Abstract
Epidemiological studies in children have reported associations between elevated dietary manganese (Mn) exposure and neurobehavioral and neurocognitive deficits. To better understand the relationship between early Mn exposure and neurobehavioral deficits, we treated neonate rats with oral Mn doses of 0, 25, or 50 mg Mn/kg/d over postnatal day (PND) 1 – 21, and evaluated behavioral performance using open arena (PND 23), elevated plus maze (PND 23), and 8-arm radial maze (PND 33–46) paradigms. Brain dopamine D1 and D2-like receptors, and DAT transporter densities were determined on PND 24, and blood and brain Mn levels were measured to coincide with behavioral testing (PND 24, PND 36). Pre-weaning Mn exposure caused hyperactivity and behavioral disinhibition in the open arena, but no altered behavior in the elevated plus maze. Manganese-exposed males committed significantly more reference and marginally more working errors in the radial arm maze compared to controls. Fewer Mn exposed males achieved the radial maze learning criterion, and they required more session days to reach it compared to controls. Manganese-exposed animals also exhibited a greater frequency of stereotypic response strategy in searching for the baited arms in the maze. These behavioral and learning deficits were associated with altered expression of the dopamine D1 and D2 receptors and the dopamine transporter in prefrontal cortex, nucleus accumbens, and dorsal striatum. These data corroborate epidemiological studies in children, and suggest that exposure to Mn during neurodevelopment significantly alters dopaminergic synaptic environments in brain nuclei that mediate control of executive function behaviors, such as reactivity and cognitive flexibility.
Keywords: Neonate, behavior, executive function, dopamine, rat, locomotor, D1, D2, DAT
Introduction
Epidemiologic and case-based studies in children have reported associations of environmental manganese (Mn) exposure with behavioral and cognitive deficits, including ADHD-like deficits in executive function affecting impulse control, cognitive flexibility, and goal-oriented behavior (Barlow 1983; Bouchard et al. 2007; Collipp et al. 1983; Pihl and Parkes 1977; Takser et al. 2003; Wasserman et al. 2006; Woolf et al. 2002; Wright et al. 2006). These studies substantiate concerns about the susceptibility of young children to elevated Mn exposure due to the vulnerability of the developing brain to chemical insult, as well as increased absorption and retention of ingested Mn compared to adults (Daubing 1968; Dorner et al. 1989; Keen et al. 1986; Kostial et al. 1978; Lai et al. 1984; Lonnerdal 1997; Miller et al. 1975; Pennington and Young 1991; Zlotkin S. H. 1986). Infants are at risk of elevated Mn exposure from contaminated well-water and soy-based infant formulas that may contain ~30 to 300-fold higher Mn levels than in breast milk (Aschner and Aschner 2005; Ericson et al. 2007; Keen et al. 1986; Ljung and Vahter 2007; Lonnerdal 1997).
Consistent with the behavioral alterations observed in Mn-exposed children, a recent study by Golub et al. (2005) reported that monkeys fed soy formula or soy formula supplemented with added Mn from birth to age 4 months exhibited increased impulsivity, altered play behavior, and more affiliative clinging in social interactions compared to controls. A number of neonate rodent studies have also reported significant impacts of pre-weaning Mn exposure using tests such as gross motor activity, passive avoidance, acoustic startle, burrowing detour, and negative geotaxis (Brenneman et al. 1999; Chandra et al. 1979; Dorman et al. 2000; Pappas et al. 1997; Reichel et al. 2006; Tran et al. 2002a, b). But, these rodent motor/behavioral tests may not be well suited for assessing the types of executive function deficits observed in Mn-exposed children.
The pre- and early post-weaning period coincides with the development of dopaminergic pathways in brain regions such as the prefrontal cortex, nucleus accumbens, and dorsal striatum that are instrumental in the regulation of executive function behaviors involving learning, memory, and attention (Arnsten 2006; Broaddus and Bennett 1990a, b; Goto and Grace 2005; Leo et al. 2003; Packard and Knowlton 2002). The dopaminergic system is also a sensitive target of Mn exposure, based on studies in adult animals and humans (Donaldson 1985; Eriksson et al. 1992; Guilarte et al. 2006; Huang et al. 2003; Kessler et al. 2003; Newland et al. 1989; Newland 1999; Normandin and Hazell 2002) and on recent studies in pre- or early post-weaning rodents (Calabresi et al. 2001; Dorman et al. 2000; McDougall, 2008; Reichel et al. 2006; Tran et al. 2002a, b;). For example, Calabresi et al. (2001) showed that early post-weaning Mn exposure in rats produced significant behavioral disinhibition, and that these behavioral changes were associated with enhanced pre-synaptic D2-like dopamine receptor expression in the corticostriatal system. Thus, the collective evidence suggests that early Mn exposure may produce deficits in learning, memory, and attention through effects on the developing dopaminergic system in specific brain areas.
The objectives of this study were to investigate the effects of oral pre-weaning Mn exposure on behavior and cognition in a rodent model, and whether these effects were associated with alterations in dopaminergic function in forebrain nuclei important in the regulation of executive function behaviors. Neonates were exposed to oral Mn doses of 0, 25, or 50 mg Mn/kg/d over PND 1 – 21, and then assessed using open arena, elevated plus maze, and 8-arm radial maze learning paradigms. Levels of dopamine D1 and D2 receptor proteins, as well as the dopamine transporter (DAT) were measured in the prefrontal cortex, nucleus accumbens, and dorsal striatum. Our results show that neonatal Mn exposure at levels comparable, on a relative basis, to those experienced by children consuming contaminated well-water or soy-based infant formula produce deficits in behavioral inhibition, and spatial and associative learning that were associated with significant alterations in D1, D2, and DAT levels.
Materials and Methods
Animals
Twenty-six primiparous pregnant Sprague-Dawley rats (gestational day 13 – 15) weighing 350 – 500g were purchased from Simonsen Laboratories (Gilroy, Ca.). At parturition, the litters were examined, sexed and weighed, and culled to adjust litter size to 10 pups composed of six male and three female pups per litter with one extra pup to complete the litter. Treatments were balanced within each litter and gender, and only one animal per litter/gender/treatment was used in an experimental outcome. Only males were used for the reported outcomes, with the exception that females were used to determine blood and brain Mn levels on PND 36 in order to infer body Mn levels in males at the beginning of radial maze testing (blood and brain Mn levels measured on PND 24 in both males and females indicated no gender-based differences). All animals were weighed daily throughout Mn exposure and at regular intervals thereafter. Animals were maintained on a reverse 12/12 hour light/dark cycle throughout the duration of the study. The animals were fed Harlan Teklad rodent chow #2018 which is reported by the manufacturer to contain 118 mg Mn/kg. Animals were given local tap water ad libitum, which contained Mn levels that were below the city’s reported detection limit. All procedures related to animal care conformed to the guidelines set forth in the Guide for the Care and Use of Laboratory Animals (NRC 1996).
Manganese Treatment
Neonate rats were orally exposed to Mn doses of 0, 25, and 50 mg Mn/kg/d over postnatal day (PND) 1 – 21. A 150 µg Mn/mL stock solution of MnCl2 was prepared by dissolving MnCl2·4H2O with Milli-Q™ water; aliquots of stock solution were diluted daily in 25% sucrose solution vehicle for oral administration to neonate pups. Oral Mn (or sucrose vehicle) was administered in a volume of ~25 µL/dose via micropipette. Control animals received only the sucrose vehicle.
These oral Mn exposure levels increased Mn intake by ~350 and 700-fold over levels consumed from lactation alone, which approximates the relative ~300 to 500-fold increases in Mn exposure suffered by infants and young children exposed to Mn contaminated water or soy-based formulas (or both), compared to Mn ingestion from human breast milk. Human breast milk contains ~6 ug Mn/L, yielding normal infant intake rates of ~0.6 ug Mn/kg/d, based on infant daily milk consumption rates of ~0.8 L/day for a 8 kg 6–9 month old infant (Arcus-Arth et al., 2005; Dewey et al., 1991; Dorner et al., 1989; Stastny, et al., 1984). By comparison rat milk Mn levels are ~200 – 300 ug Mn/L (Dorman et al., 2005; Keen et al., 1981), and pre-weaning rats consume an average of 260 mL/kg/d over PND 1–21 (Godbole et al., 1981; Yoon and Barton, 2008). Thus, pre-weanling control rats consume ~70 µg Mn/kg/d, which is ~100-times higher than normal human infant Mn intake from breast milk. Since normal daily dietary requirements for Mn are not well known for either infant humans or rats (Keen et al., 1981; Ljung and Vahter, 2007), we chose an exposure regimen that modeled the relative increase in Mn intake experienced by human infants exposed to contaminated well water or soy formulas, compared to human breast milk. For comparison, human breast milk contains an average of ~4 µg Mn/L, whereas drinking water from roughly 6% of wells monitored in the US contain over 300 µg Mn/L and can be as high as 1,500 µg Mn/L, and average Mn levels in infant formula is 400 µg Mn/L and can be as high as 1,200 µg Mn/L (Ljung and Vahter 2007; Lonnerdal 1997; Wasserman et al. 2006). These Mn exposure levels were also similar to levels used in other published studies in neonatal rats (Brenneman et al. 1999; Deskin et al. 1981a, b; Dorman et al. 2000; Kontur and Fechter 1985; Tran et al. 2002a, b).
Pup milk intake during Mn treatment
Milk intake by the nursing pups was evaluated on PND 4, 8, and 11. For this, the dam was removed from the home cage for 4 hours; immediately before returning the dam to the home cage, the pups were weighed and then allowed to nurse for 75 minutes, then reweighed to determine milk intake over the nursing bout. Data are expressed as grams milk consumed per gram body weight over the 75 minute nursing bout.
Design and Tissue Collection
On PND 23 separate cohorts of animals were evaluated in the open arena (n = 15 – 20 males/treatment) and the elevated plus maze (n = 7 males/treatment). Animals were sacrificed on PND 24 for measurement of blood and brain Mn levels (males and females, n = 8 – 12 animals/gender/treatment), and brain dopamine D1 and D2-like receptors and transporter (DAT) levels by immunohistochemical staining (n= 4 – 7 males/treatment). Another cohort of animals (n = 15 – 20 males/treatment) underwent testing in an 8-arm radial maze paradigm, using a 6 day maze acclimation period (PND 27 – 32) followed by a 14 day testing period (PND 33 – 46). A set of female animals was sacrificed on PND 36 to assess blood and brain Mn levels at a time corresponding with 8-arm maze testing.
Animals were sacrificed via decapitation and blood and brain tissues were collected. Whole blood was collected into heparinized containers for hematocrit analysis (measured immediately) and Mn concentrations (samples stored at −20°C until analysis). Hematocrits were measured using standard heparinzed hematocrit capillary tubes and centrifugation. Brain was bisected into hemispheres, and the left hemisphere was immediately frozen on dry ice for Mn concentration analyses. The right hemisphere was dedicated to immunohistochemical analysis and at collection was immediately immersed in 4% paraformaldehyde (Paraformaldehyde M.W.=90.1, #150146, MP Biomedicals) and fixed overnight at 4°C, then changed to a 10% sucrose 0.1M PBS solution and fixed overnight at 4°C, and then changed to a 30% sucrose 0.1M PBS solution for two days at 4°C for cryoprotection. Brain samples were embedded in freezing medium and stored at −70°C until further processing.
Open Arena
Spontaneous locomotor activity in the full arena and defined center and perimeter zones was assessed on PND 23 using activity chambers in connection with an automated video tracking system from San Diego Instruments (SMART System). Animals were placed individually in 60 × 60 × 30 cm open enclosure arenas in a darkened testing room and their movement was video-tracked for 30 minutes using a digital video camera under infrared light. For center and perimeter zone locomotor activity measures, individual tracks were collected and center and perimeter zones were defined in the SMART software and analyzed for time and distance spent in each zone.
Elevated Plus Maze
The elevated plus maze (EPM) was performed on PND 23 as a standard test of fear and anxiety, where anxiety-related behavior is measured by the degree to which the rodent avoids elevated, unenclosed arms of the maze and exhibits defense behaviors such as head dips and scanning posture. The maze was elevated 50 cm above ground and consisted of four arms 48 cm in length. Two opposing arms were open with no walls, while 48 cm high walls enclosed the other two opposing arms. There was a 10 × 10 cm open area at the confluence of the four arms. Testing procedures followed the Behavioral Neuroscience protocol for elevated plus maze (Current Protocols in Neuroscience 2001 John Wiley and Sons: Supplement 10 section 8.3.6 Basic Protocol 4).
Animals were kept on reverse lighting from PND 10 until the testing day. One hour before animals were put in the maze, they were placed in a darkened holding room followed by testing in a dimly lit room. Animals were placed individually in the center facing an open arm, and the rodent’s behavior recorded by video camera. Tapes were scored by a researcher blind to treatment for 1) entries into center, 2) entries into closed arms, 3) entries into distal portion of closed arms, 4) entries into open arms, 5) entries into distal portion of open arms, 6) number of head dips, 7) number of rears, 8) number of scanning postures, 9) number of grooming events.
8 arm Radial Maze
The 8-arm radial maze testing was performed over PND 33–46 to evaluate spatial memory and stimulus-response (S–R) learning after pre-weaning Mn exposure. Maze arms were 55 × 10 × 10 cm (L × W × H) and the center arena was 35 cm in diameter with 20 cm high walls. Bait reward cups were recessed at the ends of each arm so that the bait was not visible unless the animal was adjacent to the cup. Testing procedures followed the Behavioral Neuroscience protocol assessing working vs reference memory (Current Protocols in Neuroscience, 2001 John Wiley and Sons: Supplement 4 section 8.5A.1 Basic Alternate Protocol 1).
The maze was performed daily beginning with a 6-day acclimation period (PND 27 – 32) when animals were gradually conditioned to obtain the food reward (1/4 fruit loop cereal) in the recessed bait cups. Testing occurred over PND 33 – 46; food bait was placed at the ends of four arms after which the animal was placed in the center of the maze. The location of the bait remained the same over the 14 day test period for each rat. The test animal was removed only after all baits were retrieved or 10 minutes had elapsed. Order of testing in the 8-arm radial maze within each cohort was balanced by treatment within a testing day as well as across testing days. Animals were scored based on number of entries into baited and unbaited arms, time to retrieve bait rewards, and sequence of arms visited. The number of arm entries per minute was also recorded.
Blood and Brain Mn Levels
Aliquots of 180 µL whole blood were digested overnight at room temperature with 360 µL 16N HNO3 (Optima grade, Fisher Scientific). Digestion was complete after addition of 180 µL H2O2 and 1,080 µL Milli-Q™ water. After 10 minutes, digestates were centrifuged (15,000 × g for 15 min.) and the supernatant was collected for Mn analysis. Aliquots (100 mg wet weight) of homogenized brain tissue were digested with ultrapure 16N HNO3 (Optima grade, Fisher Scientific) and redissolved in 1N HNO3 for analyses, as previously described (Smith et al. 1992). Mn levels were determined using a Perkin-Elmer 4100ZL Zeeman graphite furnace AAS, with external standardization using certified SPEX standards. National Institutes of Standards and Technology SRM1577b (bovine liver) was used to evaluate procedural accuracy. The analytical detection limit for Mn was 0.1 ng/mL.
Immunohistochemistry for Dopamine Transporter (DAT), Dopamine Receptors D1 and D2, β-tubulin, and DAPI
For immunohistochemical analysis in PND 24 animals, the PFA-fixed right brain hemisphere was microtome sliced (Leica Microsystems, Inc. model CM30505) in preparation for antibody fluorescent staining for DAT, D1, and D2. Frozen brains were sectioned coronally at −20°C in 20 µm slices, mounted on superfrost/Plus slides, and stored at −20°C. Brain slices were arranged so that all three treatments were represented on each slide. Overall, 72 (for prefrontal cortex and olfactory tubercle) and 144 (for striatum and nucleus accumbens) 20 µm brain slices per region per animal per treatment were mounted on slides.
For immunostaining, mounted brain slices were blocked with 4% normal goat serum (Jackson Immunoresearch) and permeablized with 0.1% Triton for 1 hour. Tissues were then washed with PBS, and incubated with primary antibody (DAT, SC Biotech Rabbit polyclonal IgG anti-DAT (H-80) sc-14002 1:50; D1, SC Biotech Rabbit polyclonal IgG anti-D1 (H-109) sc-14001 1:50; D2, SC Biotech Rabbit polyclonal IgG anti-D2 (H-50) sc-9113 1:50; Tau 46 SC Biotech mouse monoclonal IgG anti-tubulin (46) sc-32274 1:50) overnight at 4°C. Tissues were then washed with PBS, PBST, and incubated with secondary antibody (Molecular Probes Alexa Fluor 488 goat anti-rabbit IgG highly cross-adsorbed A11034 1:1000; Molecular Probes Alexa Fluor 555 goat anti-mouse IgG highly cross-adsorbed A21424 1:500) for 1 hour. Next, slides were washed again with PBST and DAPI stained for 10 minutes (Invitrogen D21490/DAPI-Fluoro-Pure Grade, 300nM working solution). Slides were then loaded with Fluoromount GTM (Southern Biotech) and coverslipped prior to analyses by confocal microscopy.
Confocal Microscopy and Image Analysis
A total of 36 (prefrontal cortex and olfactory tubercle) or 72 (striatum and nucleus accumbens) immunostained brain slices per brain region per animal per treatment were analyzed for all proteins (i.e., every other brain slice was selected for analysis). Immunostained brain slices were analyzed using a Zeiss LSM 5 Pascal Laser Scanning Microscope. All images on each slide were taken with constant settings at 20× magnification using the same detector gain and amplifier offset for fluorescent image comparison. Subsets of 18 brain slices per region per animal were qualitatively scored for protein fluorescence, and a representative brain slice per region per animal was selected for quantification. Images from 4–7 animals per treatment, per protein, per brain region were selected for quantification and analyzed for treatment-based comparisons of fluorescent density within each slide using Metamorph software (Molecular Devices Corporation, MetaXpress™, MetaMorph 7, multiwavelength cell scoring and count nuclei module). For these analyses, brain regions were selected using standard landmarks (e.g. lateral ventricles, corpus callosum, anterior commissure) and average gray values were calculated to quantify grayscale intensity (pixel brightness) values and distribution. Average grayscale values were obtained by dividing the sum of the gray values by the number of pixels detected within the defined threshold for each slide. Fluorescence density of the Mn-treated animals in each brain region for each slide was compared to the average of all control animals in that brain region to determine Mn effects.
Data Analyses
Summary data are expressed as mean ± standard error (SE). Data were analyzed to test specific hypotheses using one-way analysis of variance (ANOVA) using JMP software (Version 7.0, 2007, SAS Institute Inc.). Multivariate analysis of variance (MANOVA) was employed to evaluate treatment effects on the cognitive and behavioral endpoints, incorporating possible covariates of performance (e.g., body weight, cohort, open arena activity bin #), all of which had no influence on the Mn effect. Individual treatment comparisons were done with Tukey’s or Dunnett’s post-hoc test. If necessary, data were log-transformed to achieve normality and variance equality. Log-transformation did not influence determination of significance. If transformation did not achieve the desired outcome, data were analyzed using the non-parametric Kruskal-Wallis test (rank sums) with one-way Chi Square approximation or WELCH ANOVA for unequal variances. A p-value of ≤0.05 for the various outcomes was considered statistically significant.
Results
Pre-weaning Oral Mn Exposure Was Not Overtly Toxic and Produced Increases in Blood Mn Levels Relevant to Human Exposure
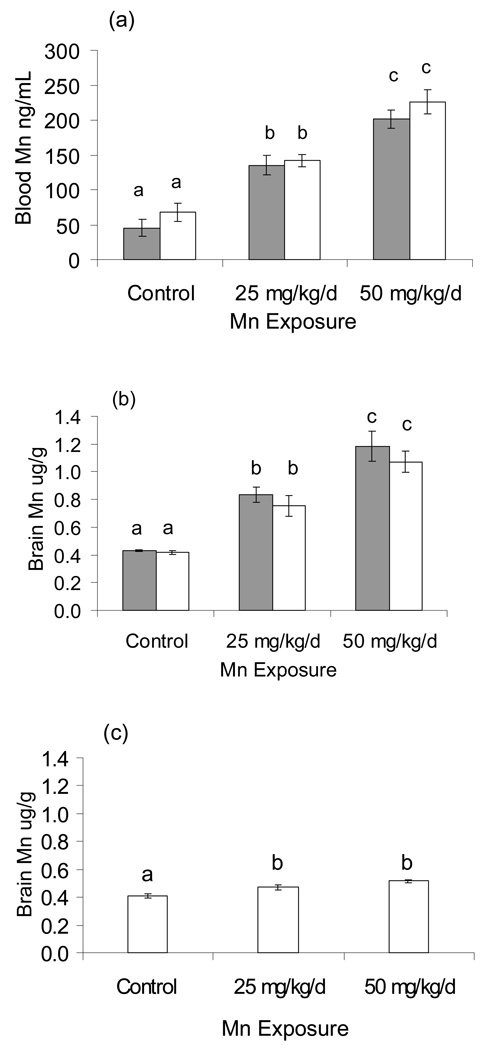
Blood and brain Mn concentrations, hematocrit (HCT), and milk intake by the nursing pups were determined to evaluate the Mn dosing regimen and its relevance to human exposure. Blood and brain Mn levels on PND 24 increased approximately two-fold following exposure to 25 mg Mn/kg/d, and about three-fold following exposure to 50 mg Mn/kg/d (WELCH ANOVA: blood F = 61.78, DFDen = 30.87, p<0.0001; brain F = 51.37, DFDen = 12.224, p<0.0001), with no difference between genders (Figure 1a, b, respectively). To determine brain Mn levels at the time of 8-arm radial maze testing, a set of female rats were sacrificed for analyses on PND 36. Results show that brain Mn levels had decreased substantially to near background levels, with the 25 and 50 mg Mn/kg/d groups being ~15% and ~27% higher than controls (F(2,24) = 17.88, p<0.001), respectively (Figure 1c).
Figure 1.
Pre-weaning Mn exposures increased Mn levels in (a) blood and (b) brain in a dose–wise fashion in PND 24 males (solid bars) and females (hatched bars) (WELCH ANOVA p<0.0001). There were no differences between genders. (c) Brain Mn levels declined to near background levels in PND 36 females. Values are mean (± SE); n=8–12 rats per treatment. Superscripts denote significant difference between treatments based on Tukey post-hoc analysis (p<0.05).
Pre-weaning oral Mn exposure did not induce an anemic state
It has been suggested that elevated Mn exposure may disrupt iron uptake and metabolism (Aschner et al. 2005), which if resulting in developmental iron deficiency could confound the Mn effects in the behavioral assays. We measured hematocrit levels on PND 24 to determine whether animals had evidence of iron deficiency anemia due to Mn exposure, and found that hematocrit levels were not different between control and Mn exposed groups (Control: 31.40 ± 0.62; 25 mg Mn/kg/d: 31.63 ± 0.42; 50 mg Mn/kg/d: 31.97 ± 0.55; Kruskal-Wallis Chi-Square = 0.332, DF = 2, p=0.85, n=8 per treatment).
Pre-weaning oral Mn exposure did not impair pre-weaning nutritional intake, but did result in slight reductions in growth rate
Pup milk intake from lactating dams was measured on PND 4, 8, and 11 to determine if the oral Mn exposure regimen altered nursing success and milk intake, which might confound Mn effects on neurodevelopment and cognitive function. There were no measurable differences in measured milk intake between treatment groups at any of these times, based on pup milk intake normalized to pup body weight (ANOVA p>0.5 for all ages and genders, n=8 animals/treatment/time point, data not shown). This indicates that the Mn exposure regimen did not impair nutritional intake. There was a small effect of Mn treatment on body weight, with the 25 and 50 mg Mn/kg/d groups weighing on average ~3% and 8% less than controls, respectively, on PND 21 (Males PND21: ANOVA F(2,95) =11.78 p<0.0001: Control 56.99 ± 0.79, 25 mg/kg/d 55.02 ± 0.71, 50mg/kg/d 51.93 ± 0.71, mean ± SE n=30/treatment). However, the random assignment of the two males/treatment/litter to different experimental outcomes (e.g., open arena, 8-arm radial maze, etc.) resulted in no differences in body weight between treatments within any of the experimental outcomes.
Pre-weaning Mn Exposure Caused Altered Locomotor Activity in the Open Arena in PND 23 Males
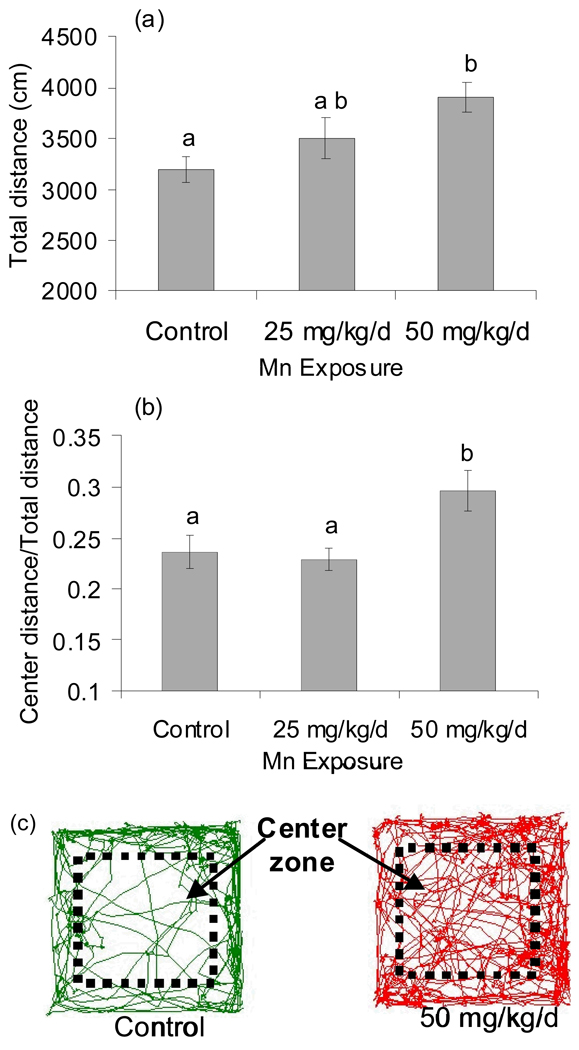
Previous studies have shown altered activity levels following elevated Mn exposure in adult and early post-weanling rodents, and in non-human primates (Brenneman et al. 1999; Chandra et al. 1979; Golub et al. 2005; Pappas et al. 1997). Here, pre-weaning oral Mn exposure caused a significant increase in distance traveled in the open arena in male rats (F(2,51)=5.06, p=0.01, total distance over 5–30 min.), with the 50 mg Mn/kg/d group exhibiting a 20% increase in total distance traveled compared to controls (p<0.05, Tukey post-hoc) (Figure 2a).
Figure 2.
Pre-weaning Mn exposure altered open arena activity in PND 23 males. (a) Mn exposure significantly increased total activity (distance in cm over 5–30 min; ANOVA p=0.01), and (b) significantly increased center zone activity (ratio center distance/total distance; WELCH ANOVA p=0.01). Values are mean (± SE); n=15–20 rats per treatment. Superscripts denote significant difference between treatments based on Tukey post-hoc analysis (p<0.05). (c) Representative activity tracks from control and 50 mg Mn/kg/d animals are shown. Activity was measured in 60 cm × 60 cm × 30 cm open enclosures using the SMART video tracking system (San Diego Instruments).
Pre-weaning Oral Mn Exposure Caused an Altered Stress Response in the Open Arena in PND 23 Males
The open arena is an established paradigm for assessing an animal’s reaction to stressful stimuli related to emotion or affective state (Prut and Belzung 2003). To determine whether pre-weaning Mn exposure altered the behavioral response to the open arena, activity tracks of each animal were analyzed to determine the total distance traveled and time spent in defined perimeter versus center zones of the open arena enclosure. Pre-weaning Mn exposure caused a significant increase in center zone activity of male rats, based on the ratio of center distance/total distance traveled over 5 – 30 min (WELCH ANOVA F=4.99, DF = 2, DFDen = 31.5, p=0.01), with the 50 mg Mn/kg/d exposure group exhibiting a 20% increase in the ratio of center/total distance traveled compared to controls (Figure 2b,c). In fact, over 70% of the increase in total distance traveled in the 50 mg Mn/kg/d group males over controls shown in figure 2a was due to increased locomotor activity in the center zone of the open arena. This increase in center zone activity in Mn exposed animals is not simply due to overall increased distance traveled in the open arena, since the center zone locomotor activity was normalized to total locomotor activity for each animal. Similar results were observed when total time spent in the center versus perimeter zones was considered (data not shown).
Pre-weaning Mn Exposure Did Not Impact Elevated Plus Maze Performance
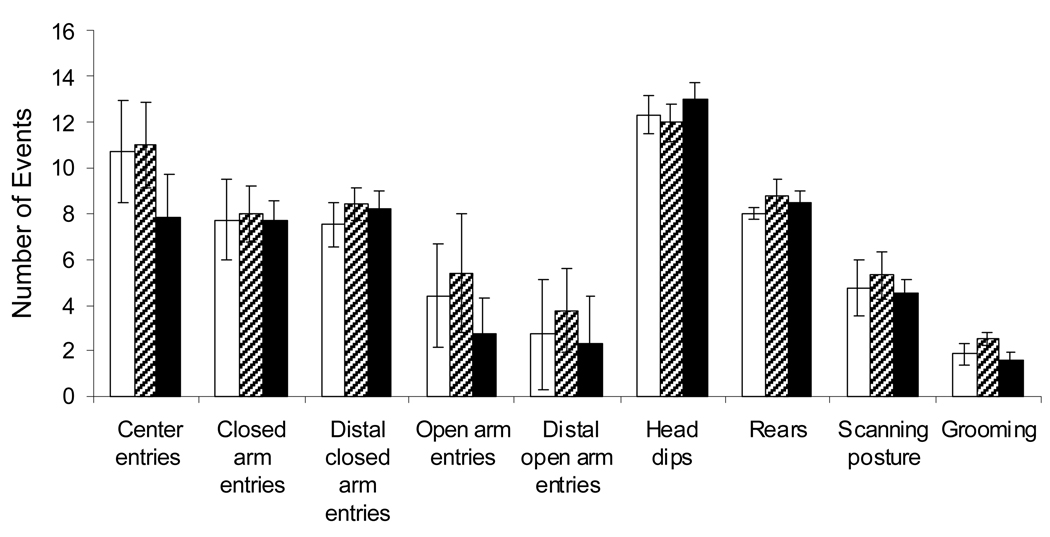
The elevated plus maze is a well-established paradigm to evaluate fear and anxiety, based on the animal’s unconditioned response to a potentially dangerous environment (Carobrez and Bertoglio 2005). Anxiety-related behavior is measured by the degree to which the animal avoids the elevated, unenclosed arms of the maze. Here, there were no measurable differences between control animals and Mn-treated groups in any of the scored behaviors (entries into closed or open arms, rears, head dips, scan posture, etc. ANOVA p>0.4 for all outcomes n=7 per treatment; Figure 3). Thus, Mn exposed rats displayed appropriate fear/anxiety responses in the elevated plus maze by avoiding elevated open arms and exhibiting normal defense behaviors, compared to controls.
Figure 3.
Pre-weaning Mn Exposure Did Not Impact Elevated Plus Maze Performance. There were no measurable differences between control animals and Mn-treated groups in any of the scored behaviors (ANOVA p>0.4 for all outcomes n=7 per treatment).
Pre-weaning Mn Exposure Cause Impaired Learning/Memory Performance in Males in the 8-Arm Radial Maze over PND 33 – 46
The 8-arm radial maze is a well-established learning paradigm to assess spatial memory and stimulus-response (S–R) learning abilities involving both hippocampus-dependent and dorsal caudate (striatum)-dependent learning systems. Both spatial and S–R learning systems are important for success in the radial arm maze. Spatial learning can be assessed via working (short-term) memory, and S–R learning can be assessed via reference (long-term) memory (McDonald and White 1993; Packard et al. 1989; Packard and White 1990; Packard and Knowlton 2002).
Mn exposure caused a significant delay or failure to reach learning criterion
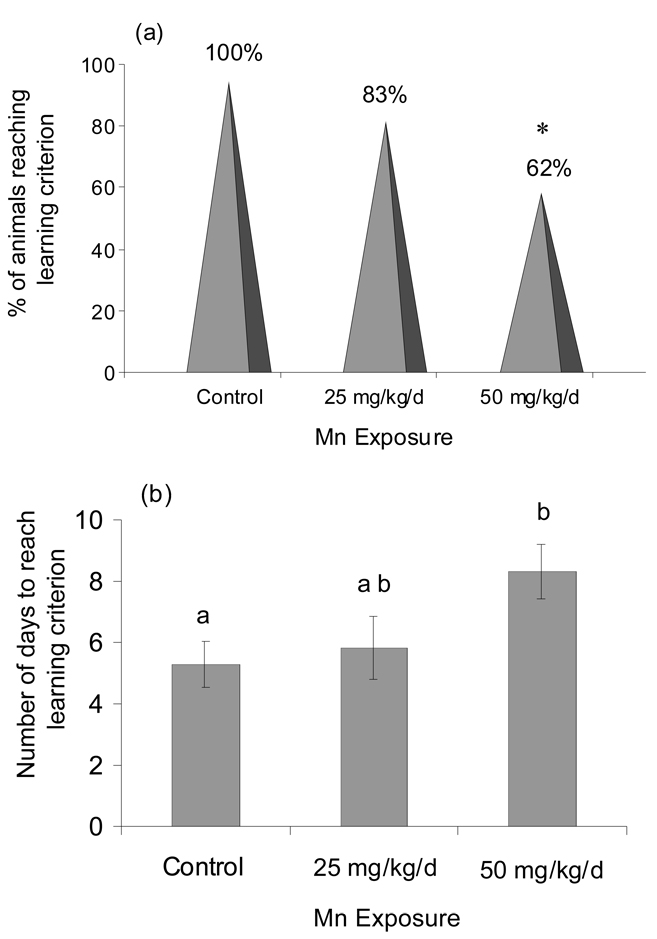
The learning criterion, established as ≤4 total errors over 3 consecutive session days, was based on the lowest number of total errors achieved by 100% of the control animals over 3 consecutive test-session days. Fewer Mn-exposed animals reached the learning criterion over the 14 day testing period; 100% of controls reached criterion compared to only 83% or 62% of animals exposed to 25 or 50 mg Mn/kg/d, respectively (Figure 4a). The 50 mg Mn/kg/d treatment group differed significantly from the control and the 25 mg/kg/d treatment groups (p<0.05, Fisher’s exact test). Further, the Mn-exposed animals that did achieve the learning criterion were significantly delayed in reaching the criterion (Kruskal-Wallis Chi Square = 8.37, DF = 2, p=0.01) (Figure 4b). Control animals reached the learning criterion on session day 5 (avg. 5.3 ± 0.7 SE), while the 25 mg Mn/kg/d and 50 mg Mn/kg/d groups required 5.8 ± 1.0 and 8.3 ± 0.9 session days to achieve criterion, respectively.
Figure 4.
(a) Fewer male rats exposed to daily oral Mn met the radial maze learning criterion (learning criterion of ≤4 total errors/day for 3 consecutive days over 14 day test period, see text). Data expressed as the proportion (%) of treatment group animals who reached criterion (n=18 – 20 rats per treatment). Asterisk denotes significantly different from control and 25 mg/kg groups (p<0.05, Fisher’s exact test). (b) Mn-exposed animals that reached criterion required more session days to reach it (Kruskal-Wallis, p=0.01). Values are mean (± SE); n=11–16 rats per treatment. Superscripts denote significant difference between treatments based on Tukey post-hoc analysis (p<0.05).
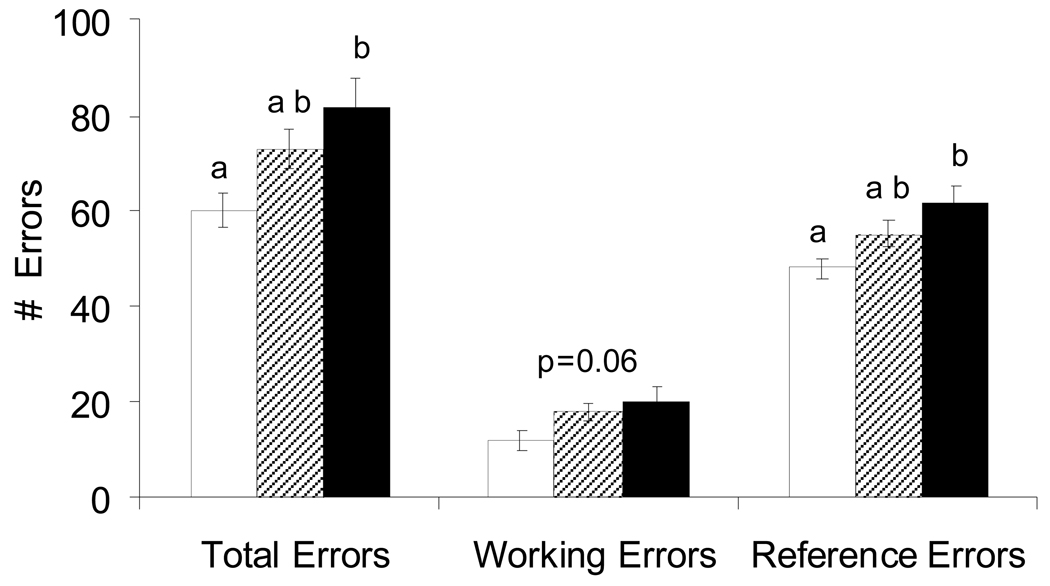
Mn-exposed males committed significantly more learning errors
Pre-weaning Mn exposure caused a significant increase in reference errors (F(2,36) = 5.25, on log transformed data p=0.01), and total errors (F(2,36) = 5.25, on log transformed data p=0.01), and a borderline significant increase in working errors (F(2,36) = 3.02, p=0.06) in the 8-arm radial maze (Figure 5).
Figure 5.
Pre-weaning Mn exposure caused a significant increase in reference errors (ANOVA, on log transformed data p=0.01), total errors (ANOVA, on log transformed data p=0.01), and borderline increase in working errors (ANOVA p=0.06) in males in the 8-arm radial maze (open bars, Control; hatched bars, 25 mg/kg/d; solid bars, 50 mg Mn/kg/d). Working, reference, and total errors were determined for each daily session for all animals running the maze, regardless of success in reaching the learning criterion. Values are mean (± SE); n=20 rats per treatment. Superscripts denote significant difference between treatments based on Tukey post-hoc analysis (p<0.05).
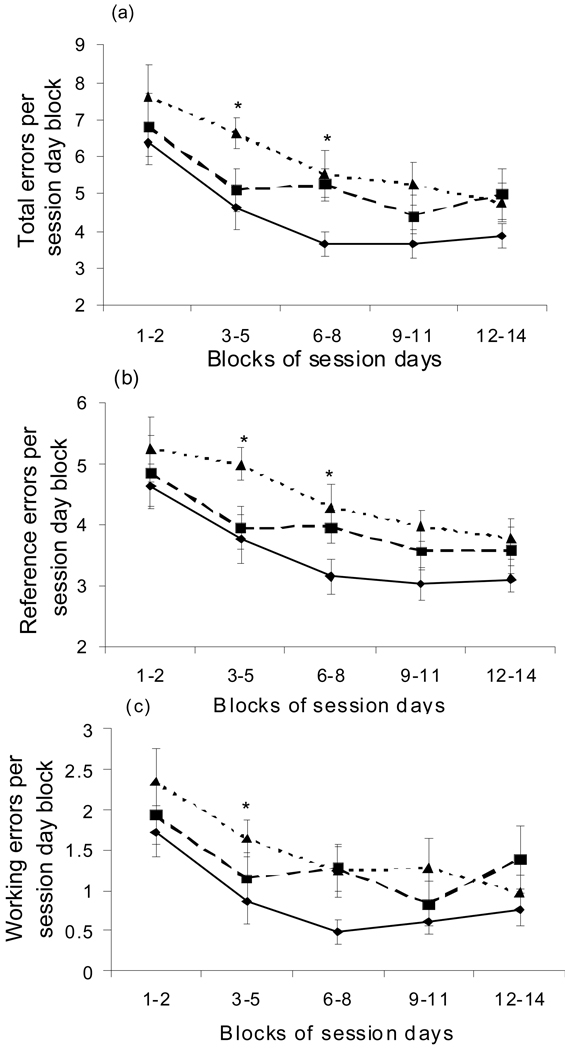
Analysis of learning/memory errors across blocks of test session days (i.e. session day blocks 1 – 2, 3 – 5, 6 – 8, etc.) revealed that the 50 mg Mn/kg/d exposure group committed significantly more total and reference errors compared to controls in session day blocks 3 – 5 and 6 – 8. In the latter day block session blocks, the treatment groups did not significantly differ and the performance was generally asymptotic (i.e. session day blocks 9 – 11, 12 – 14). These results indicate that the significantly increased learning errors suffered by Mn-exposed animals occurred over the learning acquisition phase of maze testing, and not over the later asymptotic performance phase of testing. Statistical analyses of treatment group errors within session day blocks showed that Mn-exposed animals committed significantly more reference (F(2,35) = 6.73, p=0.003), working (WELCH ANOVA F=3.56 DF=2, DFDen=19.48, on log transformed data p=0.04), and total (Kruskal-Wallis Chi-Square = 9.07 DF=2, p=0.01) errors than controls on session day block 3 – 5, and significantly more reference (F(2,34) = 6.02, on log transformed data p=0.045) and total (F(2,36) = 3.24, p=0.048) errors than controls on session block 6 – 8 (Figure 6a, b, c). The number of learning errors committed by the Mn exposed animals was also somewhat higher than controls over the asymptotic performance phase (day blocks 9 – 11, 12 – 14), though those differences did not reach statistical significance. Collectively, these radial maze results suggest that Mn compromises both spatial and S–R learning systems, resulting in significant deficits during the active learning phase of the test period.
Figure 6.
Mn exposure significantly increased maze errors committed by males over the ‘learning acquisition’ phase of 8-arm radial maze testing (see text). Pre-weaning Mn-treated animals committed significantly more (a) total, (b) reference, and (c) working errors than controls over the ‘learning’ phase of maze testing (e.g., session day block 3 – 5 and/or 6 – 8), but not over the asymptotic ‘performance’ phase of maze testing (session day blocks 9 – 11, 12 – 14). Treatment groups are controls (diamonds, solid line), 25 mg Mn/kg/d (squares, dashed line), and 50 mg Mn/kg/d (triangles, dotted line). Data are mean (± SE); n=20 rats per treatment. Asterisk denotes significant difference between 50 mg/kg/d group versus controls, based on Tukey post-hoc analysis (p<0.05). Some analyses done on log-transformed data to achieve normality and equality of variances across treatments, though data are graphed in original scale units. There was no treatment × session day block interactions for any of the outcomes.
To determine whether maze performance was affected by differences in motivation between treatment groups, we examined the number of maze arm entries over the testing period. We found no differences between control and Mn-treated groups in the number of maze arms entered per minute per session day across the 14 day test session (Kruskal-Wallis ChiSquare = 2.47 DF = 2, p>0.29, data not shown). This suggests that there were no differences in motivation to perform the maze across the treatment groups.
Pre-weaning Mn exposure caused a shift in goal-oriented behavior in males
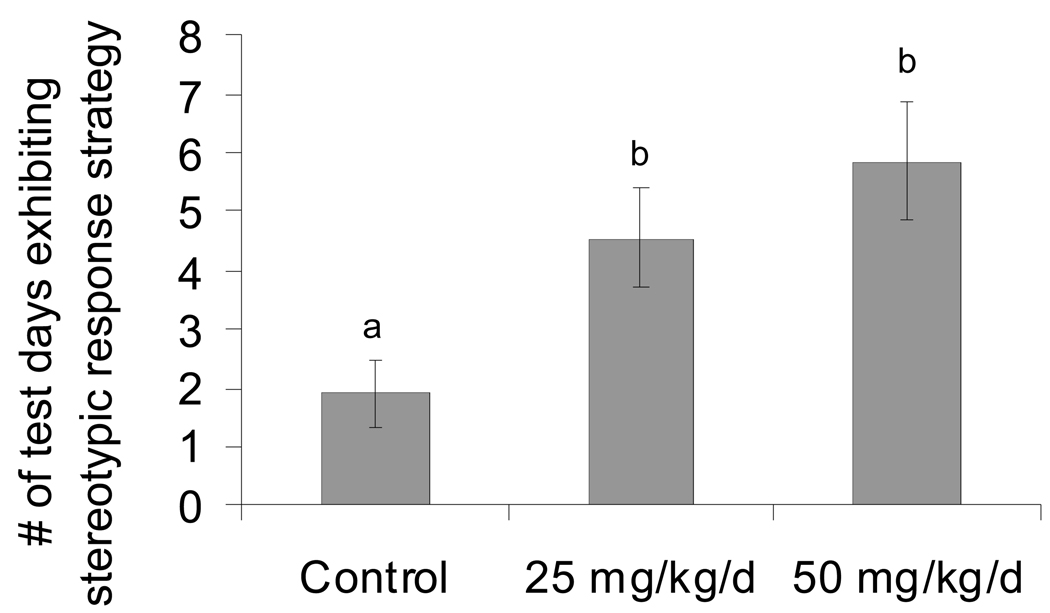
Performance in the 8-arm radial maze was analyzed to evaluate specific learning strategies based on the sequence of maze arms entered on each session day. It has been shown that rodents with impaired spatial memory will utilize a stereotypic response strategy (i.e., successively entering adjacent arms) in searching for bait rewards that manifests as preference for choice of direction (Lanke et al. 1993). Here, pre-weaning Mn-exposed male rats exhibited a stereotypic response strategy on a significantly greater number of session days compared to controls (Kruskal-Wallis Chi-Square = 8.47, DF = 2, p=0.01) (Figure 7). This is consistent with the notion that Mn exposed animals suffered deficits in spatial/associative learning processes, defaulting to a stereotypic response strategy to maximize success.
Figure 7.
Mn exposed male rats exhibited a significant increase in stereotypic response strategy to obtain bait rewards in the 8-arm radial maze. Data are the mean number of maze test session days (± SE; n=20 rats per treatment) that animals exhibited a stereotypic strategy over the 14 day maze test period. Superscript denotes significant difference between treatments based on Dunnett’s test (p<0.05).
Pre-weaning Mn Exposure Altered Dopamine Receptor and Transporter Protein Levels in Males on PND 24
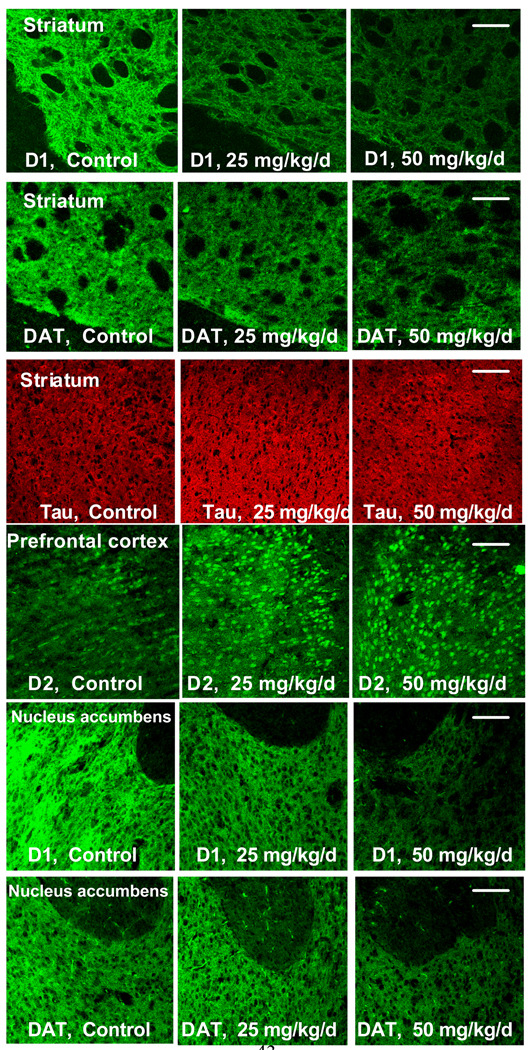
Pre-weaning Mn exposure led to altered levels of D1, D2, and DAT proteins compared to controls in the prefrontal cortex, nucleus accumbens and dorsal striatum brain regions that stained positively for these proteins in PND 24 male rats (Table 1). In the dorsal striatum, D1 receptor levels in both the 25 and 50 mg Mn/kg/d groups and DAT levels in the 50 mg Mn/kg/d group decreased significantly to ~50 – 70% of control group levels (D1 p=0.01 for 25 mg Mn/kg/d and p=0.001 for 50 mg Mn/kg/d; DAT p=0.01 for 50 mg Mn/kg/d, based on Dunnett’s test) (Table 1, Figure 8). In the nucleus accumbens, D1 receptors and DAT in the 50 mg Mn/kg/d group decreased significantly to ~60% of control group levels (D1, p=0.01; DAT, p=0.003 based on Dunnett’s test) (Table 1, Figure 8). D2 receptor levels in the striatum and the nucleus accumbens did not measurably change with Mn treatment. In contrast, D2 receptor immunostaining in the prefrontal cortex increased significantly to 430% of control group levels in the 50 mg Mn/kg/d treatment group (p<0.0001, based on Dunnett’s test) (Table 1, Figure 8). β-tubulin levels in the striatum did not measurably change with Mn treatment (Figure 8).
Table I. Pre-weaning Mn Exposure Altered Dopamine Receptor and Transporter Protein Levels on PND 24.
Levels of D1-like and D2-like dopamine receptors and dopamine transporter (DAT) in the prefrontal cortex, dorsal striatum, and nucleus accumbens of Mn exposed male rats, expressed as percent of control group animals (n= 4 – 7/treatment). Protein levels were determined using fluorescence immunohistochemical staining and a Zeiss LSM 5 Pascal Laser Scanning Microscope, and quantified using MetamorphTM software (see text for details).
| Brain Region | Mn dose → |
Control | 25 mg/kg | 50mg/kg | |
|---|---|---|---|---|---|
| D1 | Pre-frontal cortex | weak staining | |||
| Striatum | 100 ± 5 | 71 ± 7* | 54 ± 9** | ||
| Accumbens | 100 ± 6 | 83 ± 14 | 62 ± 9* | ||
| D2 | Pre-frontal cortex | 100 ± 28 | 200 ± 37 | 429 ± 37** | |
| Striatum | 100 ± 11 | 123 ± 14 | 103 ± 12 | ||
| Accumbens | 100 ± 20 | 134 ± 18 | 103 ± 20 | ||
| DAT | Pre-frontal cortex | weak staining | |||
| Striatum | 100 ± 8 | 95 ± 7 | 67 ± 11* | ||
| Accumbens | 100 ± 6 | 85 ± 9 | 61 ± 5** | ||
Values are average (±SE) changes expressed as percent of average of all control animals in that brain region (* p<0.05, ** p < 0.01, based on Dunnett’s comparison with control versus each Mn group).
Striatal β-tubulin was immunostained as a negative control and showed no measurable change with Mn treatment.
Figure 8.
Representative immunohistochemistry (IHC) photpmicrographs demonstrating that pre-weaning Mn exposure altered levels of D1, D2, and DAT proteins compared to controls in the prefrontal cortex, nucleus accumbens and dorsal striatum in PND 24 male rats (images reflect data displayed as Table 1). In the dorsal striatum and nucleus accumbens, D1 receptor and DAT levels decreased significantly, while β-tubulin levels were unaltered. In contrast, D2 receptor immunostaining increased significantly in the prefrontal cortex. IHC slides were prepared and stained with three animals/slide balanced by treatment and photographed at 20× magnification under defined illumination conditions (see Materials and Methods for details). Scale bar = 100um for all images.
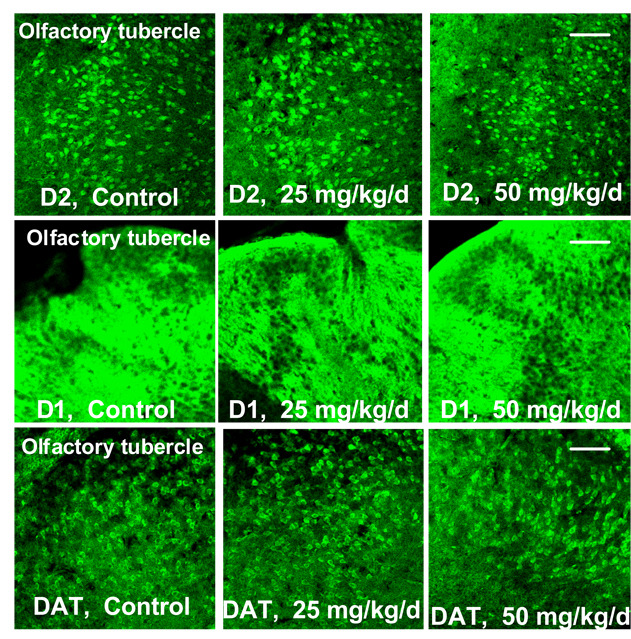
Finally, the olfactory tubercle stained positively for D1, D2, and DAT but did not show measurable changes in protein levels due to pre-weaning Mn treatment (Figure 9), serving as a negative control for these immunohistochemical fluorographs.
Figure 9.
Representative immunohistochemistry (IHC) photomicrographs showing that pre-weaning Mn exposure had no measurable effect on D1, D2, and DAT levels in the olfactory tubercle of PND 24 male rats. Slides were prepared and stained with three animals/slide balanced by treatment and photographed at 20× magnification. Scale bar = 100um for all images.
Brain regions histochemically stained for DAPI did not show measurable differences between treatments (data not shown), indicating that Mn treatments did not cause overt cell loss in the striatum, nucleus accumbens, or prefrontal cortex.
Discussion
We investigated the effects of early Mn exposure on behavior and learning and associated effects on expression of dopamine-related proteins in a rodent model of environmentally relevant Mn exposure. Pre-weaning oral Mn exposure resulted in behavioral hyperactivity and disinhibition in the open arena, and learning deficits in the 8-arm radial maze as well as alterations in dopamine receptors/transporter D1, D2, and DAT in prefrontal cortex, nucleus accumbens, and dorsal striatum. These data suggest exposure to Mn during neurodevelopment significantly alter dopaminergic synaptic environments in brain nuclei that mediate control of executive function behaviors, such as impulsivity, hyper-reactivity, and cognitive flexibility. These results corroborate epidemiological studies in children reporting associations between elevated Mn exposure and neurocognitive deficits and ADHD-like behaviors such as impulsiveness, hyper-reactivity, attention deficits, behavioral disinhibition, and visual-spatial and goal-oriented behavior deficits (Barkley 1997; Bouchard et al. 2007; Collipp et al. 1983; Ericson et al. 2007; Oades et al. 2005; Wasserman et al. 2006; Winstanley et al. 2006; Wright et al. 2006).
Pre-weaning oral Mn exposure caused a significant 20% increase in locomotor activity in the open arena on PND 23 (Figure 2a), consistent with motor hyperactivity effects of Mn reported in other neonate animal studies (Brenneman et al. 1999; Chandra et al. 1979; Pappas et al. 1997). More detailed zonal analyses of locomotor activity in the open arena revealed that Mn-exposed animals also traveled significantly more and spent more time in the center zone of the arena compared to control animals, as reflected in the significantly increased center activity/total activity ratio (Figure 2b). In fact, over 70% of the increase in total open arena locomotor activity exhibited by animals exposed to 50 mg Mn/kg/d (versus controls, Figure 2) occurred in the center zone of the arena. This suggests that the hyperactivity exhibited by Mn-exposed animals was due largely to hyper-reactivity and/or disinhibition of exploratory behavior, and not simply to increased activity (hyperactivity) per se. This suggestion is consistent with the observations of Calabresi et al. (2001), who found that post-weaning Mn exposure led to behavioral disinhibition and hyperactivity in animals as young adults.
In contrast to the Mn effects on open arena behavior, pre-weaning Mn exposure had no effect on the animals’ innate fear response in the elevated plus maze. Manganese-exposed animals avoided open arms in the elevated plus maze as much as control animals. In addition, there were no differences in other defensive behavioral responses (e.g., scan posture and head dips) across treatments (Figure 3). The elevated plus maze and the open arena are both considered screening tests for emotional reactivity (Ducottet and Belzung 2005), but there is a fundamental difference between the two paradigms. The open arena introduces a novel environment with stressors of a wide-open unfamiliar space, as well as isolation from cage mates. Normally, animals show a preference for thigmotaxic (wall touching) behavior in response to these stress cues, but in the absence of normal inhibition of exploratory behavior in this novel environment, animals will more readily venture into the center of the enclosure (Prut and Belzung 2003), as we observed here. The elevated plus maze also presents a novel environment and isolation from cagemates, but includes additional stress factors in the elevated open arms that are absent of thigmotaxic cues and introduce a potentially harmful situation (Carobrez and Bertoglio 2005).
Disinhibition of exploratory behavior in the open arena, but appropriate innate fear response in the elevated plus maze may suggest differential susceptibilities of dopamine systems controlling these behaviors to early Mn exposure. Inhibitory control of exploratory behavior is governed in part by dopamine release in the accumbens and prefrontal cortex (Arnsten and Goldman-Rakic 1998; Bandyopadhyay et al. 2005; Grace 2000), but innate fear conditions, such as those presented by the elevated plus maze, elicit dopaminergic release in relatively primitive structures such as the amygdale and bypass prefrontal cortex influence, resulting in greater autonomic control of behavioral responses (Arnsten 2000; Corcoran and Quirk 2007; LeDoux 2000; LeDoux 2003). This may suggest that behavioral tests that rely only on innate or conditioned fear responses to possible injury, such as shock avoidance, may not be as sensitive for detecting effects of Mn exposure.
Behavioral disinhibition, observed as increased center zone activity in the open arena (Figure 2), was associated with decreased levels of D1 receptors and DAT in the nucleus accumbens and dorsal striatum, and increased D2 receptors in the prefrontal cortex of Mn-exposed animals (Table 1, Figure 8). It is possible that these effects on dopamine-related proteins resulted in deregulation of dopaminergic control over suppression of outward exploratory behavior in the open arena, leading to increased center zone activity. The dopamine system normally functions in the prefrontal cortex and nucleus accumbens to modulate neuronal activity to elicit appropriate behavioral responses to relevant stimuli, such as a novel stressful environment, and for suppression of neuronal activity that might otherwise lead to contextually inappropriate behavioral responses (Arnsten and Goldman-Rakic 1998; Arnsten 2006; Russell 2003). Alteration of the levels/function of these dopamine-related proteins in the Mn-exposed animals may have led to impairment of proper inhibitory control of contextually inappropriate behavior. The lack of a Mn effect in the elevated plus maze (Figure 3), and the observation that Mn had no effect on dopamine receptors or DAT levels in the olfactory tubercle (Figure 9), both support the suggestion that early Mn exposure targets specific dopaminergic nuclei, while sparing others.
Pre-weaning oral Mn exposure also led to significant learning deficits in the 8-arm radial maze, as evidenced by the significantly greater number of learning errors (Figure 5), and the significant delay or failure of Mn-exposed animals to achieve the learning criterion (Figure 4). These deficits may reflect lasting effects of early Mn exposure, since they were measured at a time (PND 33 – 46) when brain Mn levels had declined to near-control levels (Figure 1). The 8-arm radial maze is a standard test for assessment of spatial memory and stimulus-response learning abilities, which are controlled in part by the hippocampus, prefrontal cortex, nucleus accumbens, and striatum (McDonald and White 1993; Packard and White 1989; Packard and White 1990; Packard and Knowlton 2002; White and McDonald 2002). Neural structures of a few key learning and memory systems in the brain have access to the same information and process this information at the same time, known as parallel processing (White and McDonald, 2002). The neural system (hippocampal/pre-frontal cortex driven) that can store information quickly based on a brief, single experience processes information about relations among spatial cues in the environment within some limited time defined as temporal information processing, also known as short-term - or working memory (White and McDonald, 2002). Another neural system at work (dorsal striatum driven) aids in establishing associations between neural response of a stimulus and behavioral response, which are strengthened with repeated exposure of the stimulus and reinforcement (e.g. food bait). This type of learning is defined as stimulus-response learning to increase probability that the stimulus will elicit a response on future occasions, known as long-term - or reference memory (Packard et al., 1989; Packard and White, 1990, White and McDonald, 2002). Both of these neural processes are necessary for proper execution of the maze and occur simultaneously.
An animal’s normal initial response in the radial maze utilizes declarative, short-term, working memory when an environmental cue is associated with reinforcement such as a food bait reward (Packard and Knowlton, 2002). The stimulus-response associations develop and strengthen with repeated presentation of the reinforcement for long-term, reference memory applications (Packard and White 1990; White and McDonald 2002). Thus, the significantly greater number of reference errors and borderline greater number of working errors committed by Mn-exposed animals evidences deficits in both short and long-term learning abilities (Figure 5). Notably, these deficits were most pronounced during the active learning (acquisition) phase of the radial maze test period (i.e., session days 3 – 8, Figure 6), and were not evident in the ‘performance’ phase of maze testing (session days 9 – 14) where Mn-exposed animals did not differ significantly from controls.
These radial maze learning deficits are consistent with the significant changes in levels of D1, D2, and DAT measured in Mn-exposed animals on PND 24. In addition to regulating reactivity to external stimuli, the ascending dopamine system is involved in the integration of external stimuli necessary for goal-directed learning (Arnsten 2006; Goldman-Rakic et al. 2000; Grace 2000; Grace et al. 2007; Seamans et al. 2001; Williams and Goldman-Rakic 1995; Williams and Goldman-Rakic 1998). An intact dopaminergic cortico-striato-thalamo-cortical loop is essential for proper evaluation of external stimuli in goal-directed behaviors, and is the main interface for the dopaminergic system’s influence on behavior (Carr et al. 1999; Pattij et al. 2007). Thus, the altered D1, D2, and DAT protein levels observed here may be an underlying contributor to the significant learning deficits in Mn-exposed animals, and together suggest an impaired ability to regulate reactivity, establish appropriate contextual associations with environmental cues, and process and establish stimulus-reward associations required in learning the maze (Haber et al. 2000; Johansen and Sagvolden 2004).
The significantly increased use of a stereotypic response strategy by Mn exposed animals in the 8-arm maze (Figure 7) is further evidence of disrupted learning behavior. Impaired spatial/associative learning in rats has been shown to result in animals choosing arms in the maze in a stereotypic manner (sequentially entering adjacent arms), reflecting a shift from the more efficient allocentric stimulus-response learning strategy (utilizing environmental cues) to a pre-conditioned ‘default’ egocentric strategy (preference of direction) to recover the bait reward (Lanke et al. 1993; Soblosky et al. 1996). Amygdala function is important in mediating this pre-conditioned approach-reward stereotypic strategy, which does not involve spatial or associative memory or depend upon a learned response (McDonald and White 1993; White and McDonald 2002). The increased use of a stereotypic response strategy is consistent with the suggestion above that early Mn exposure caused deficits in spatial/associative learning in dopaminergic pathways of the cortico-striato-thalamo-cortical loop modulating executive function.
This exposure regimen is comparable on a relative basis to estimated elevated environmental and dietary (infant formula) exposure suffered by human infants (Aschner and Aschner 2005; Dorman et al. 2000; Ljung and Vahter 2007). Further, it produced 2 – 3-fold increases in blood Mn levels that are comparable with relative increases in blood Mn levels reported for Mn exposed adult humans (Aschner et al. 2005; Bowler et al. 2007; Lucchini et al. 1995; Mizoguchi et al. 2001; Myers et al. 2003;).
In summary, pre-weaning Mn exposure produced deficits in behavioral inhibition, and spatial and associative learning that were associated with significant alterations in dopamine receptors and DAT levels in selected brain regions. These results, together with animal studies showing that Mn targets the dopaminergic system (Chen et al. 2006; Donaldson 1985; Eriksson et al. 1992; Guilarte et al. 2006; Newland et al. 1989; Newland 1999; Yamada et al. 1986), and epidemiologic studies in children showing associations of cognitive deficits and ADHD-like behaviors with elevated Mn exposure (Bouchard et al. 2007; Collipp et al. 1983; Ericson et al. 2007; Wasserman et al. 2006; Wright et al. 2006), supports the notion that early elevated Mn exposure produces behavioral deficits by targeting dopaminergic pathways of executive function. This suggestion is consistent with animal model studies linking disruption of the dopaminergic system to ADHD-like behavioral deficits in executive function (Giedd et al. 2001; Oades et al. 2005; Schrimsher et al. 2002; Swanson et al. 1998), and with recent human studies reporting altered DAT binding in striatum, sustantia nigra, and ventral tegmentum in adults and children with ADHD (Jucaite et al. 2005; Larisch et al. 2006; Madras et al. 2005; Spencer et al. 2007). Together, these results support a need for further animal model and human studies to establish the causal relationship between early Mn exposure and persistent cognitive and ADHD-like deficits, and the mechanistic basis of these effects.
Acknowledgements
This study was supported by the National Institute of Environmental Health Sciences (grant #ES010788), the University of California Toxic Substances Research and Teaching Program, a scholarship to Cynthia Kern from the Achievement Rewards for College Scientists, the President’s Dissertation-Year Fellowship from the UCSC Graduate Council, and the Violence Research Foundation. Gregg Stanwood receives financial support from the Vanderbilt Kennedy Center. We thank Barbara Strupp for advice on the behavioral paradigms and statistical analysis. We also thank student researchers Stefanie Hood, Tyra Thorstad, Vadim Keyfes, Yasmine Pilz, Richard Cathey, Charles Goodhue, and Sylvia Mau for their invaluable assistance with behavioral testing, and Kimberley Best at Molecular Devices for technical assistance with Metamorph software. Finally, we thank Roberto Gwiazda and Tom Jursa for their laboratory support and invaluable advice throughout the study.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3(3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Arcus-Arth A, Krowech G, Zeise L. Breast milk and lipid intake distributions for assessing cumulative exposure. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:357–365. doi: 10.1038/sj.jea.7500412. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67 Suppl 8:7–12. [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55(4):362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26(4–5):353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35(1):1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Gonzalez-Islas C, Hablitz JJ. Dopamine enhances spatiotemporal spread of activity in rat prefrontal cortex. J Neurophysiol. 2005;93(2):864–872. doi: 10.1152/jn.00922.2004. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. J Dev Behav Pediatr. 1997;18(4):271–279. [PubMed] [Google Scholar]
- Barlow PJ. A pilot study on the metal levels in the hair of hyperactive children. Med Hypotheses. 1983;11(3):309–318. doi: 10.1016/0306-9877(83)90094-4. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64(3):167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20(2–3):477–487. [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Brain Res Dev Brain Res. 1990a;52(1–2):265–271. doi: 10.1016/0165-3806(90)90244-s. [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. II. Effects of neonatal 6-hydroxydopamine treatments on D1 and D2 receptors, adenylate cyclase activity and presynaptic dopamine function. Brain Res Dev Brain Res. 1990b;52(1–2):273–277. doi: 10.1016/0165-3806(90)90245-t. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Ammassari-Teule M, Gubellini P, Sancesario G, Morello M, Centonze D, Marfia GA, Saulle E, Passino E, Picconi B, Bernardi G. A synaptic mechanism underlying the behavioral abnormalities induced by manganese intoxication. Neurobiol Dis. 2001;8(3):419–432. doi: 10.1006/nbdi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carr DB, O'Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19(24):11049–11060. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Saxena DK. Manganese-induced behavioral dysfunction and its neurochemical mechanism in growing mice. J Neurochem. 1979;33(6):1217–1221. doi: 10.1111/j.1471-4159.1979.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27(2):229–236. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409(1):38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann Nutr Metab. 1983;27(6):488–494. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubing J. The development of the blood-brain barrier. Prog Brain Res. 1968;29:417–427. doi: 10.1016/S0079-6123(08)64172-2. [DOI] [PubMed] [Google Scholar]
- Deskin R, Bursian SJ, Edens FW. The effect of chronic manganese administration on some neurochemical and physiological variables in neonatal rats. Gen Pharmacol. 1981a;12(4):279–280. doi: 10.1016/0306-3623(81)90058-6. [DOI] [PubMed] [Google Scholar]
- Deskin R, Bursian SJ, Edens FW. Neurochemical alterations induced by manganese chloride in neonatal rats. Neurotoxicology. 1981b;2(1):65–73. [PubMed] [Google Scholar]
- Dewey KG, Heinig MJ, Nommsen LA, Lönnerdal B. Adequacy of energy intake among breast-fed infants in the DARLING study: relationships to growth velocity, morbidity, and activity levels. Davis Area Research on Lactation, Infant Nutrition and Growth. J Pediatr. 1991;119(4):538–547. doi: 10.1016/s0022-3476(05)82401-1. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Barbeau A. Metal ions in neurology and psychiatry. In: Gabay SHJ, Ho BT, editors. Manganese neurotoxicity: possible clues to the etiology of human brain disorders. New York: Alan R. Liss Inc.; 1985. pp. 259–285. [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol. 2000;20(3):179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dorman DC, McElveen AM, Marshall MW, Parkinson CU, James RA, Struve MF, Wong BA. Tissue manganese concentrations in lactating rats and their offspring following combined in utero and lactation exposure to inhaled manganese sulfate. Toxicol Sci. 2005;84(1):12–21. doi: 10.1093/toxsci/kfi060. [DOI] [PubMed] [Google Scholar]
- Dorner K, Dziadzka S, Hohn A, Sievers E, Oldigs HD, Schulz-Lell G, Schaub J. Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow's milk formulas. Br J Nutr. 1989;61(3):559–572. doi: 10.1079/bjn19890143. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res. 2005;156(1):153–162. doi: 10.1016/j.bbr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29(2):181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Gillberg PG, Aquilonius SM, Hedstrom KG, Heilbronn E. Receptor alterations in manganese intoxicated monkeys. Arch Toxicol. 1992;66(5):359–364. doi: 10.1007/BF01973632. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- Godbole VY, Grundleger ML, Pasquine TA, Thenen SW. Composition of rat milk from day 5 to 20 of lactation and milk intake of lean and preobese Zucker pups. J Nutr. 1981 Mar;111(3):480–487. doi: 10.1093/jn/111.3.480. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27(4):615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202(2):381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Gwiazda R, Lucchini R, Smith D. Adequacy and consistency of animal studies to evaluate the neurotoxicity of chronic low-level manganese exposure in humans. J Toxicol Environ Health A. 2007;70(7):594–605. doi: 10.1080/10937400600882897. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Weng YH, Lu CS, Chu NS, Yen TC. Dopamine transporter binding in chronic manganese intoxication. J Neurol. 2003;250(11):1335–1339. doi: 10.1007/s00415-003-0214-1. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T. Response disinhibition may be explained as an extinction deficit in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2004;149(2):183–196. doi: 10.1016/s0166-4328(03)00229-8. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry. 2005;57(3):229–238. doi: 10.1016/j.biopsych.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Keen CL, Bell JG, Lonnerdal B. The effect of age on manganese uptake and retention from milk and infant formulas in rats. J Nutr. 1986;116(3):395–402. doi: 10.1093/jn/116.3.395. [DOI] [PubMed] [Google Scholar]
- Keen CL, Lonnerdal B, Clegg M, Hurley LS. Developmental changes in composition of rat milk: trace elements, minerals, protein, carbohydrate and fat. J Nutr. 1981;111(2):226–236. doi: 10.1093/jn/111.2.226. [DOI] [PubMed] [Google Scholar]
- Kessler KR, Wunderlich G, Hefter H, Seitz RJ. Secondary progressive chronic manganism associated with markedly decreased striatal D2 receptor density. Mov Disord. 2003;18(2):217–218. doi: 10.1002/mds.10325. [DOI] [PubMed] [Google Scholar]
- Kontur PJ, Fechter LD. Brain manganese, catecholamine turnover, and the development of startle in rats prenatally exposed to manganese. Teratology. 1985;32(1):1–11. doi: 10.1002/tera.1420320102. [DOI] [PubMed] [Google Scholar]
- Kostial K, Kello D, Jugo S, Rabar I, Maljkovic T. Influence of age on metal metabolism and toxicity. Environ Health Perspect. 1978;25:81–86. doi: 10.1289/ehp.782581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Leung TK, Lim L. Differences in the neurotoxic effects of manganese during development and aging: some observations on brain regional neurotransmitter and non-neurotransmitter metabolism in a developmental rat model of chronic manganese encephalopathy. Neurotoxicology. 1984;5(1):37–47. [PubMed] [Google Scholar]
- Lanke J, Mansson L, Bjerkemo M, Kjellstrand P. Spatial memory and stereotypic behaviour of animals in radial arm mazes. Brain Res. 1993;605(2):221–228. doi: 10.1016/0006-8993(93)91744-d. [DOI] [PubMed] [Google Scholar]
- Larisch R, Sitte W, Antke C, Nikolaus S, Franz M, Tress W, Muller HW. Striatal dopamine transporter density in drug naive patients with attention-deficit/hyperactivity disorder. Nucl Med Commun. 2006;27(3):267–270. doi: 10.1097/00006231-200603000-00010. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(45):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leo D, Sorrentino E, Volpicelli F, Eyman M, Greco D, Viggiano D, di Porzio U, Perrone Capano C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci Biobehav Rev. 2003;27(7):661–669. doi: 10.1016/j.neubiorev.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007;115(11):1533–1538. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnerdal B. Nutritional aspects of soy formula. Acta Paediatr Suppl. 1994;402:105–108. doi: 10.1111/j.1651-2227.1994.tb13371.x. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol Rev. 1997;77(3):643–669. doi: 10.1152/physrev.1997.77.3.643. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B, Keen CL, Hurley LS. Iron, copper, zinc, and manganese in milk. Annu Rev Nutr. 1981;1:149–174. doi: 10.1146/annurev.nu.01.070181.001053. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21(2):143–149. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107(1):3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol. 1975;229(4):1080–1084. doi: 10.1152/ajplegacy.1975.229.4.1080. [DOI] [PubMed] [Google Scholar]
- Mizoguchi N, Nishimura Y, Ono H, Sakura N. Manganese elevations in blood of children with congenital portosystemic shunts. Eur J Pediatr. 2001;160(4):247–250. doi: 10.1007/s004310000720. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Naik I, Theodorou P, Esswein E, Tassell H, Daya A, Renton K, Spies A, Paicker J, Young T, Jeebhay M, Ramushu S, London L, Rees DJ. The utility of biological monitoring for manganese in ferroalloy smelter workers in South Africa. Neurotoxicology. 2003;24(6):875–883. doi: 10.1016/S0161-813X(03)00082-2. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese's neurotoxicity. Neurotoxicology. 1999;20(23):415–432. [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp Neurol. 1989;106(3):251–258. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Normandin L, Hazell AS. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab Brain Dis. 2002;17(4):375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- NRC. National Research Council. Guide for the care and use of laboratory animals. Washington D.C.: National Academy Press; 1996. [Google Scholar]
- Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev Sci. 2005;8(2):122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9(5):1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacol Biochem Behav. 1989;33(3):511–518. doi: 10.1016/0091-3057(89)90378-x. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Lesions of the caudate nucleus selectively impair "reference memory" acquisition in the radial maze. Behav Neural Biol. 1990;53(1):39–50. doi: 10.1016/0163-1047(90)90780-a. [DOI] [PubMed] [Google Scholar]
- Papp A, Pecze L, Vezer T. Acute effects of lead, mercury and manganese on the central and peripheral nervous system in rats in combination with alcohol exposure. Arh Hig Rada Toksikol. 2005;56(3):241–248. [PubMed] [Google Scholar]
- Pappas BA, Zhang D, Davidson CM, Crowder T, Park GA, Fortin T. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19(1):17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191(3):587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Pennington JA, Young BE. Total diet study nutritional elements, 1982–1989. J Am Diet Assoc. 1991;91(2):179–183. [PubMed] [Google Scholar]
- Pihl RO, Parkes M. Hair element content in learning disabled children. Science. 1977;198(4313):204–206. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci. 2006;93(1):114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Wacan JJ, Farley CM, Stanley BJ, Crawford CA, McDougall SA. Postnatal manganese exposure attenuates cocaine-induced locomotor activity and reduces dopamine transporters in adult male rats. Neurotoxicol Teratol. 2006;28(3):323–332. doi: 10.1016/j.ntt.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Russell VA. Dopamine hypofunction possibly results from a defect in glutamate stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder--the spontaneously hypertensive rat. Neurosci Biobehav Rev. 2003;27(7):671–682. doi: 10.1016/j.neubiorev.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Billingsley RL, Jackson EF, Moore BD., 3rd Caudate nucleus volume asymmetry predicts attention-deficit hyperactivity disorder (ADHD) symptomatology in children. J Child Neurol. 2002;17(12):877–884. doi: 10.1177/08830738020170122001. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Osterloh JD, Niemeyer S, Flegal AR. Stable isotope labeling of lead compartments in rats with ultralow lead concentrations. Environ Res. 1992;57(2):190–207. doi: 10.1016/s0013-9351(05)80079-9. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Tabor SL, Matthews MA, Davidson JF, Chorney DA, Carey ME. Reference memory and allocentric spatial localization deficits after unilateral cortical brain injury in the rat. Behav Brain Res. 1996;80(1–2):185–194. doi: 10.1016/0166-4328(96)00034-4. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62(9):1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny D, Vogel RS, Picciano MF. Manganese intake and serum manganese concentration of human milk-fed and formula-fed infants. Am J Clin Nutr. 1984;39(6):872–878. doi: 10.1093/ajcn/39.6.872. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, Lerner M, Williams L, LaHoste GJ, Wigal S. Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol Psychiatry. 1998;3(1):38–41. doi: 10.1038/sj.mp.4000354. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24(4–5):667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Lonnerdal B, Le L, Parker M, Chicz-Demet A, Crinella FM. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology. 2002;23(4–5):645–651. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8(4):321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ Health Perspect. 2002;110(6):613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27(2):210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70(3–4):273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Yoon M, Barton HA. Predicting maternal rat and pup exposures: how different are they? Toxicol Sci. 2008;102(1):15–32. doi: 10.1093/toxsci/kfm286. Epub 2007 Nov 17. [DOI] [PubMed] [Google Scholar]
- Zlotkin SHBBE. Manganese intakes in intravenously fed infants-dosages and toxicity studies. Biol Trace Elem Res. 1986;9:271. [Google Scholar]