proteins have recently been localized to the mitochondrial rich ellipsoid region of the inner segments, another region of high oxidative stress within the cell (Bhosale, et al., 2004; Bhosale et al., 2009; Yang, et al., 2003).
Figure 2.
Absorption spectra of lutein (red) and zeaxanthin (blue) in olive oil. A mixture of lutein plus zeaxanthin (dashed black line) closely approximates the absorption spectrum of the macular pigment in the living human eye.
Lutein and zeaxanthin consumption correlates with increased serum levels
Lutein and zeaxanthin, like all other carotenoids, are not synthesized in the body. Thus, lutein and zeaxanthin must be obtained solely from the diet where the richest sources are dark green, leafy vegetables such as spinach and kale and various orange and yellow fruits and vegetables (Mangels, et al., 1993). Egg yolks provide a highly bioavailable source of lutein presumably due to the associated fat content, even though the lutein concentration is relatively low (Handelman, et al., 1999). Supplements containing lutein are widely available, while zeaxanthin supplements are less common. The most recent studies evaluating the impact of lutein and/or zeaxanthin supplementation upon eye health have utilized 10 mg or more per day (Bahrami, Melia & Dagnelie, 2006; Chew, 2007; Khachik, et al., 2006; Kvansakul, et al., 2006; Neelam, et al., 2008; Parisi, et al., 2008; Richer, Devenport & Lang, 2007; Richer, et al., 2004; Rodriguez-Carmona, et al., 2006; Rosenthal, et al., 2006; Schalch, et al., 2007; Stringham & Hammond, 2008; van het Hof, et al., 1999; Wenzel, et al., 2007). This dosage is well below the 2 mg/kg acceptable daily intake (ADI) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). In addition, there are no reported toxic effects of long-term exposure to lutein and zeaxanthin from dietary sources.
Following ingestion and absorption, lutein and zeaxanthin are deposited in a number of tissues including the eyes, skin, breast, cervix, and brain. It is likely that an active transport mechanism exists for macular deposition of lutein and zeaxanthin as levels are ~10,000-fold higher in the macula as compared to the serum. Specific xanthophyll-binding proteins recently identified may play a role in this transport mechanism (Bernstein, et al., 1997; Bhosale et al., 2004; Bhosale et al., 2009; Matthews, et al., 2006). Serum and ocular concentrations of lutein and zeaxanthin have been shown to increase following increased intake of foods rich in these carotenoids (Hammond, et al., 1997a; Johnson, et al., 2000; Wenzel, et al., 2006b) or ingestion of supplements (Bone, et al., 2000; Bone, et al., 2003; Landrum, et al., 1997; Rosenthal et al., 2006). It is estimated that the average daily intake of lutein+zeaxanthin in the U.S. is ~2 mg, which is far below that shown to reduce the risk of age-related eye disease (Centers for Disease Control and Prevention). Based on the information at hand, increased consumption of foods or supplements containing lutein and zeaxanthin may benefit populations where consumption is generally low in order to reach levels associated with benefits to eye health.
Increased serum lutein and zeaxanthin levels are associated with decreased risk for AMD
The first indication that lutein and zeaxanthin impacted AMD risk was an epidemiological study from the Eye Disease Case Control (EDCC) Study Group published in 1993, and a follow-up study by Seddon and colleagues on a subset of EDCC patients published in 1994 (Seddon, et al., 1994; The Eye Disease Case-Control Study Group, 1993). Results indicated that individuals with the highest blood levels and highest dietary intake of lutein and zeaxanthin had a 57% risk reduction for AMD (Seddon et al., 1994). Since then, there have been numerous observational studies evaluating the relationship between dietary lutein intake, serum lutein levels, and AMD risk that have generally been consistent with these initial findings (Delcourt, et al., 2006; Gale, et al., 2003; Moeller, et al., 2006; Snellen, et al., 2002; Tan, et al., 2008; The Eye Disease Case-Control Study Group, 1993; Vu, et al., 2006). These epidemiological studies in aggregate provide ample evidence from an observational perspective to conclude that consumption of lutein and zeaxanthin from food is associated with reduced risk for AMD; however, controlled intervention studies will be necessary to establish a causal relationship.
Two prospective trials are currently evaluating the progression of AMD following supplementation with lutein and zeaxanthin in a large population diagnosed with early-AMD: CARMA and AREDS2. The Carotenoids in Age-Related Maculopathy (CARMA) study is investigating the efficacy of lutein and zeaxanthin supplementation (6 mg and 0.3 mg, respectively) for 36 months upon distance visual acuity, contrast sensitivity, photopic interferometric acuity, and shape discrimination in 433 individuals with early AMD. Secondary aims of the trial are to assess progression of early AMD, evaluate macular pigment optical density, and determine serum lutein and zeaxanthin concentrations.
The Age-Related Eye Disease Study 2 (AREDS2) adds the carotenoids lutein (10 mg per day) and zeaxanthin (2 mg per day), alone or in combination with the omega-3 fatty acids, DHA (350 mg per day) and EPA (650 mg per day) to the original AREDS supplement formulation to assess their influence on the progression to advanced AMD in individuals at high risk for the disease with bilateral large soft drusen and/or advanced AMD in one eye. The decision to add lutein and zeaxanthin at a typical dietary ratio of 5:1 to the original AREDS supplement formula was in part due to analysis of the dietary intake of lutein and zeaxanthin in the AREDS participants, which showed that those individuals with the highest intake had the lowest risk for AMD (AREDS Report No. 22, 2007). In June 2008, 80 participating U.S. centers completed recruitment of over 4,000 AMD patients for the study with each patient slated to receive his or her assigned treatment in a randomized, placebo-controlled, double-blind manner for five years. In addition to evaluating the rate of AMD progression, other outcomes simultaneously evaluated include the effects of supplementation on cognitive function, cataract development, cardiovascular disease, vision loss, and visual function.
The AREDS2 study is designed to provide definitive evidence of the efficacy of lutein and zeaxanthin supplementation in the prevention of progression to advanced AMD in high risk eyes (bilateral large drusen, extrafoveal geographic atrophy, and/or advanced AMD in the fellow eye), but to demonstrate efficacy in the prevention of AMD in lower risk eyes (i.e. young people with a family history of AMD) will be much more difficult because studies would potentially have to be decades long and involve tens of thousands of subjects. Still, in clinical practice, these are the people most likely to request guidance on whether they are consuming adequate levels of lutein and zeaxanthin in the diet and whether or not they might benefit from supplementation. In order to address this issue, the lead author (PSB) convened a panel of fellow experts with extensive experience in carotenoid physiology and/or macular pigment measurement in normal and AMD eyes (the co-authors) to come to a consensus as to the value of noninvasive macular pigment measurement as a screening tool for AMD risk based on a review of the literature and their clinical experience.
Macular Pigment Optical Density (MPOD)
The epidemiology studies discussed above generally used serum carotenoid levels or dietary surveys to group patients for statistical analyses, but there is ample evidence that these methods are, at best, weak indicators of actual tissue levels in the macula. Thus, there is considerable interest in incorporating various non-invasive techniques to measure the amount and corresponding spatial distribution of the macular carotenoids to enhance research and clinical care for populations at risk for visual loss from AMD. Traditional laboratory methods used to measure lutein and zeaxanthin, namely high performance liquid chromatography (HPLC), cannot be used on living eyes, so a surrogate optical indicator of xanthophyll levels in the eye is often employed, macular pigment optical density (MPOD).
MPOD is a measurement of the attenuation of blue light by macular pigment and is linearly related to the amount (concentration×pathlength×area) of lutein and zeaxanthin in the macula if integrated over the region where macular pigment is deposited. Optical density levels, or density units (d.u.) typically encountered in the center of the human macula vary between 0 and 1 (Bone & Landrum, 1992; Snodderly, Handelman & Adler, 1991). Since lutein and zeaxanthin have been associated epidemiologically with a protective role against age-related macular degeneration and cataract, it is reasonable to infer that central MPOD levels could be an indicator of AMD risk (Delcourt et al., 2006; Gale et al., 2003; Moeller et al., 2006; Seddon et al., 1994; Snellen et al., 2002; Tan et al., 2008; The Eye Disease Case-Control Study Group, 1993; Vu et al., 2006). In fact, some studies have shown that healthy individuals have higher central MPOD levels than those afflicted with AMD (Beatty, et al., 2001; Bernstein, et al., 2002; Obana, et al., 2008).
A recent epidemiological study in 828 healthy Irish subjects found a positive and significant relationship between dietary intake of lutein and zeaxanthin, serum concentrations of the respective carotenoids, and central MPOD (Nolan, et al., 2007a). A statistically significant inverse relationship between central MPOD levels and risk factors for AMD including age, tobacco use, and family history of age-related maculopathy (ARM) was also demonstrated in this group suggesting that increased risk of ARM may be attributable in part to a relative lack of macular pigment (Nolan, et al., 2007b). The optical density at 0.5-degrees eccentricity in these subjects ranged from 0 to 0.87 with a mean value of 0.30 (± 0.17) which is consistent with previous studies showing wide variation in MPOD levels among individuals (Bone, Landrum & Cains, 1992; Bone & Sparrock, 1971; Hammond, Fuld & Curran-Celentano, 1995; Hammond & Fuld, 1992; Pease, Adams & Nuccio, 1987). It should be noted, however, some studies have indicated that macular pigment appears to be reduced in obese subjects, subjects with a higher % body fat, and females (Hammond, Ciulla & Snodderly, 2002; Nolan, et al., 2004; Richer et al., 2004). This implies that one or more of these attributes may reduce the visual and ocular effects of lutein and/or zeaxanthin supplementation. It also argues for prescriptive carotenoid dosing, rather than single dose recommendations for all patients.
Macular Pigment Distributions
Mapping of the spatial distribution of macular pigment definitely provides a more complete and accurate representation of macular pigment levels and may enable the correlation of distribution with developing pathology. Determination of the spatial distribution of MPOD is especially important, not only because some distributions may be associated with particular eye diseases, but because quantitation of central MPOD levels alone may underestimate or overestimate the potential role of macular pigment in protection against AMD. This was confirmed by Robson et al. in 2003 when it was reported that central MPOD levels are only poorly correlated with the total amount of macular pigment present, and they concluded that the total amount of macular pigment cannot be reliably predicted from only central attenuation (Robson, et al., 2003). Also, as discussed below, most MPOD methods measure the density at the center of the retina relative to some eccentric location where macular pigment concentrations are assumed to be negligible. If an individual’s macular pigment is broadly distributed over many degrees, if it has an irregular distribution, or if supplementation increases lutein or zeaxanthin concentrations in the peripheral retina substantially, this assumption may not hold true (Bhosale, Zhao da & Bernstein, 2007b).
In 1997, Hammond and colleagues described macular pigment as having a central peak that on average, decreases exponentially to undetectable levels at 6 to 8 degrees eccentricity as determined by heterochromatic flicker photometry (HFP) (Hammond, Wooten & Snodderly, 1997b), and more recent HFP studies have indicated secondary shoulders on the exponential decay may be detectable in some individuals (Kirby, et al., 2009). The shape of the spatial distribution of macular pigment was thought to be accounted for, in part, by the distribution of the cone photoreceptors which decreases rapidly from the center of the fovea outward or by foveal architecture, particularly foveal width (Elsner, et al., 1998; Kirby et al., 2009). Likewise, Robson et al. showed a variety of macular pigment (MP) distribution patterns using motion photometry (Robson et al., 2003). Higher resolution optical imaging techniques enable not only refined local measurements of MPOD levels but can at once display topographical variations in macular pigment as a three-dimensional image of MPOD levels which can be integrated to calculate total macular xanthophyll content (Berendschot & van Norren, 2006; Delori, et al., 2006; Elsner et al., 1998; Gellermann, et al., 2002; Robson et al., 2003; Trieschmann, et al., 2003).
Two recent studies in normal subjects using confocal and non-confocal autofluorescence imaging (Berendschot & van Norren, 2006; Delori et al., 2006) and reflectance imaging (Berendschot & van Norren, 2006) found that approximately half of the subjects had a ring-like distribution superimposed on the flanks of a central peak. The pattern of Maxwell’s spot also corresponded with the pattern detected by autofluorescence imaging. One study (Delori et al., 2006), but not the other, found that women were more likely to exhibit the ring-like distribution. Furthermore, evidence was presented that differences in macular pigment distribution may be related to anatomical differences in the shape of the foveal depression (Delori et al., 2006). This variability in the distributions of the macular pigment was also observed in monkeys by Snodderly et al. (Snodderly et al., 1984a). This led him to hypothesize that this variability was related to variations of the size of the fovea and that contributions of macular pigment in the cone axons and in the inner plexiform layer of the retina could combine to produce variations in the overall macular pigment distribution.
Baseline levels and distributions of MPOD levels are being measured using two wavelength, non-mydriatic, autofluorescence imaging (AFI) (Sharifzadeh, Bernstein & Gellermann, 2006) in a subset of patients enrolled in AREDS2 as part of an ancillary study from Utah which will provide insight into the relationship between MPOD levels and topography and AMD progression (Bernstein, et al., 2009). Preliminary data from this AREDS2 subset and from a group of 70 age-matched healthy Utah subjects suggest that there are five categories (A–E) of macular pigment distributions as measured by autofluorescence imaging (Bernstein et al., 2009; Sharifzadeh et al., 2006). Of the healthy subjects, eleven percent of the subjects fell into category A, in which their central MPOD was very low (less than 0.05). Twenty-two percent of subjects fell into category B in which the macular pigment distribution was laterally extended with a slightly enhanced central region. Category C featured a sole, sharp, central distribution observed in 28% of subjects. Category D was characterized by a sharp, central distribution and an additional ring around the foveal region in 17% of subjects. Finally, category E had a relatively uniform, laterally extended distribution of macular pigment seen in 12% of the subjects. The Utah AREDS2 subjects had similar distributions among the categories but with higher-than-average baseline central MPOD which may have been due to the subjects’ high rate of regular consumption of lutein and/or zeaxanthin supplements prior to enrollment (Bernstein et al., 2009).
Trieschmann et al. measured macular pigment in 400 subjects by single wavelength AFI and found four separate classes of macular pigment distributions (Trieschmann et al., 2003). Reduced levels of macular pigment in subjects with early AMD were observed in this study. In addition, type 3 (only central macular pigment) and type 4 (only paracentral macular pigment) distributions were more often observed in subjects with early AMD.
A unique distribution of macular pigment has been associated with macular telangiectasia (MacTel) type 2 (Charbel Issa, et al., 2008; Helb, et al., 2008). This distribution was characterized by reduction of central MPOD within the macula with a surrounding ring of preserved macular pigment at about 6 degrees eccentricity. Interestingly, two subjects taking lutein and zeaxanthin supplements had more pronounced macular pigment in the eccentric ring suggesting xanthophyll supplementation might help accumulate macular pigment in this population (Charbel Issa, et al., 2009).
Determination of the relationship between foveal architecture and MPOD spatial profiles in normal and diseased human subjects may provide further insights into the relationships between MPOD levels and pathophysiology of the human eye (Delori et al., 2006; Snodderly et al., 1991). Several recent studies have attempted to correlate macular pigment levels and distributions with foveal architecture measured by optical coherence tomography (OCT) (Kirby et al., 2009; Nolan, et al., 2008). A greater understanding of MPOD topography may also elucidate mechanisms of macular pigment deposition.
MPOD Measurement
There are a number of techniques available to measure central MPOD, four of which will be described here. The most commonly used noninvasive test to quantify central macular pigment levels are psychophysical methods: heterochromatic flicker photometry (HFP) (Beatty, et al., 2000; Snodderly, et al., 2004) and, less commonly, motion photometry which is based on normal color discrimination measured on a modified anomaloscope and is easier to perform as compared to HFP (Moreland, Robson & Kulikowski, 2001; Moreland, et al., 1998; Robson, et al., 2005; Robson et al., 2003). HFP was originally described in the 1970’s, and its use has increased with improved equipment. The HFP measurement procedure involves a test stimulus, typically a disk or ring shape, that alternates between a wavelength absorbed by macular carotenoids (typically blue at 460 nm) and a wavelength that is not absorbed (typically green at 540 nm). To the subject, this alternating stimulus appears as a flickering disk or ring, subtending, most often, one-degree of visual angle. The subject adjusts the intensity of blue light until the perception of flicker is minimized or eliminated. The average intensity of the blue light aimed at the foveal region at minimal flicker (Bfov) is recorded. The test is then repeated with the stimulus aimed at an eccentric fixation point where it is assumed that the macular pigment optical density is negligible (Bref). The central MPOD level is then calculated with the equation: MPOD = log (Bfov / Bref). A subject with a high central MPOD then will require a greater blue light intensity in the fovea to compensate for the attenuation of blue light by the macular pigment, relative to the parafovea compared to an individual with low MPOD. Spatial mapping of off-central MPOD levels can be approximated in a limited manner by adjusting the visual angle of the test stimulus. Although HFP may be a minimally invasive measure of macular pigment levels, as it does not require pupillary dilation and uses advantageously low light levels, it is a psychophysical procedure, necessitating both proper training of the subject and his or her attention while performing the measurement. Further, subjects must have normal corrected or uncorrected visual acuity to fixate the central and peripheral targets and similar long (L) and medium (M) wavelength cone ratios at these measurement loci; however, recent adaptive optics studies show that Land M cone distributions can be quite irregular even in normal subjects (Hofer, et al., 2005) and these irregularities could be substantially worse with aging or macular disease. Additionally, some individuals have measurable amounts of carotenoids in the peripheral region beyond the traditional reference point of 7 degrees (Bhosale et al., 2007b), which could lead to an underestimation of foveal MPOD (Wenzel et al., 2007).
Another technique to quantify macular pigment is fundus reflectometry. Fundus reflectance spectroscopy has been used for more than 50 years to study the macular pigment (Brindley & Willmer, 1952). A number of different variations in the technique exist. In the imaging mode, fundus reflectometry measurement of macular pigment is typically obtained at the foveal and parafoveal region using a fundus camera attached to a charge-couple device (CCD), and a scanning laser ophthalmoscope (SLO) (Kilbride, et al., 1989; Wustemeyer, et al., 2002). Images are recorded using blue (480–488 nm) and green (515–540 nm) wavelengths of light. Light directed at the fovea will traverse the macular pigment twice and also is reflected and/or absorbed at layers posterior to the macular pigment including the photoreceptors, the retinal pigment epithelium, the choroid, and sclera (van de Kraats, Berendschot & van Norren, 1996). As macular pigment absorbs blue light more than green light, subtraction of the aligned green and blue images after logarithmic transformation along with light-propagation-model dependent scaling of the density differences can be used to approximate the topographic distribution and quantification of macular pigment. This method is objective and has the capability to map the spatial distribution of macular pigment, but is based on the assumption that all the reflected light that is detected has been attenuated by the macular pigment; however, since other absorbers exist in the eye, it cannot be considered chemically specific. Imaging fundus reflectometry also requires pupil dilation in some implementations, expensive equipment, and technical expertise which limit its widespread use, although a less expensive method based on a non-mydriatic digital fundus camera has recently been reported (Bone, Brener & Gibert, 2007). More recently, MPOD levels over a small central area have been measured by spectral reflectometry in conjunction with an optical model of the fundus layers (van de Kraats & van Norren, 2008). This method uses multiple wavelengths and requires neither reference point measurement nor pupil dilation, but it is non-imaging (van de Kraats, et al., 2006). Significantly, a pilot study using this approach claims that separate measurement of lutein and zeaxanthin optical density may be possible (van de Kraats, et al., 2008).
A third macular pigment measurement method is resonance Raman spectroscopy (RRS) which measures the excitation of bond vibrations within molecules and which is directly proportional to the concentration of macular pigment compound existing in the irradiated macular area. The use of RRS to measure carotenoids in retinal tissue was first described in 1998 by Bernstein, Gellermann, and colleagues on human cadaver and monkey eyes (Bernstein, et al., 1998), and several years later, a device was developed for human clinical studies (Bernstein et al., 2002; Ermakov, et al., 2004; Zhao, et al., 2003). For measurement by RRS, a subject fixates on a 1 mm spot of argon laser light which resonantly excites the macular carotenoids for ~0.2 sec. The intensity of the Raman scattered light at the carotenoid conjugated carbon double bond stretch frequency of 1525 cm−1 is quantified after subtraction of background fluorescence. The intensity levels of xanthophyll carotenoid Raman scattering at this frequency are linearly associated with total macular carotenoid content in the region illuminated, and this can be confirmed by HPLC-based correlation measurements of excised retinal tissue samples (Bernstein et al., 1998; Ermakov, et al., 2001). The Raman signal intensity is typically expressed as photon counts rather than optical density, but with proper external calibration using a carotenoid solution whose absorption/ optical density can be measured, the two values can be correlated. One advantage of RRS as compared to HFP, reflectometry, and AFI is its high chemical specificity since the resonant Raman scattering effect of the macular pigment carotenoids at 1525 cm−1 is many orders of magnitude stronger than that of any other retinal compound. More recently, these same investigators have developed an imaging mode of ocular RRS to map the distribution of macular pigment at high spatial resolution (Sharifzadeh, et al., 2008). RRS measurements of macular pigment do have limitations, however. In particular, absorbance or scattering by the lens can attenuate the Raman signal, and wide pupil dilation is generally required for measurement. Also, rather high light levels are used, and a relatively expensive laser light source is required at present which so far has impeded its broad use.
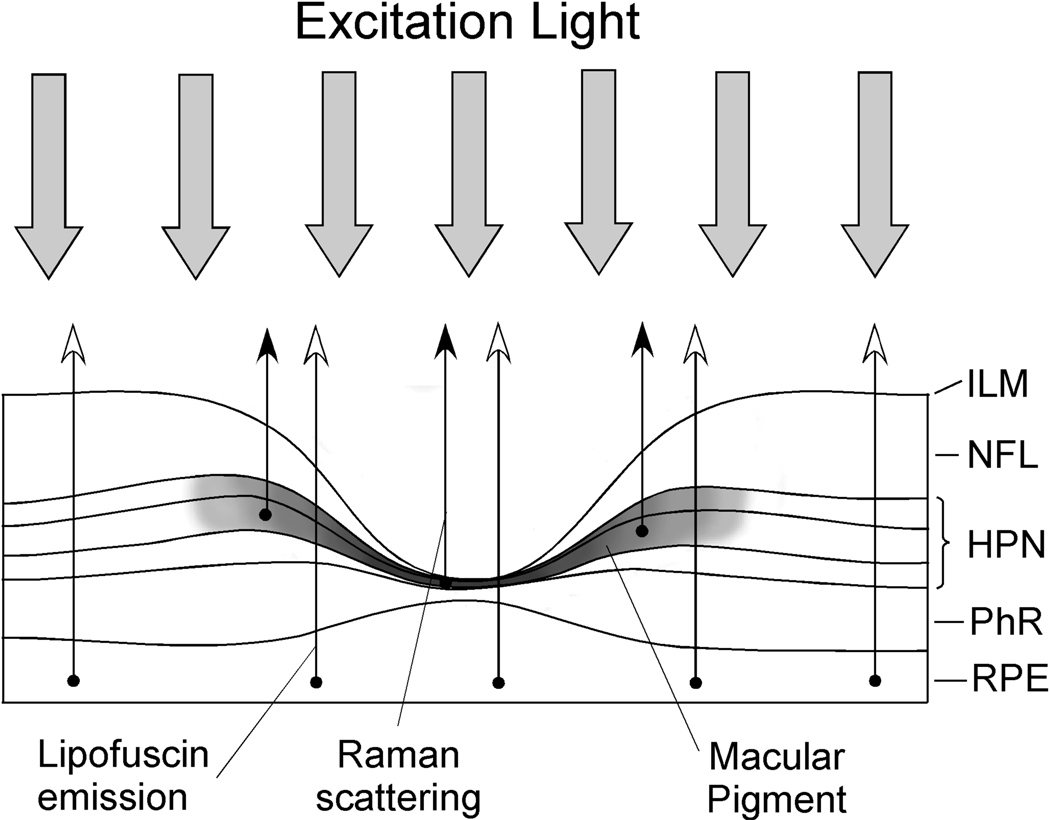
A fourth macular pigment measurement technique is autofluorescence imaging (AFI). This technique was first described in a non-imaging configuration in 1994 to determine the integrated single-path absorption of macular pigment (Delori, 1994) and later in an imaging mode (Wustemeyer et al., 2002). This method measures MPOD levels by determining the macular pigment’s attenuation of the fluorescence of lipofuscin in the retinal pigment epithelium (RPE) (See Figure 3 for a comparison of the principles of the various methods of macular pigment measurement). The best characterized fluorophore of lipofuscin is A2E, a compound formed by the condensation of two retinaldehyde molecules with phosphatidylethanolamine (Sparrow, et al., 1999). Lipofuscin absorbs in the blue wavelength region which overlaps with that of carotenoids and emits in the orange-red region, well beyond the absorption of carotenoids (Sparrow et al., 1999). Since there is virtually no intrinsic fluorescence of macular pigment (Gellermann et al., 2002), it is possible to excite the lipofuscin emission within and outside the absorption range of macular pigment (Delori, 1994). To measure macular pigment with this technique, the fluorescence of lipofuscin is usually excited at two commonly available laser wavelengths: 488 nm and 514 nm (argon laser) or 532 nm (YAG laser). Both lipofuscin and macular pigment absorb light at 488 nm; however, 514 and 532 nm wavelengths are less absorbed by carotenoids but do excite lipofuscin. In order to avoid intrinsic lens fluorescence, AFI imaging of macular pigment can be done on a confocal scanning laser ophthalmoscope (SLO) platform (Wustemeyer et al., 2002). Alternatively, a recently developed approach showed that confounding lens fluorescence effects could be avoided by still using blue excitation wavelengths (488 nm laser or 473 nm LED), but restricting the lipofuscin detection to fluorescence wavelengths above ~650 nm (i.e. to wavelengths lying outside the fluorescence range of the lens) (Sharifzadeh et al., 2006). Several examples of AFI images using this technique are shown in Figure 4. The macular pigment optical density levels are calculated from the difference in lipofuscin fluorescence intensities at foveal and extrafoveal sites (typically 7 degrees eccentricity) (Delori, 1994; Delori, et al., 1995; Delori, et al., 2001). In normal subjects or those with limited macular pathology where lipofusin autofluorescence is relatively uniform in the macula, single wavelength imaging with blue light is usually sufficient; however when significant AMD pathology is present, lipofuscin may no longer be uniformly distributed, and dual wavelength imaging is advisable. AFI has a number of distinct advantages in that it can map spatial variation in macular pigment without pupil dilation, is objective, rapid, requires little training of the subject, and minimizes confounding scattering effects from the anterior ocular media.
Figure 3.
Schematic diagram of the light pathways used in the various methods to measure macular pigment in the living human eye. The dark band corresponds to the location of the macular carotenoids in the fovea. In heterochromatic flicker photometry (HFP), the photoreceptors detect incoming light which is attenuated differentially in the fovea and parafovea depending on the amount of macular pigment encountered. In reflectometry, incoming light is reflected off of various retinal structures of the outer retina, RPE/choroid, and sclera. The double-pass attenuation by the macular pigment is then calculated. In resonance Raman spectroscopy (RRS), incoming light is Raman scattered by the macular carotenoids of the inner retina which is then optically collected and analyzed spectroscopically or displayed in an imaging mode. In autofluorescence imaging (AFI), incoming blue light causes RPE lipofuscin to fluoresce which is subsequently imaged by a scanning laser ophthalmoscope or a CCD camera. The macular pigment of the fovea attenuates the incoming blue light but does not attenuate the longer wavelength fluorescence. This attenuation is imaged and quantified.
Abbreviations: inner plexiform layer (ILM); nerve fiber layer (NFL); Henle fiber, plexiform, and nuclear layers (HPN); photoreceptor layer (PhR); retinal pigment epithelium (RPE). (Reprinted from Sharifzadeh, et al., (2006) with permission).
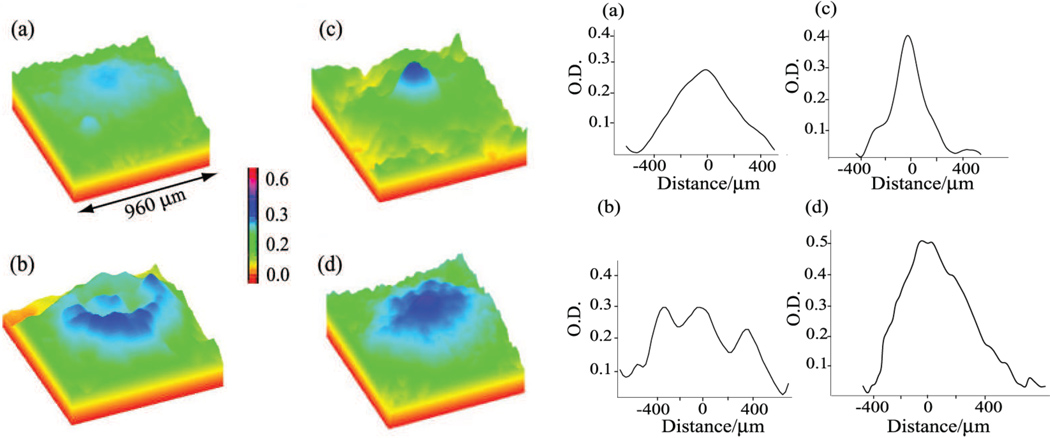
Figure 4.
Varied macular pigment distributions measured by single-wavelength autofluorescence imaging. Pseudocolor images of the macular carotenoid pigments from four individuals are on the left showing narrow and broad distributions and ring structures. Line plots along the horizontal axis for the same subjects are shown on the right. (Reprinted from Sharifzadeh, et al., (2006) with permission).
As AFI is a relatively new technique, a number of studies have sought to determine whether MPOD levels determined with AFI correlated with those established by other methods. It has been demonstrated that MPOD levels measured with AFI had a significant positive correlation with measurements determined by RRS, motion photometry, reflectometry, and HFP (Canovas, et al., 2009; Delori et al., 2001; Egan, Robson & Moreland, 2009; Robson et al., 2003; Sharifzadeh et al., 2006; Tanito, et al., 2009). In addition to measuring MPOD levels, AFI can be used clinically to evaluate patients with dry AMD, especially if they have geographic atrophy, as it can image irregularities of the RPE and define active borders of geographic atrophy (Schmitz-Valckenberg, et al., 2004). This technique can also be useful to diagnose and monitor macular dystrophy patients such as those with Stargardt disease, cone dystrophies, and pattern dystrophies as well as for evaluating patients with ocular inflammation such as uveitis. Additionally, AFI has been found to be an acceptable method with which to evaluate photopigment abnormalities caused by disorders of the outer retina (Sekiryu, et al., 2009).
Lutein and zeaxanthin consumption correlates with increased MPOD
Over two dozen studies have been published demonstrating an increase in macular carotenoids following lutein and/or zeaxanthin supplementation of 2–30 mg per day or a high carotenoid diet (Aleman, et al., 2001; Berendschot, et al., 2000; Bernstein et al., 2002; Bone, 2007; Bone et al., 2003; Cardinault, et al., 2003; Duncan, et al., 2002; Francoise, et al., 2006; Hammond et al., 1997a; Johnson, et al., 2008; Johnson et al., 2000; Koh, et al., 2004; Kopsell, et al., 2006; Kvansakul et al., 2006; Landrum et al., 1997; Morganti, Fabrizi & Bruno, 2004; Richer et al., 2004; Rodriguez-Carmona et al., 2006; Schalch et al., 2007; Schweitzer, et al., 2002; Stringham & Hammond, 2008; Trieschmann, et al., 2007; Vishwanathan, et al., 2009; Wenzel et al., 2006b; Wenzel et al., 2007; Zeimer, et al., 2009). One of the largest clinical trials evaluating the impact of lutein upon MPOD was the Lutein Antioxidant Supplementation Trial (LAST) (Richer et al., 2004). Ninety males with atrophic AMD were supplemented with 10 mg of lutein, 10 mg of lutein plus antioxidants, or a placebo over the course of one year. Subjects in the lutein-only treatment group experienced a 36% increase in MPOD, whereas a 43% increase was observed in those who received lutein plus antioxidants. These results suggest that MPOD can be modulated even in a population suffering from AMD. Additionally, visual acuity, visual function, photo-stress recovery time, and contrast sensitivity were significantly improved as well.
Another large intervention trial, the Carotenoids and Co-Antioxidants in Age-Related Maculopathy (CARMA) study, has recently finished (Neelam et al., 2008). Similar to AREDS2, this study is evaluating the impact of lutein and zeaxanthin along with co-antioxidants in a large population of AMD patients (433 total). Early results have shown significant increases in MPOD in subjects supplemented with lutein for 36 months as compared to the placebo group with the most dramatic increase occurring at 6 months (Beatty, et al., 2009). There was also a positive, significant association between serum lutein levels and MPOD.
Another study evaluated the dose response of subjects supplemented with 5, 10, or 20 mg of lutein upon MPOD (Landrum, et al., 2004). With increasing lutein dose, subjects exhibited a general increase in serum lutein, and with increasing dose the number of subjects with a positive MPOD response rose, suggesting that lutein intake is positively associated with MPOD levels. Increases in MPOD were suggested to be specific to lutein as opposed to zeaxanthin when an investigation by Tanito et al. found that supplementation with 10 mg lutein significantly increased MPOD, while supplementation with 10 mg zeaxanthin did not have an effect (Tanito et al., 2009). However, other researchers have found that zeaxanthin does indeed raise MPOD in quail and humans (Garnett, et al., 2002). In the LUXEA study, supplementation of zeaxanthin alone produced similar pigment accumulation in the fovea and parafovea, confounding the MPOD measurements (Schalch et al., 2007). After correction for this, a 14% MPOD increase resulted for zeaxanthin alone. The authors concluded that differential spatial accumulation of lutein relative to zeaxanthin may be relevant to retinal health. In summary, the differential effects of lutein versus zeaxanthin supplementation remain controversial. This is undoubtedly due in part to the difficulty in controlling dietary intake of carotenoids in clinical study subjects compounded by the fact that commercial lutein supplements typically contain about 6% zeaxanthin.
The degree of increase in MPOD levels following lutein or zeaxanthin supplementation varies widely. This is likely due to subject demographics, disease state, measurement method used, diet, and supplementation regimen. Though the majority of subjects show an increase in MPOD when increasing their lutein and/or zeaxanthin consumption, some individuals show no MPOD response even when levels of lutein and zeaxanthin in the serum increase (Hammond et al., 1997a). The reason for this lack of response is unknown; however, there is speculation that xanthophyll binding proteins may already be saturated with ligand in these indviduals (Trieschmann et al., 2007).
Increased MPOD correlates with improvements in visual performance
The antioxidant and blue light filtering functions of lutein and zeaxanthin have an impact upon eye health beyond just decreasing the risk of age-related eye disease. Macular pigment has been shown to influence visual function and comfort as well. Blue wavelengths of visible light are scattered to a greater degree than longer wavelengths of light (Rayleigh, 1871). Forward scatter in the eye can produce disability glare and a reduction of contrast in the retinal image. Scatter is heightened in individuals with lens opacification that occurs with cataract. Absorption of short wavelengths of visible light by the macular pigment may reduce disability glare. In line with this hypothesis, recent findings demonstrated that supplementation with lutein and zeaxanthin increased MPOD in a healthy population and led to an improvement in tolerance to glaring light and decreased photostress recovery time (Stringham & Hammond, 2008). It has also been proposed that the macular pigment may play a role in reducing the effects of blue haze which is observed when viewing objects in the distance, thereby reducing their visibility (Wooten & Hammond, 2002). The anatomical localization of macular carotenoids is ideal for them to act as an optical filter. Lutein and zeaxanthin supplementation has been shown to enhance this filtering property to improve vision in low light conditions and during glare situations. These data suggest MPOD can potentially serve as a biomarker not only for predicting the risk for eye disease but also for visual function.
Improvements in visual function and comfort parameters have been associated with lutein and zeaxanthin supplementation and subsequent increases in MPOD. High levels of MPOD have been linked directly to preserved foveal function in patients with annular maculopathy and to preserved visual sensitivity in healthy subjects (Haegerstrom-Portnoy, 1988; Hammond, Wooten & Snodderly, 1998; Parisi et al., 2008; Weiter, Delori & Dorey, 1988). In addition, macular pigment, likely via its blue light filtering property, contributes to better visual acuity, glare recovery, and contrast sensitivity in healthy individuals as well as those with age-related eye diseases (Bahrami et al., 2006; Cangemi, 2007; Kvansakul et al., 2006; Massacesi, et al., 2001; Olmedilla, et al., 2003; Richer, 1999; Richer et al., 2004). High levels of macular pigment additionally attenuate chromatic aberration and photophobia (Wenzel, et al., 2006a) .
Highlighting the importance of the relationship between MPOD and visual performance is the fact that numerous trials are being conducted to increase the body of knowledge in this area. Both the AREDS2 and the CARMA studies are evaluating visual function in at least a subset of their respective study populations. Additionally, a large trial involving 120 healthy subjects has recently been completed in Ireland, the Collaborative Optical Macular Pigment Assessment (COMPASS) study (Nolan, 2009). This placebo-controlled, randomized and double-blind clinical trial was designed to assess whether baseline MPOD levels relate to visual performance and whether augmentation of MPOD through supplementation with lutein and zeaxanthin enhances visual performance. The Zeaxanthin and Visual Function (ZVF) Study is evaluating MPOD, visual acuity, contrast sensitivity, shape discrimination, color vision, glare recovery, and lipofuscin pattern changes following supplementation with 9 mg lutein, 8 mg zeaxanthin, or a combination of the two xanthophylls per day for 12 months (Richer, et al., 2008).
Increased MPOD is associated with decreased risk for some eye diseases
The retina contains highly unsaturated lipids susceptible to oxidative damage in a region of high oxygen tension and light exposure. AMD is believed to be in part a disease of oxidative stress, so antioxidant nutrients may play a role in protection against AMD. There is a growing body of evidence suggesting a relationship between levels of macular pigment and risk of age-related eye diseases, although a direct link has not been established. For example, risk factors for AMD including tobacco use, light iris color, age, obesity, and gender are associated with low levels of MPOD (Beatty et al., 2001; Hammond, et al., 1996; Hammond, Fuld & Snodderly, 1996; Hammond & Caruso-Avery, 2000; Hammond et al., 2002; Hammond, Wooten & Snodderly, 1996; Nolan et al., 2004). As mentioned previously, an inverse relationship between MPOD and age, current and prior use of tobacco, and family history of age-related maculopathy was found in a study of 828 healthy subjects (Nolan et al., 2007b). Some studies have shown no association with MPOD and age; however, these results did not take into account the other previously mentioned variables believed to be related to MPOD (Bone et al., 1988; Werner, Donnelly & Kliegl, 1987).
High levels of MPOD may be a protective factor against photo-oxidative damage caused by blue light and potentially reduce one’s risk for AMD. This hypothesis is supported by a number of observational studies. When lutein and zeaxanthin were extracted from healthy and AMD donor eyes and examined via HPLC, concentrations of lutein and zeaxanthin were significantly lower in AMD eyes compared to healthy eyes (Bone, et al., 2001). Beatty and colleagues examined MPOD in a Northern European population using HFP and discovered significantly less macular pigment in eyes at high risk for AMD because of advanced disease in the fellow eye as compared to eyes with no known risk (Beatty et al., 2001). A study of Japanese adults using RRS found that macular carotenoid levels in age-related maculopathy (ARM) patients were significantly lower than those in healthy volunteers, and MPOD levels in later ARM (AMD) patients were significantly lower than levels found in patients with early ARM (Obana et al., 2008). It was also observed in this study that subjects with lower MPOD levels exhibited a greater progression of disease in the fellow eye, implying that lower MPOD may be one risk factor of ARM progression. The CAREDS study of 1698 women aged 54 to 86 years found an indirect association (though not significant) between MPOD and AMD risk after exclusion of subjects with unstable diets and conditions related to AMD risk (LaRowe, et al., 2008). In a separate study, MPOD was measured in 93 AMD eyes and 220 normal eyes, and results showed that AMD patients who were not taking lutein supplements had 32% lower MPOD than healthy subjects. Interestingly, AMD patients taking high-dose lutein supplements after initial AMD diagnosis were found to have MPOD levels indistinguishable from those of control patients (Bernstein et al., 2002).
The low MPOD observed in AMD patients in these studies appears to be specific for this retinal disorder, as patients with retinitis pigmentosa and choroideremia had macular pigment levels similar to healthy controls (Aleman et al., 2001; Alexander, et al., 1987; Duncan et al., 2002; Zhao et al., 2003). On the other hand, five patients with Stargardt macular dystrophy also had significantly lower MPOD as compared to healthy controls, suggesting that MPOD level could be an indicator for risk of other eye diseases as well (Zhao et al., 2003). Establishing a direct link between MPOD and AMD risk is quite challenging due to numerous variables including measurement method, diet and supplementation assessment, age, and disease state within the study population. Due to these inherent confounding variables, there are inconsistencies in the existing available data as two studies have found no association between MPOD and AMD risk (Berendschot, et al., 2002; Jahn, et al., 2005). Clearly, the relationship between MPOD and AMD risk is just now being elucidated. There is a great need for simplified clinical MPOD instruments as well as randomized, double-blind, placebo-controlled studies to firmly establish MPOD as a risk factor for AMD and other retinal disorders.
One important question that arises is: Are low amounts of lutein and zeaxanthin in the AMD-affected macula merely a consequence of the disease? There is no conclusive answer yet, but Bone and colleagues provide evidence to the contrary. They observed lower lutein and zeaxanthin concentrations in the peripheral retina of autopsy eyes from persons with AMD, relative to controls, suggesting that pathology in the central macular did not explain their finding that AMD eyes have significantly less macular pigment than healthy eyes (Bone et al., 2001). It has been suggested that metabolism of lutein and zeaxanthin into various secondary products may account for the changes in MPOD in AMD (Kalariya, et al., 2008). This hypothesis is not widely accepted and requires further investigation.
Conclusions of the Macular Pigment Consensus Panel
The current body of knowledge regarding macular lutein and zeaxanthin and their roles in protection against age-related eye diseases support the hypothesis that AMD is in part a manifestation of an ocular deficiency of lutein and/or zeaxanthin and that higher macular levels of these xanthophyll carotenoids may protect against AMD. The suggestion that low macular pigment amount or certain spatial distribution profiles are risk factors for AMD has profound implications. As macular pigment is comprised of constituents derived solely from the diet, dietary modification could prevent or delay the most common cause of irreversible blindness in Western society. Macular pigment measurement by various methods has the potential to become a commonly tested biomarker to measure risk for eye disease and visual function. Knowledge of one’s MPOD level and spatial distribution may enable individuals to take easy, safe, cost-effective measures to improve their vision and quality of life.
The panel concluded that it might be possible to identify individuals at reduced, medium, and elevated risk for age-related eye disease based on high, medium, and low central MPOD levels, respectively, although macular pigment distribution profiles may be even more important (see below) because central MPOD is only weakly proportional to the total amount of the xanthophyll pigments and ranges from undetectable to over 1.0 optical density units (d.u.) at the peak. The panel members agreed that a central MPOD below 0.2 d.u. should be considered low, between 0.2 d.u. and 0.5 d.u. is mid-range, and levels above 0.5 d.u. as high. When compiling data from numerous studies across a diverse U.S. population (n = 846), approximately 43% have a central MPOD below 0.2 d.u. and about 16% have an MPOD level below 0.1 d.u. (Wooten & Hammond, 2002). These data are also consistent with the mean central MPOD level of a study of 828 healthy Irish subjects (Nolan et al., 2007b). In this population, the mean central MPOD was 0.30 d.u. which is comparable to values determined in other studies (ranging from 0.21 to 0.44 d.u.) using similar age groups and testing conditions (Berendschot & van Norren, 2005; Ciulla, et al., 2001; Hammond & Caruso-Avery, 2000; Liew, et al., 2006; Mellerio, et al., 2002; Nolan et al., 2007b). The panel acknowledged that whether or not central MPOD declines with age remains controversial. Since discordant age-MPOD correlations have been observed with a variety of techniques, these differing results may be related to population selection (i.e. clinic-based versus recruited volunteers) rather than methodological differences. In any event, it is important to consider potential agerelated MPOD changes in any case-control study or in mass public screening of MPOD levels.
The panel members agreed that the diverse methods to measure macular pigment provide great value to the study of the role of nutrients in combating age-related eye disease. As with any scientific technique, each one has its own strengths and weaknesses. The true “gold standard” of macular pigment measurement, HPLC, cannot be made non-invasive and suffers from limited spatial resolution, but it can be used to cross validate other techniques, as has been done for RRS on human cadaver and living monkey eyes. HFP has been employed widely in macular pigment research because the apparatus can be made relatively inexpensively, does not require pupil dilation, and, with proper operator and subject training, it can yield reproducible results. HFP can be difficult for some subjects to perform, and spatial mapping of macular pigment is predicated on a limited number of eccentricities. Reflectometry has a much shorter clinical and research track record but is the only non-invasive technique in which it has been claimed that lutein and zeaxanthin might be distinguishable in a non-imaging variant of the method. This method relies on mathematical models and assumptions, and is not particularly chemically specific, especially in an imaging mode. RRS has exquisite chemical specificity for carotenoids, and, like reflectometry, can be implemented in an integral mode or an imaging mode. The apparatus can be expensive and complex, however, and there are concerns about age-related lens and vitreous changes that can attenuate the Raman signal. AFI has significant potential since it is well suited to a high resolution imaging mode, and recent technological advances have demonstrated that a non-confocal, non-mydriatic, LED-based system can be made at a reasonable cost relative to much more expensive SLO-based systems. AFI is relatively specific if performed correctly, but like all other optical methods can be difficult to implement in subjects with significant macular pathology. Despite this wide array of macular pigment measurement methods, it is reassuring that there is generally good agreement when these techniques are used in the same populations. The panel members agreed that clinical studies of the role of the macular pigment in ocular health and disease should use the chosen measurement technique in a consistent manner
Screening for macular pigment central optical density levels may best be accomplished using HFP or spectral reflectance methods because of their relative simplicity, low cost, and no requirement of pupil dilation as long as proper operator and subject training are employed in the case of HFP. Single wavelength autofluorescence imaging may also be used for screening, perhaps at a higher cost. For more detailed clinical studies in aging subjects and patients with AMD, all methods are applicable as long as the assumptions on which each method is based are not flagrantly violated. Among all the methods, resonant Raman spectroscopy (RRS) may be most appropriate in advanced AMD patients because it is highly specific and does not depend upon anatomical and functional integrity of the macular region as long as central fixation is maintained or video targeting is implemented. The panel also acknowledged that a number of studies have suggested that spatial distribution of macular pigment may be associated with the risk for AMD. Resonance Raman imaging and AFI are particularly effective in obtaining spatial distribution profiles in addition to central MPOD values. An assessment of total macular pigment amount and its spatial distribution may more accurately describe AMD risk as compared to peak MPOD alone.
More clinical studies are necessary to more accurately define the range of MPOD values and distributions that might correlate with disease risk; however, the body of evidence that supports a link between a lack of macular pigment and AMD risk is growing. As the ability to more efficiently and cost effectively measure MPOD levels improves, a more precise relationship between optical density values and spatial distribution of macular pigment and eye health will emerge. The goal of this understanding is to enable individuals with low levels of MPOD, and possibly greater risk for eye disease, to take actions to improve their eye health such as improving their diet by consuming more fruits, vegetables and healthy fats, not smoking, and maintaining a healthy weight. Indeed, the greatest rate of increase in macular pigment is found in patients with the lowest baseline who may not be obtaining enough plant food in their diet (Richer et al., 2007; Stringham & Hammond, 2008).
In those who are unable to make these lifestyle changes in a sufficient manner, supplementation with lutein and zeaxanthin is a compelling option based upon available science. The ability to measure distributions of the macular pigment as well as to estimate the amounts of lutein and zeaxanthin in the retina, offers new tools for detailed studies of the anatomical localization of the carotenoids and for the optimization of supplementation strategies. Increased intake of lutein and zeaxanthin through diet or supplementation has been demonstrated in many studies to increase MPOD levels, improve visual function, and reduce the risk of age-related eye diseases. Their antioxidant and blue light filtering properties provide a strong basis for investigating their potential roles in preventing or slowing the progression of certain age-related eye diseases that manifest in part from cumulative oxidative damage. Expansion of the scientific data and improvements in methodology and equipment necessary to measure MPOD levels in a diverse population will hopefully bring about a paradigm shift in the way we recognize, diagnose, and treat those at risk for AMD and other age-related eye diseases.
Acknowledgments
This work was supported by NEI grant EY-11600 and by an unrestricted departmental grant to the Moran Eye Center of the University of Utah and to the Department of Ophthalmology & Visual Sciences of the University of Texas Medical Branch by Research to Prevent Blindness (New York, NY). Critical reading of the manuscript was provided by Dr. Werner Gellermann. The authors greatly appreciate the support of Kemin Health (Des Moines, IA) for facilitating the meeting of the Macular Pigment Consensus Panel. Dr. Bernstein and the University of Utah hold patent rights for measurement of carotenoids in biological tissues using resonance Raman spectroscopy and autofluorescence attenuation in several configurations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, Steinberg JD, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in retinitis pigmentosa and usher syndrome. Investigative Ophthalmology and Visual Science. 2001;42(8):1873–1881. [PubMed] [Google Scholar]
- Alexander KR, Kilbride PE, Fishman GA, Fishman M. Macular pigment and reduced foveal short-wavelength sensitivity in retinitis pigmentosa. Vision Research. 1987;27(7):1077–1083. doi: 10.1016/0042-6989(87)90022-8. [DOI] [PubMed] [Google Scholar]
- AREDS Report No. 22. The Relationship of Dietary Carotenoid and Vitamin A, E, and C Intake With Age-Related Macular Degeneration in a Case-Control Study: AREDS Report No. 22. Archives of Ophthalmology. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [ NCT00029289] BMC Ophthalmol. 2006;6(1):23. doi: 10.1186/1471-2415-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. British Journal of Ophthalmology. 1999;83(7):867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Koh HH, Carden D, Murray IJ. Macular pigment optical density measurement: a novel compact instrument. Ophthalmic and Physiological Optics. 2000;20(2):105–111. [PubMed] [Google Scholar]
- Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Investigative Ophthalmology and Visual Science. 2001;42(2):439–446. [PubMed] [Google Scholar]
- Beatty S, Nolan J, Kavanagh H, O'Donovan O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Archives of Biochemistry and Biophysics. 2004;430(1):70–76. doi: 10.1016/j.abb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Beatty S, Stevenson M, Nolan JM, Woodside J, The CSG, Chakravarthy U. Longitudinal Relationships Between Macular Pigment and Serum Lutein in Patients Enrolled in the CARMA Clinical Trial (Carotenoids and Co-antioxidants in Age-Related Maculopathy)(Abstract) Investigative Ophthalmology and Visual Science. 2009;50 E-Abstract 1719. [Google Scholar]
- Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Investigative Ophthalmology and Visual Science. 2000;41(11):3322–3326. [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. On the age dependency of the macular pigment optical density. Experimental Eye Research. 2005;81(5):602–609. doi: 10.1016/j.exer.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, van Norren D. Macular pigment shows ringlike structures. Investigative Ophthalmology and Visual Science. 2006;47(2):709–714. doi: 10.1167/iovs.05-0663. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, Willemse-Assink JJ, Bastiaanse M, de Jong PT, van Norren D. Macular pigment and melanin in age-related maculopathy in a general population. Investigative Ophthalmology and Visual Science. 2002;43(6):1928–1932. [PubMed] [Google Scholar]
- Bernstein PS, Ahmed F, Liu A, Sharifzadeh M, Ermakov I, Gellermann W, Sheng X, Zhang K, Allman S, Harmon J. Macular Pigment Imaging in AREDS II Participants: Baseline Studies From an Ancillary Study of AREDS II Subjects Enrolled at the Moran Eye Center. Investigative Ophthalmology and Visual Science. 2009;50 doi: 10.1167/iovs.12-10275. E-Abstract 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Balashov NA, Tsong ED, Rando RR. Retinal tubulin binds macular carotenoids. Investigative Ophthalmology and Visual Science. 1997;38(1):167–175. [PubMed] [Google Scholar]
- Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Investigative Ophthalmology and Visual Science. 1998;39(11):2003–2011. [PubMed] [Google Scholar]
- Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109(10):1780–1787. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. Journal of Biological Chemistry. 2004;279(47):49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48(22):4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Serban B, Zhao da Y, Bernstein PS. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry. 2007a;46(31):9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Zhao da Y, Bernstein PS. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Investigative Ophthalmology and Visual Science. 2007b;48(2):543–549. doi: 10.1167/iovs.06-0558. [DOI] [PubMed] [Google Scholar]
- Bone RA. Macular pigment response to a supplement containing mesozeaxanthin, lutein and zeaxanthin. Nutrition and Metabolism. 2007;4(12) doi: 10.1186/1743-7075-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, Brener B, Gibert JC. Macular pigment, photopigments, and melanin: distributions in young subjects determined by four-wavelength reflectometry. Vision Research. 2007;47(26):3259–3268. doi: 10.1016/j.visres.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, Landrum JT. Distribution of macular pigment components, zeaxanthin and lutein, in human retina. Methods in Enzymology. 1992;213:360–366. doi: 10.1016/0076-6879(92)13137-m. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Research. 1992;32(1):105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Experimental Eye Research. 2000;71(3):239–245. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Investigative Ophthalmology and Visual Science. 1988;29(6):843–849. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Experimental Eye Research. 1997;64(2):211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and Zeaxanthin Dietary Supplements Raise Macular Pigment Density and Serum Concentrations of these Carotenoids in Humans. Journal of Nutrition. 2003;133(4):992–998. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Investigative Ophthalmology and Visual Science. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Investigative Ophthalmology and Visual Science. 2001;42(1):235–240. [PubMed] [Google Scholar]
- Bone RA, Sparrock JM. Comparison of macular pigment densities in human eyes. Vision Research. 1971;11(10):1057–1064. doi: 10.1016/0042-6989(71)90112-x. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Willmer EN. The reflexion of light from the macular and peripheral fundus oculi in man. J Physiol. 1952;116(3):350–356. doi: 10.1113/jphysiol.1952.sp004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangemi FE. TOZAL Study: an open case control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC ophthalmology. 2007;7:3. doi: 10.1186/1471-2415-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovas R, Lima VC, Garcia PM, Morini C, Prata TS, Rosen R. Comparison between Macular Pigment Optical Density Measurements Using Two-Wavelength Autofluorescence and Heterochromatic Flicker Photometry Techniques. Investigative Ophthalmology and Visual Science. 2009 doi: 10.1167/iovs.09-3608. In press. [DOI] [PubMed] [Google Scholar]
- Cardinault N, Gorrand JM, Tyssandier V, Grolier P, Rock E, Borel P. Short-term supplementation with lutein affects biomarkers of lutein status similarly in young and elderly subjects. Experimental Gerontology. 2003;38(5):573–582. doi: 10.1016/s0531-5565(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2001–2002. http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm.
- Charbel Issa P, Berendschot TT, Staurenghi G, Holz FG, Scholl HP. Confocal blue reflectance imaging in type 2 idiopathic macular telangiectasia. Investigative Ophthalmology and Visual Science. 2008;49(3):1172–1177. doi: 10.1167/iovs.07-0636. [DOI] [PubMed] [Google Scholar]
- Charbel Issa P, van der Veen RL, Stijfs A, Holz FG, Scholl HP, Berendschot TT. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Experimental Eye Research. 2009;89(1):25–31. doi: 10.1016/j.exer.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Chew E. Age-Related Eye Disease Study 2 (AREDS2): A Multi-center, Randomized Trial of Lutein, Zeaxanthin, and Omega-3 Long-Chain Polyunsaturated Fatty Acids (Docosahexaenoic Acid [DHA] and Eicosapentaenoic Acid [EPA]) in Age-Related Macular Degeneration. 2007. IND # 74,781, Version 5.1, 3 December 2007. [Google Scholar]
- Ciulla TA, Curran-Celantano J, Cooper DA, Hammond BR, Jr, Danis RP, Pratt LM, Riccardi KA, Filloon TG. Macular pigment optical density in a midwestern sample. Ophthalmology. 2001;108(4):730–737. doi: 10.1016/s0161-6420(00)00655-2. [DOI] [PubMed] [Google Scholar]
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- De La Paz M, Anderson RE. Region and age-dependent variation in susceptibility of the human retina to lipid peroxidation. Investigative Ophthalmology and Visual Science. 1992;33(13):3497–3499. [PubMed] [Google Scholar]
- Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Investigative Ophthalmology and Visual Science. 2006;47(6):2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- Delori FC. Spectrophotometer for noninvasive measurement of intrinsic fluorescence and reflectance of the ocular fundus. Applied Optics. 1994;33:7439–7452. doi: 10.1364/AO.33.007439. [DOI] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investigative Ophthalmology and Visual Science. 1995;36(3):718–729. [PubMed] [Google Scholar]
- Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. Journal of the Optical Society of America. 2001;18(6):1212–1230. doi: 10.1364/josaa.18.001212. [DOI] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. Journal of the Optical Society of America A. Optics, Image Science, and Vision. 2006;23(3):521–538. doi: 10.1364/josaa.23.000521. [DOI] [PubMed] [Google Scholar]
- Duncan JL, Aleman TS, Gardner LM, De Castro E, Marks DA, Emmons JM, Bieber ML, Steinberg JD, Bennett J, Stone EM, MacDonald IM, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in choroideremia. Experimental Eye Research. 2002;74(3):371–381. doi: 10.1006/exer.2001.1126. [DOI] [PubMed] [Google Scholar]
- Egan CA, Robson AG, Moreland JD. Comparison of Motion Photometry and 2-Wavelength Fundus Autofluorescence Assessments of Macular Pigment Spatial Profiles in Healthy Subjects (Abstract) Investigative Ophthalmology and Visual Science. 2009;50 E-Abstract 1714. [Google Scholar]
- Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone photopigment distribution: small alterations associated with macular pigment distribution. Investigative Ophthalmology and Visual Science. 1998;39(12):2394–2404. [PubMed] [Google Scholar]
- Ermakov I, Ermakova M, Gellermann W, Bernstein PS. Macular pigment Raman detector for clinical applications. Journal of Biomedical Optics. 2004;9(1):139–148. doi: 10.1117/1.1627776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakov IV, McClane RW, Gellermann W, Bernstein PS. Resonant Raman detection of macular pigment levels in the living human retina. Optics Letters. 2001;26(4):202–204. doi: 10.1364/ol.26.000202. [DOI] [PubMed] [Google Scholar]
- Francoise JL, Askew EW, Lang LC, Bernstein PS. Serum and macular responses to antioxidant supplementation versus a carotenoid-rich dietary intervention in the elderly. Current Topics in Nutraceutical Research. 2006;4(1):69–78. [Google Scholar]
- Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Investigative Ophthalmology and Visual Science. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- Garnett KM, Guerra LH, Lamb JD, Epperson JL, Greenbury DL, Dorey K, Craft NE. Serum and Macular Pigment Responses to Supplementation With Lutein or Zeaxanthin. Investigative Ophthalmology and Visual Science. 2002;43(12) E-Abstract 2820. [Google Scholar]
- Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS. In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. Journal of the Optical Society of America A. Optics, Image Science, and Vision. 2002;19(6):1172–1186. doi: 10.1364/josaa.19.001172. [DOI] [PubMed] [Google Scholar]
- Gruszecki WI, Sielewiesiuk J. Orientation of xanthophylls in phosphatidylcholine multibilayers. Biochimica et Biophysica Acta. 1990;1023(3):405–412. doi: 10.1016/0005-2736(90)90133-9. [DOI] [PubMed] [Google Scholar]
- Haegerstrom-Portnoy G. Short-wavelength-sensitive-cone sensitivity loss with aging: a protective role for macular pigment? Journal of the Optical Society of America A. Optics and Image Science. 1988;5(12):2140–2144. doi: 10.1364/josaa.5.002140. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, Snodderly DM. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Research. 1996;36(13):2001–2012. doi: 10.1016/0042-6989(95)00290-1. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Fuld K, Curran-Celentano J. Macular pigment density in monozygotic twins. Investigative Ophthalmology and Visual Science. 1995;36(12):2531–2541. [PubMed] [Google Scholar]
- Hammond BR, Fuld K, Snodderly DM. Iris color and macular pigment optical density. Experimental Eye Research. 1996;62(3):293–297. doi: 10.1006/exer.1996.0035. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Investigative Ophthalmology and Visual Science. 1997a;38(9):1795–1801. [PubMed] [Google Scholar]
- Hammond BR, Jr, Caruso-Avery M. Macular pigment optical density in a Southwestern sample. Investigative Ophthalmology and Visual Science. 2000;41(6):1492–1497. [PubMed] [Google Scholar]
- Hammond BR, Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Investigative Ophthalmology and Visual Science. 2002;43(1):47–50. [PubMed] [Google Scholar]
- Hammond BR, Jr, Fuld K. Interocular differences in macular pigment density. Investigative Ophthalmology and Visual Science. 1992;33(2):350–355. [PubMed] [Google Scholar]
- Hammond BR, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Research. 1996;36(18):3003–3009. doi: 10.1016/0042-6989(96)00008-9. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. Journal of the Optical Society of America A. Optics and Image Science. 1997b;14(6):1187–1196. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Wooten BR, Snodderly DM. Preservation of visual sensitivity of older subjects: association with macular pigment density. Investigative Ophthalmology and Visual Science. 1998;39(2):397–406. [PubMed] [Google Scholar]
- Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Investigative Ophthalmology and Visual Science. 1988;29(6):850–855. [PubMed] [Google Scholar]
- Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. American Journal of Clinical Nutrition. 1999;70(2):247–251. doi: 10.1093/ajcn.70.2.247. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Snodderly DM, Krinsky NI, Russett MD, Adler AJ. Biological control of primate macular pigment. Biochemical and densitometric studies. Investigative Ophthalmology and Visual Science. 1991;32(2):257–267. [PubMed] [Google Scholar]
- Helb HM, Charbel Issa P, RL VDV, Berendschot TT, Scholl HP, Holz FG. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008;28(6):808–816. doi: 10.1097/IAE.0b013e31816d81aa. [DOI] [PubMed] [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. Journal of Neuroscience. 2005;25(42):9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn C, Wustemeyer H, Brinkmann C, Trautmann S, Mossner A, Wolf S. Macular pigment density in age-related maculopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2005;243(3):222–227. doi: 10.1007/s00417-004-0995-7. [DOI] [PubMed] [Google Scholar]
- JECFA Lutein from Tagetes erecta specifications prepared at the 63rd JECFA (2004) and published in FNP52. 2004;12:35–37. [Google Scholar]
- Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. American Journal of Clinical Nutrition. 2008;87(5):1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. American Journal of Clinical Nutrition. 2000;71(6):1555–1562. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional Manipulation of Primate Retinas, III: Effects of Lutein or Zeaxanthin Supplementation on Adipose Tissue and Retina of Xanthophyll-Free Monkeys. Investigative Ophthalmology and Visual Science. 2005;46(2):692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Experimental Eye Research. 2008;86(1):70–80. doi: 10.1016/j.exer.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F, Beecher GR, Goli MB, Lusby WR. Separation and quantitation of carotenoids in foods. Methods in Enzymology. 1992a;213:347–359. doi: 10.1016/0076-6879(92)13136-l. [DOI] [PubMed] [Google Scholar]
- Khachik F, Beecher GR, Goli MB, Lusby WR, Smith JC., Jr Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Analytical Chemistry. 1992b;64(18):2111–2122. doi: 10.1021/ac00042a016. [DOI] [PubMed] [Google Scholar]
- Khachik F, de Moura FF, Chew EY, Douglass LW, Ferris FL, 3rd, Kim J, Thompson DJ. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Investigative Ophthalmology and Visual Science. 2006;47(12):5234–5242. doi: 10.1167/iovs.06-0504. [DOI] [PubMed] [Google Scholar]
- Kilbride PE, Alexander KR, Fishman M, Fishman GA. Human macular pigment assessed by imaging fundus reflectometry. Vision Research. 1989;29(6):663–674. doi: 10.1016/0042-6989(89)90028-x. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Galea M, Loane E, Stack J, Beatty S, Nolan JM. Foveal anatomic associations with the secondary peak and the slope of the macular pigment spatial profile. Investigative Ophthalmology and Visual Science. 2009;50(3):1383–1391. doi: 10.1167/iovs.08-2494. [DOI] [PubMed] [Google Scholar]
- Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Experimental Eye Research. 2004;79(1):21–27. doi: 10.1016/j.exer.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kopsell DA, Lefsrud MG, Kopsell DE, Wenzel AJ, Gerweck C, Curran-Celentano J. Spinach cultigen variation for tissue carotenoid concentrations influences human serum carotenoid levels and macular pigment optical density following a 12-week dietary intervention. Journal of Agricultural and Food Chemistry. 2006;54(21):7998–8005. doi: 10.1021/jf0614802. [DOI] [PubMed] [Google Scholar]
- Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Kopcke W, Schalch W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic and Physiological Optics. 2006;26(4):362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Archives of Biochemistry and Biophysics. 2001;385(1):28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Dixon Z, Etienne-Levielle V, Formosa M, Saint-Louis M. Influence of Lutein Dosage on Macular Pigment Response (Abstract) Investigative Ophthalmology and Visual Science. 2004;45 E-Abstract 1290. [Google Scholar]
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Experimental Eye Research. 1997;65(1):57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- LaRowe TL, Mares JA, Snodderly DM, Klein ML, Wooten BR, Chappell R. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study. An ancillary study of the women's health initiative. Ophthalmology. 2008;115(5):876–883. doi: 10.1016/j.ophtha.2007.06.015. e871. [DOI] [PubMed] [Google Scholar]
- Liew SH, Gilbert CE, Spector TD, Mellerio J, Van Kuijk FJ, Beatty S, Fitzke F, Marshall J, Hammond CJ. Central retinal thickness is positively correlated with macular pigment optical density. Experimental Eye Research. 2006;82(5):915–920. doi: 10.1016/j.exer.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. Journal of the American Dietetic Association. 1993;93(3):284–296. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- Massacesi AL, Faletra R, Gerosa F, Staurenghi G, Orzales N. The effect of oral supplementation of macular carotenoids ( lutein and zeaxanthin) on the prevention of age-related macular degeneration: A 18 month follow up study (Abstract) IOVS. 2001;42(4):S234. [Google Scholar]
- Matthews SJ, Ross NW, Lall SP, Gill TA. Astaxanthin binding protein in Atlantic salmon. Comparative Biochemistry and Physiology. Part B, Biochemistry and Molecular Biology. 2006;144(2):206–214. doi: 10.1016/j.cbpb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Mellerio J, Ahmadi-Lari S, van Kuijk F, Pauleikhoff D, Bird A, Marshall J. A portable instrument for measuring macular pigment with central fixation. Current Eye Research. 2002;25(1):37–47. doi: 10.1076/ceyr.25.1.37.9961. [DOI] [PubMed] [Google Scholar]
- Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Archives of Ophthalmology. 2006;124(8):1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- Moreland JD, Robson AG, Kulikowski JJ. Macular pigment assessment using a colour monitor. Color Research & Application. 2001;26(S1):S261–S263. [Google Scholar]
- Moreland JD, Robson AG, Soto-Leon N, Kulikowski JJ. Macular pigment and the colour-specificity of visual evoked potentials. Vision Research. 1998;38(21):3241–3245. doi: 10.1016/s0042-6989(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Morganti P, Fabrizi G, Bruno C. Protective effects of oral antioxidants on skin and eye function. Skinmed. 2004;3(6):310–316. doi: 10.1111/j.1540-9740.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Neelam K, Hogg RE, Stevenson MR, Johnston E, Anderson R, Beatty S, Chakravarthy U. Carotenoids and co-antioxidants in age-related maculopathy: design and methods. Ophthalmic Epidemiology. 2008;15(6):389–401. doi: 10.1080/09286580802154275. [DOI] [PubMed] [Google Scholar]
- Nolan J, O'Donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S. Macular pigment and percentage of body fat. Investigative Ophthalmology and Visual Science. 2004;45(11):3940–3950. doi: 10.1167/iovs.04-0273. [DOI] [PubMed] [Google Scholar]
- Nolan JM Collaborative Optical Macular Pigment ASsessment Study. 2009 ISRCTN35481392. [Google Scholar]
- Nolan JM, Stack J, O'Connell E, Beatty S. The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Investigative Ophthalmology and Visual Science. 2007a;48(2):571–582. doi: 10.1167/iovs.06-0864. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Stack J, O OD, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Experimental Eye Research. 2007b;84(1):61–74. doi: 10.1016/j.exer.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Nolan JM, Stringham JM, Beatty S, Snodderly DM. Spatial profile of macular pigment and its relationship to foveal architecture. Investigative Ophthalmology and Visual Science. 2008;49(5):2134–2142. doi: 10.1167/iovs.07-0933. [DOI] [PubMed] [Google Scholar]
- Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115(1):147–157. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19(1):21–24. doi: 10.1016/s0899-9007(02)00861-4. [DOI] [PubMed] [Google Scholar]
- Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115(2):324–333. doi: 10.1016/j.ophtha.2007.05.029. e322. [DOI] [PubMed] [Google Scholar]
- Pease PL, Adams AJ, Nuccio E. Optical density of human macular pigment. Vision Research. 1987;27(5):705–710. doi: 10.1016/0042-6989(87)90067-8. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On the scattering of light by small particles. Philosophical Magazine. 1871;41:447–454. [Google Scholar]
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Archives of Ophthalmology. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- Richer S. ARMD--pilot (case series) environmental intervention data. Journal of the American Optometric Association. 1999;70(1):24–36. [PubMed] [Google Scholar]
- Richer S, Devenport J, Lang JC. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78(5):213–219. doi: 10.1016/j.optm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75(4):216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- Richer SP, Stiles W, Graham K, Thomas C, Clouser L, Nyland J, Touzeau P, Bekritsky J, Richer D, Park D. The Zeaxanthin and Atrophic AMD Visual Function Study (ZVF)- Investigator Initiated FDA IND #78,973. Investigative Ophthalmology and Visual Science. 2008;49(5) E-Abstract 4973. [Google Scholar]
- Robson AG, Harding G, van Kuijk FJ, Pauleikhoff D, Holder GE, Bird AC, Fitzke FW, Moreland JD. Comparison of fundus autofluorescence and minimum-motion measurements of macular pigment distribution profiles derived from identical retinal areas. Perception. 2005;34(8):1029–1034. doi: 10.1068/p5196. [DOI] [PubMed] [Google Scholar]
- Robson AG, Moreland JD, Pauleikhoff D, Morrissey T, Holder GE, Fitzke FW, Bird AC, van Kuijk FJ. Macular pigment density and distribution: comparison of fundus autofluorescence with minimum motion photometry. Vision Research. 2003;43(16):1765–1775. doi: 10.1016/s0042-6989(03)00280-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Carmona M, Kvansakul J, Harlow JA, Kopcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic and Physiological Optics. 2006;26(2):137–147. doi: 10.1111/j.1475-1313.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal JM, Kim J, de Monastario F, Thompson DJ, Bone RA, Landrum JT, de Moura FF, Khachik F, Chen H, Schleicher RL, Ferris FL, 3rd, Chew EY. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Investigative Ophthalmology and Visual Science. 2006;47(12):5227–5233. doi: 10.1167/iovs.05-1513. [DOI] [PubMed] [Google Scholar]
- Schalch W. Nutritional And Environmental Influences on the Eye. Boca Raton: CRC; 1999. The Carotenoids of the Human Retina; pp. 215–250. [Google Scholar]
- Schalch W, Cohn W, Barker FM, Kopcke W, Mellerio J, Bird AC, Robson AG, Fitzke FF, van Kuijk FJ. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin - the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Archives of Biochemistry and Biophysics. 2007;458(2):128–135. doi: 10.1016/j.abb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Bultmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Investigative Ophthalmology and Visual Science. 2004;45(12):4470–4476. doi: 10.1167/iovs.03-1311. [DOI] [PubMed] [Google Scholar]
- Schweitzer D, Lang GE, Beuermann B, Remsch H, Hammer M, Thamm E, Spraul CW, Lang GK. Objektive bestimmung der optischen dichte von xanthophyll nach supplementation von lutein. Ophtalmologe. 2002;99:270–275. doi: 10.1007/s003470100533. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- Sekiryu T, Iida T, Maruko I, Horiguchi M. Clinical application of autofluorescence densitometry with a scanning laser ophthalmoscope. Investigative Ophthalmology and Visual Science. 2009;50(6):2994–3002. doi: 10.1167/iovs.08-2774. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. Journal of the Optical Society of America. 2006;23(10):2373–2387. doi: 10.1364/josaa.23.002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. Resonance Raman imaging of macular pigment distributions in the human retina. Journal of the Optical Society of America A. Optics, Image Science, and Vision. 2008;25(4):947–957. doi: 10.1364/josaa.25.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmologica Scandinavica. 2002;80(4):368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. American Journal of Clinical Nutrition. 1995;62(6) Suppl:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Investigative Ophthalmology and Visual Science. 1984a;25(6):674–685. [PubMed] [Google Scholar]
- Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investigative Ophthalmology and Visual Science. 1984b;25(6):660–673. [PubMed] [Google Scholar]
- Snodderly DM, Handelman GJ, Adler AJ. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Investigative Ophthalmology and Visual Science. 1991;32(2):268–279. [PubMed] [Google Scholar]
- Snodderly DM, Mares JA, Wooten BR, Oxton L, Gruber M, Ficek T. Macular pigment measurement by heterochromatic flicker photometry in older subjects: the carotenoids and age-related eye disease study. Investigative Ophthalmology and Visual Science. 2004;45(2):531–538. doi: 10.1167/iovs.03-0762. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Investigative Ophthalmology and Visual Science. 1999;40(12):2988–2995. [PubMed] [Google Scholar]
- Stringham JM, Hammond B. Macular Pigment and Visual Performance Under Glare Conditions. Optometry and Vision Science. 2008;85(2):82–88. doi: 10.1097/OPX.0b013e318162266e. [DOI] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115(2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Tanito M, Obana A, Okazaki S, Ohira A, Gellermann W. Change of Macular Pigment Density Quantified With Resonance Raman Spectrophotometry and Autofluorescence Imaging in Normal Subjects Supplemented With Oral Lutein or Zeaxanthin (Abstract) Investigative Ophthalmology and Visual Science. 2009;50 E-Abstract 1716. [Google Scholar]
- The Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Archives of Ophthalmology. 1993;111(1):104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Experimental Eye Research. 2007;84(4):718–728. doi: 10.1016/j.exer.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Trieschmann M, Spital G, Lommatzsch A, van Kuijk E, Fitzke F, Bird AC, Pauleikhoff D. Macular pigment: quantitative analysis on autofluorescence images. Graefes Archive for Clinical and Experimental Ophthalmology. 2003;241(12):1006–1012. doi: 10.1007/s00417-003-0796-4. [DOI] [PubMed] [Google Scholar]
- Trieschmann M, van Kuijk FJ, Alexander R, Hermans P, Luthert P, Bird AC, Pauleikhoff D. Macular pigment in the human retina: histological evaluation of localization and distribution. Eye. 2008;22(1):132–137. doi: 10.1038/sj.eye.6702780. [DOI] [PubMed] [Google Scholar]
- van de Kraats J, Berendschot TT, Valen S, van Norren D. Fast assessment of the central macular pigment density with natural pupil using the macular pigment reflectometer. Journal of Biomedical Optics. 2006;11(6):064031. doi: 10.1117/1.2398925. [DOI] [PubMed] [Google Scholar]
- van de Kraats J, Berendschot TT, van Norren D. The pathways of light measured in fundus reflectometry. Vision Research. 1996;36(15):2229–2247. doi: 10.1016/0042-6989(96)00001-6. [DOI] [PubMed] [Google Scholar]
- van de Kraats J, Kanis MJ, Genders SW, van Norren D. Lutein and zeaxanthin measured separately in the living human retina with fundus reflectometry. Investigative Ophthalmology and Visual Science. 2008;49(12):5568–5573. doi: 10.1167/iovs.08-1939. [DOI] [PubMed] [Google Scholar]
- van de Kraats J, van Norren D. Directional and nondirectional spectral reflection from the human fovea. Journal of Biomedical Optics. 2008;13(2):024010. doi: 10.1117/1.2899151. [DOI] [PubMed] [Google Scholar]
- van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RP, van Dusseldorp M, Weststrate JA, Eskes TK, Hautvast JG. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. American Journal of Clinical Nutrition. 1999;70(2):261–268. doi: 10.1093/ajcn.70.2.261. [DOI] [PubMed] [Google Scholar]
- Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. American Journal of Clinical Nutrition. 2009 doi: 10.3945/ajcn.2009.28013. [DOI] [PubMed] [Google Scholar]
- Vu HT, Robman L, McCarty CA, Taylor HR, Hodge A. Does dietary lutein and zeaxanthin increase the risk of age related macular degeneration? The Melbourne Visual Impairment Project. British Journal of Ophthalmology. 2006;90(3):389–390. doi: 10.1136/bjo.2005.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Human Vision and the Spectrum. Science. 1945;101(2635):653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- Weiter JJ, Delori F, Dorey CK. Central sparing in annular macular degeneration. American Journal of Ophthalmology. 1988;106(3):286–292. doi: 10.1016/0002-9394(88)90363-7. [DOI] [PubMed] [Google Scholar]
- Wenzel AJ, Fuld K, Stringham JM, Curran-Celentano J. Macular pigment optical density and photophobia light threshold. Vision Research. 2006a;46(28):4615–4622. doi: 10.1016/j.visres.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Wenzel AJ, Gerweck C, Barbato D, Nicolosi RJ, Handelman GJ, Curran-Celentano J. A 12-wk egg intervention increases serum zeaxanthin and macular pigment optical density in women. Journal of Nutrition. 2006b;136(10):2568–2573. doi: 10.1093/jn/136.10.2568. [DOI] [PubMed] [Google Scholar]
- Wenzel AJ, Sheehan JP, Gerweck C, Stringham JM, Fuld K, Curran-Celentano J. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic and Physiological Optics. 2007;27(4):329–335. doi: 10.1111/j.1475-1313.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Research. 1987;27(2):257–268. doi: 10.1016/0042-6989(87)90188-x. [DOI] [PubMed] [Google Scholar]
- Wooten BR, Hammond BR. Macular pigment: influences on visual acuity and visibility. Progress in Retinal and Eye Research. 2002;21(2):225–240. doi: 10.1016/s1350-9462(02)00003-4. [DOI] [PubMed] [Google Scholar]
- Wustemeyer H, Jahn C, Nestler A, Barth T, Wolf S. A new instrument for the quantification of macular pigment density: first results in patients with AMD and healthy subjects. Graefes Archive for Clinical and Experimental Ophthalmology. 2002;240(8):666–671. doi: 10.1007/s00417-002-0515-6. [DOI] [PubMed] [Google Scholar]
- Yang JH, Basinger SF, Gross RL, Wu SM. Blue light-induced generation of reactive oxygen species in photoreceptor ellipsoids requires mitochondrial electron transport. Investigative Ophthalmology and Visual Science. 2003;44(3):1312–1319. doi: 10.1167/iovs.02-0768. [DOI] [PubMed] [Google Scholar]
- Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and coantioxidants. The LUNA Study. Ophthalmologe. 2009;106(1):29–36. doi: 10.1007/s00347-008-1773-4. [DOI] [PubMed] [Google Scholar]
- Zhao DY, Wintch SW, Ermakov IV, Gellermann W, Bernstein PS. Resonance Raman measurement of macular carotenoids in retinal, choroidal, and macular dystrophies. Archives of Ophthalmology. 2003;121(7):967–972. doi: 10.1001/archopht.121.7.967. [DOI] [PubMed] [Google Scholar]