Abstract
Chemoprevention is a practical and translational approach to reduce the risk of various cancers including colorectal cancer (CRC) which is a major cause of cancer-related deaths in the United States. Accordingly, here we assessed chemopreventive efficacy and associated mechanisms of long-term silibinin feeding on spontaneous intestinal tumorigenesis in APCmin/+ mice model. Six-week old APCmin/+ mice were orally-fed with vehicle control (0.5% carboxymethyl celluloseand 0.025% Tween 20 in distilled water) or 750 mg silibinin/kg body weight in vehicle for five days/week for 13 weeks and then sacrificed. Silibinin feeding strongly prevented intestinal tumorigenesis in terms of polyps formation in proximal, middle and distal portions of small intestine by 27% (P<0.001), 34% (P<0.001) and 49% (P<0.001), respectively. In colon we observed 55% (P<0.01) reduction in number of polyps by silibinin treatment. In size distribution analysis, silibinin showed significant decrease in large size polyps (>3 mm) by 66% (P<0.01) and 88% (P<0.001) in middle and distal portions of small intestine, respectively. More importantly, silibinin caused a complete suppression in >3 mm size polyps and 92% reduction in >2–3 mm size polyps in colon. Molecular analyses of polyps suggested that silibinin exerts its chemopreventive efficacy by inhibiting cell proliferation, inflammation and angiogenesis; inducing apoptosis; decreasing β-catenin levels and transcriptional activity; and modulating the expression profile of cytokines. These results for the first time show the efficacy and associated mechanisms of long-term oral silibinin-feeding against spontaneous intestinal tumorigenesis in APCmin/+ mice model, suggesting for its chemopreventive potential against intestinal cancers including CRC.
Keywords: Chemoprevention, colon cancer, silibinin
Introduction
Chemoprevention has emerged as a pragmatic approach to reduce the risk of various cancers including colorectal cancer (CRC); one of the most common malignancies in the Western world (1). Familial adenomatous polyposis (FAP), a hereditary CRC predisposition syndrome (2), is caused by mutations in adenomatous polyposis coli (APC) gene and is characterized by progressive development of numerous adenomas in colon progressing to CRC during later stages (2, 3). Approximately 90% of FAP patients also develop small intestinal adenomas; overall, these patients are at ~330 times higher risk to develop small intestinal adenomas than normal population (4). Moreover, with increased survival seen in FAP patients following prophylactic colectomy, small intestinal adenomas are now a common cause of death in FAP patients (4, 5). Thus, animal models of intestinal tumorigenesis are needed to study the pathogenesis and to develop the strategies to control the malignancy including chemoprevention. APCmin/+ mouse, one of the most studied models of intestinal tumorigenesis, harbors a dominant germ-linemutation at codon 850 of mouse homologue of human APC gene, which is similar to that in FAP patients (6, 7). APCmin/+ micedevelop multiple adenomas in whole intestinal tract primarily in small intestine and fewer in colon (7). Thus, APCmin/+ mouse model is considered an analogue of human intestinal tumorigenesis, and therefore is extensively used in both mechanistic and chemoprevention/intervention efficacy studies (7, 8).
Silibinin is used traditionally to treat various liver conditions and is largely nontoxic (9–11). In last 20-years, we have shown silibinin efficacy in various in vitro and in vivo models of skin, prostate, bladder and lung cancers (12–17). Regarding CRC, silibinin exhibits anticancer effects in HT29 cells in culture (11) and xenografts (18), and its dietary feeding inhibits azoxymethane-induced colonic aberrant crypt foci formation in rats without any adverse health effects (19). Together, these results suggested that silibinin could be an effective chemopreventive agent against CRC. Accordingly, for the first time, here we assessed the efficacy and associated mechanisms of long-term silibinin feeding on spontaneous intestinal tumorigenesis in APCmin/+ mice.
Materials and Methods
Animals and chemoprevention study protocol
C57BL/6J-APCmin/+ and wild-type male mice (5-week age) werefrom Jackson Laboratory (Bar Harbor, Maine). Silibinin was from Sigma with >98% purity (20). One week after acclimation, APCmin/+ mice (6-week old) were divided into two groups of 18 animals each, and given0.2 ml vehicle (0.5% w/v carboxy methyl celluloseand 0.025% Tween 20 in distilled water) or 750 mg silibinin/kg body weight by oral gavage in 0.2 ml vehicle for five days/week for 13 weeks. Negative controls (n = 9/group) of wild-type mice were given vehicle or same silibinin treatment as for APCmin/+ mice. Selection of silibinin dose (750 mg/kg bw) was based on published reports (14, 15, 17, 19, 21). Specifically, silibinin dose is extrapolated from the mice consuming up to 1% (w/w) silibinin in diet that did not show any apparent toxicity, which we have used in several animal studies (14, 15, 17, 19, 21). Mice in all groups received AIN-76A diet and water ad libitum throughout, and food consumption and mice body weights were recorded weekly. Animal care and treatments were in accordance with approved protocol and institutional guidelines. Experiment was terminated after 13 weeks of treatment-period (at 19-week age) to minimize mortality risk caused by severe anemia and intestinal obstruction which is more common in APCmin/+ mice at this age (22). Following euthanasia, small intestine and colon were removed, opened longitudinally and rinsed withsaline. Small intestine was divided by length into three equal sections (proximal, middle and distal segments) and spread onto microscope slides. Polypson intestinal segments including colon were counted and their sizes measured with digital caliper under a dissecting microscope.
Immunohistochemistry staining and quantification
Paraffin-embedded sections (5-μm thick) were deparaffinized and stained using specific antibodies followed by DAB as described (14, 18). Primary antibodies used were mouse monoclonal anti-PCNA (1:250 dilution; Dako), HIF-1α (1:100 dilution; Novus) and anti-nestin (1:100 dilution; SantaCruz), and rabbit polyclonal anti-cleaved caspase-3 (CC3) (1:50dilution; Cell signaling), anti-VEGF-A (1:100 dilution; Neomarkers), anti-eNOS (1:100 dilution, Abcam), anti-β-catenin (1:100 dilution; Santa Cruz), anti-cyclin D1 (1:100 dilution; Neomarkers) and anti-COX-2 (1:100dilution; Cell Signaling). Secondary antibodies used were rabbit anti-mouse IgG (Dako) and goat anti-rabbit IgG (SantaCruz). Apoptotic cells were identified by TUNEL staining using Dead End Colorometric TUNEL System (Promega Corp). PCNA, TUNEL, CC3, nuclear β-catenin and cyclin D1 positive cells were quantified as described recently (14, 18). eNOS, VEGF, HIF-1α and COX-2 were quantified by immunoreactivity (represented by intensityof brown staining) and scored as 0 (no staining), +1 (very weak),+2 (weak), +3 (moderate), and +4 (strong) at 5 randomly selected fields at 400X magnification in each sample. Newly formed nestin-positive microvessels were quantified as mean number of positive vessels at 5 randomly selected fields at 400X magnification in each sample. All microscopic histological and IHC analyses were done by Zeiss Axioskop 2 microscope(Carl Zeiss, Inc.) and photomicrographs were captured by Carl Zeiss AxioCam MRC5 camera.
Western blot analysis
Tissue lysates of distal part of intestinal polyps from control and silibinin groups were analyzed by immunoblotting (14, 18). Densitometric analyses of bands are adjusted with β-actin as loading control.
Assay for prostaglandin (PGE2) levels
Small intestinal polyps were homogenized in 0.1 M phosphate buffer, pH 7.4, containing 1 mM EDTA and 10 μM indomethacin with polytron-type homogenizer. Tissue extracts were acidified with HClto pH 2.5, vortexed for 1 minute after adding 1 mL of ethylacetate, and centrifuged at 3,000 × g for 5 minutes. Organic layer was collected, evaporated under N2, and stored at −80°C. Each sample was reconstituted in 1 mL enzyme immunoassay buffer (Cayman Chemical), and PGE2 levels measured using ELISA kit.
Mouse cytokine array
Tissue lysates of intestinal polyps from three randomly selected animals/group were applied to a Mouse Cytokine Antibody Array (RayBiotech, Inc) to analyze the expression of various cytokine molecules. Expression of each protein was represented in duplicate on the membrane, which were scanned and quantified by ScionImage Program, and densitometric data analyzed using antibody array analysis tool (RayBiotech, Inc).
Reporter gene assay
Transcriptional activity of β-catenin was measured employing TOP/FOPFlash reporter activity assay. HT29 cells were plated to 35% confluency and then co-transfected with 1.2 μg of 8XTOPFLash/FOPFlash (from Dr. Randall Moon) and 250ng of pRL-CMV for 12 h. Cells were then transfected with control or β-catenin siRNA and 12 h later treated with DMSO or 100 μM silibinin for 48 h. Luciferase activity was measured using Promega’s Dual Luciferase Reporter Assay system. Final reporter activity was normalized for transfection efficiency using renilla luciferase activity.
Statistical analysis
Statistical analyses weredone using SigmaStat software version 3.5 (Jandel Scientific). Quantitative data are presented as mean and standard error of mean (SEM). Statistical significance of difference between APCmin/+ control and silibinin treatment groups was determined by unpaired Student’s t-test and P< 0.05 was considered statistically significant.
Results
Silibinin feeding prevents intestinal tumorigenesis in APCmin/+ mice
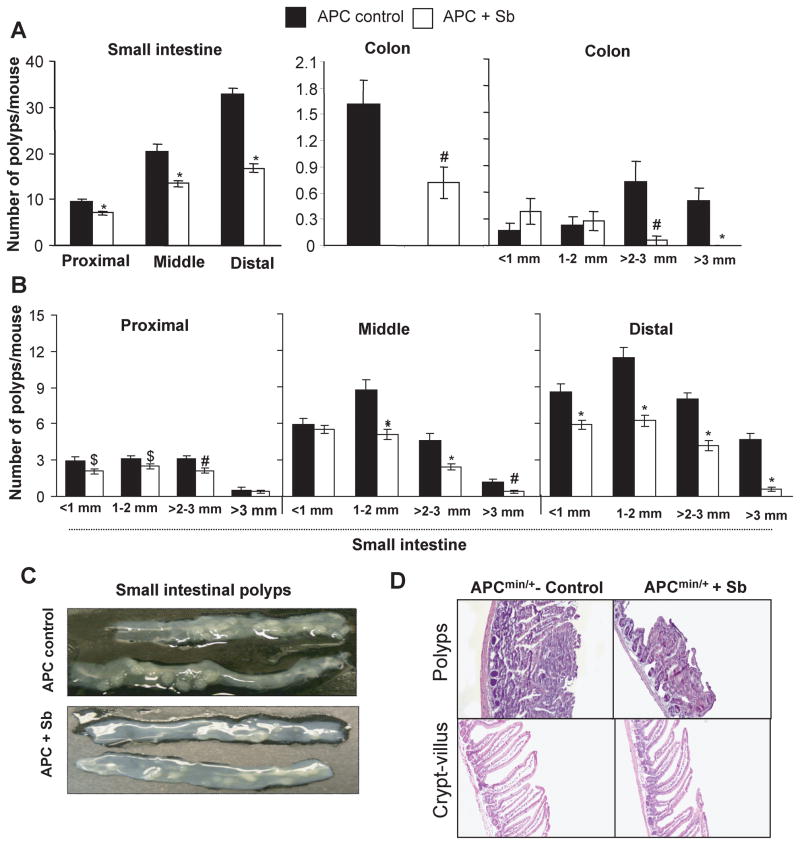
Silibinin treatment of APCmin/+ mice resulted in a strong inhibition in intestinal tumorigenesis in terms of decreased poly number, size and appearance in small intestine (Fig. 1A–C). Specifically, control APCmin/+ mice developed 10, 20 and 33 polyps in proximal, middle and distal portions of small intestine; however, silibinin feeding for 13 weeks significantly prevented polyps number in these portions of small intestine by 27% (P<0.001), 34% (P<0.001), and 49% (P<0.001), respectively (Fig. 1A). Size distribution analysis of polyps in small intestine showed differential silibinin efficacy depending on small intestine segment and polyp size. For example, silibinin reduced number of <1 mm size polyps by 30% (P<0.05) in proximal and 32% (P<0.001) in distal but no effect in middle segment; 1–2 mm size polyps by 19% (P<0.05), 41% (P<0.001) and 45% (P<0.001) in proximal, middle and distal portions; and >2–3 mm size polyps by 32% (P<0.01) in proximal, 47% (P<0.001) in middle and 48% (P<0.001) in distal portions, respectively (Fig. 1B). Most prominent silibinin effect was observed on bigger-size polyps (>3 mm) which decreased by 66% (P<0.01) and 88% (P<0.001) in middle and distal portions, respectively (Fig. 1B).
Figure 1.
Silibinin-feeding prevents spontaneous intestinal polyposis in APCmin+ mice. At the end of the silibinin efficacy study, results are shown for A, number of polyps/mouse in small intestine and colon, and polyp size distribution in colon; B, size distribution of polyps in proximal, middle and distal portions of small intestine; C, representative pictures of distal small intestinal polyps; and D, H&E stained sections from polyps and normally appearing crypt-villus axis from control and silibinin-treated APCmin/+ mice (100x). Bars shown in each case are mean ± SEM from 18 animals in each group. $, P<0.05; #, P<0.01; *, P<0.001 versus control; Sb, silibinin.
Regarding its chemopreventive efficacy in colon, silibinin significantly decreased (55%, P<0.01) number of colonic polyps in APCmin/+ mice (Fig. 1A). Silibinin effect was most profound on colonic polyps size where it very strongly arrested the growth of larger size polyps (>2 mm) than smaller size (<1 mm and 1–2 mm) polyps (Fig. 1A). Size-distribution analysis of colonic polyps showed a strong decrease (92%, P<0.001) in >2–3 mm size polyps in silibinin-fed group compared to controls (Fig. 1A). In terms of the incidence, 8 mice in control group had >2–3 mm size polyps versus only 1 mouse in silibinin-fed group (n=18) (data not shown). More importantly, none of the mice showed >3 mm size polyps in silibinin-fed group accounting for a complete suppression, whereas 8 out of 18 mice in control group showed colonic polyps >3 mm (up to 3.7 mm) in size (Fig. 1A). Importantly, observed decrease in larger-size polyps in silibinin-fed group was accompanied with almost 2-fold increase in <1 mm size polyps and a slight increase in 1–2 mm size polyps, suggesting that silibinin inhibits the growth and progression of smaller colonic polyps in to the larger ones.
In APCmin/+ mice, all polyps were histologically identified as adenomas (Fig. 1D); however, their number and diameter were significantly reduced in silibinin-fed mice. We did not observe any other histological changes in crypt-villus axis between control and silibinin-fed APCmin/+ mice (Fig. 1D). In wild-type C57BL/6J mice, both control and silibinin-fed groups did not develop any polyps throughout intestine including colon, and showed normal intestinal histology (data not shown). Also, silibinin feeding did not show any considerable change in food consumption and gain in body weight compared to controls (data not shown) during entire treatment, which is consistent with previous studies (14, 15, 17–19).
Collectively, above efficacy study results clearly showed that silibinin feeding strongly prevents number of polyps in small intestine of APCmin/+ mice together with a significant decrease in polyp size. Notably, silibinin more strongly prevented incidence, multiplicity and burden of colonic polyps. Whereas this effect of silibinin supports its translational potential, it limited our ability to perform mechanistic studies in colonic polyps because of their fewer numbers (an average of 1.6 polyps in control APCmin/+ group and 0.76 polyps in silibinin-fed group) and very small size specifically in silibinin-fed group. Accordingly, we selected only small intestinal polyps for mechanistic studies as reported by others (22, 23).
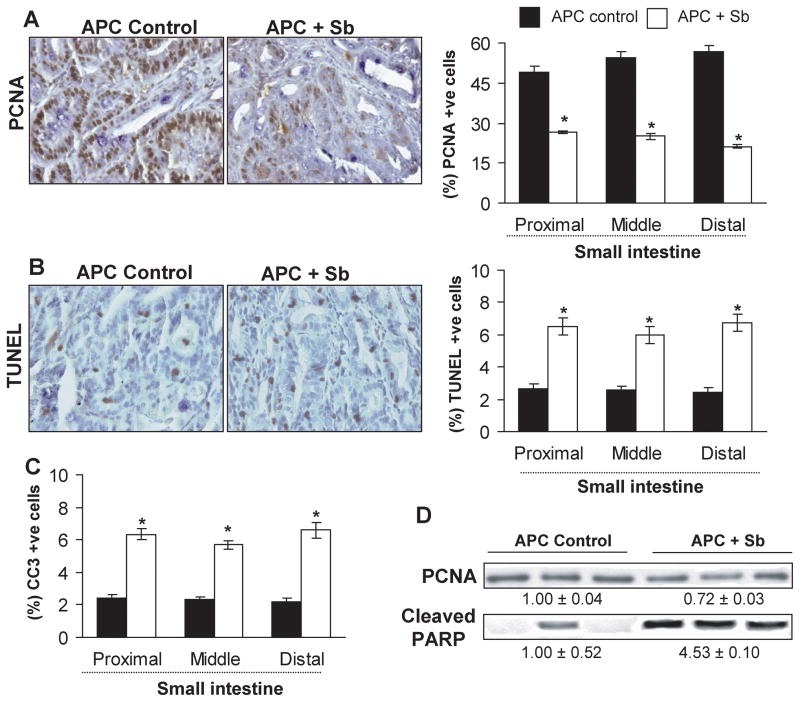
Silibinin feeding inhibits proliferation and induces apoptosis selectively in small intestinal polyps of APCmin/+ mice
PCNA, TUNEL and CC3 are widely used markers/players for cell proliferation and apoptosis (24–25). To assess whether silibinin efficacy is associated with its in vivo antiproliferative and pro-apoptotic effects, all three segments of small intestine were analyzed for PCNA, TUNEL and CC3 immunostaining. Microscopic examination of tissue sections showed a decrease in PCNA (Fig. 2A) but an increase in TUNEL (Fig. 2B) and CC3-positive cells (IHC staining data not shown) in polyps from silibinin-fed compared to control APCmin/+ mice. Quantification of PCNA staining showed 46–63% (P<0.001) decrease in proliferation indices; however, TUNEL and CC3 positive cells increased by 2.4–3 fold (P<0.001) in proximal, middle and distal segments of small intestinal polyps from silibinin-fed mice compared to controls (Fig. 2A–C). These results were further confirmed by immunoblotting (Fig. 2D), where densitometric analysis of bands (adjusted with β-actin as loading control) showed 28% (P<0.05) decrease in PCNA expression and 4.5 fold (P<0.01) increase in cleaved-PARP expression in intestinal polyps from silibinin-treated mice. We did not observe any difference in PCNA, TUNEL and CC3-positive cells in crypt-villus regions throughout small intestine of control and silibinin-fed APCmin/+ as well as wild-type C57BL/6J mice (data not shown). Together, these results clearly suggest in vivo anti-proliferative and pro-apoptotic effects of silibinin selectively in polyps during its chemopreventive efficacy against spontaneous intestinal tumorigenesis in APCmin/+ mice.
Figure 2.
Silibinin-feeding reduces proliferation but induces apoptosis selectively in small intestinal polyps of APCmin/+ mice. Small intestinal segments were processed for PCNA, TUNEL and CC3 staining. Tissue sections from APCmin/+ control and silibinin-treated groups show brown-colored (A) PCNA- and (B) TUNEL-positive cells in polyps (400x). Quantitative data for (A) proliferative and (B and C) apoptotic indices were determined as number of PCNA, TUNEL-or CC3-positive cells × 100/total number of cells, respectively, and represent mean ± SEM of six animals. *, P<0.001 versus control. Polyps from distal portion of small intestine from each group were also analyzed by immunoblotting for PCNA and cleaved–PARP levels. Values of band intensity adjusted with β-actin. Sb, silibinin; CC3, cleaved caspase 3.
Silibinin feeding inhibits angiogenesis selectively in small intestinal polyps of APCmin/+ mice
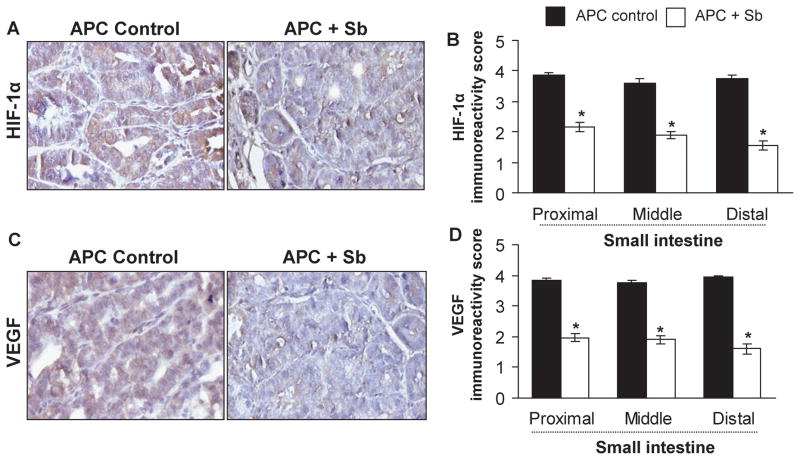
Neoangiogenesis, formation of new blood vessels in tumor, plays a critical role in malignancy (26). Both hypoxia and VEGF are reported as most predominant and specific factors that stimulate neoangiogenesis. Hypoxia triggers induction of transcription factor HIF (27). Importantly, HIF-1α, which is induced under hypoxic condition, is considered a primary regulator of VEGF expression and angiogenesis (28). Thus, to determine whether silibinin affects polyp angiogenesis, we examined HIF-1α and VEGF expression by IHC. Our immunostaining results showed intense immunoreactivity for HIF-1α and VEGF in small intestinal polyps of APCmin/+ mice, but strongly decreased levels in silibinin-fed group (Fig. 3A and 3C). Quantitative data showed that silibinin decreases HIF-1α and VEGF immunoreactivity scores in polyps by 44% (P<0.001) and 49% (P<0.001) in proximal, 47% (P<0.001) and 49% (P<0.001) in middle, and 58% (P<0.001) and 59% (P<0.001) in distal portions of small intestine, respectively (Fig. 3B and 3D).
Figure 3.
Silibinin-feeding inhibits HIF-1α and VEGF expression selectively in small intestinal polyps of APCmin/+ mice. Small intestinal segments were processed for HIF-1α and VEGF staining. Tissue sections from APCmin/+ control and silibinin-treated groups show brown-colored (A) HIF-1α- and (C) VEGF-positive cells in polyps (400x). Quantitative data for (B) HIF-1α and (D) VEGF are shown based on their intensity of cytoplasmic brown staining, and represent mean ± SEM of six animals. *, P<0.001 versus control; Sb, silibinin.
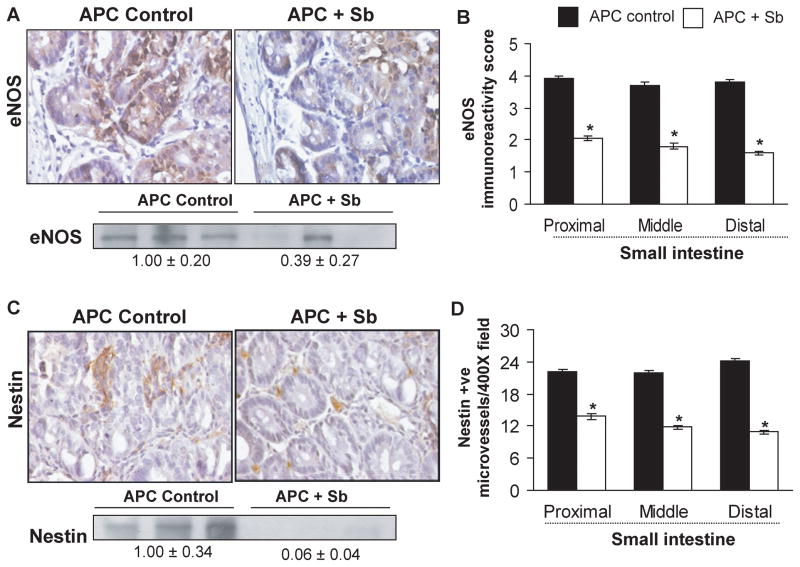
Over expression of eNOS, that catalyzes the production of nitric oxide (NO), a key regulator of angiogenesis, is reported during carcinogenesis even though it is constitutively expressed in vascular endothelial cells (29); VEGF also activates eNOS expression (30). Nestin, a class VI intermediate filament protein and neuro-epithelial stem cell marker, is expressed on newly formed microvessels (31), and is a novel angiogenic marker for newly formed blood vessels in CRC (32). To gain additional mechanistic insights regarding anti-angiogenic effect of silibinin, expression of these two important angiogenic molecules, eNOS and nestin, was also examined by IHC staining. We observed intense immunoreactivity for eNOS in polyps from control APCmin/+ mice, but strongly decreased expression in silibinin-fed group (Fig. 4A). Quantification showed that silibinin reduces eNOS immunoreactivity score in polyps from proximal, middle and distal regions of small intestine by 48% (P<0.001), 51% (P<0.001) and 58% (P<0.001), respectively (Fig. 4B). Regarding nestin, microscopic examination showed numerous nestin-positive microvessels in polyps from control APCmin/+ mice, but a significant reduction in silibinin-fed group (Fig. 4C). Quantification of nestin-positive cells showed that silibinin also decreases nestin-positive microvessels by 37% (P<0.001), 46% (P<0.001), and 55% (P<0.001) in the polyps from proximal, middle and distal segments of small intestine, respectively (Fig. 4D). These results were further confirmed by immunoblotting which showed that silibinin feeding decreases eNOS and nestin expression by 61% (P<0.05) and 94% (P<0.01) in intestinal polyps, respectively (Fig. 4A and 4C).
Figure 4.
Silibinin feeding inhibits eNOS expression and nestin-positive microvessels selectively in small intestinal polyps of APCmin/+ mice. Small intestinal segments were processed for eNOS and nestin staining. Tissue sections from APCmin/+ control and silibinin-treated groups show brown-colored (A) eNOS- and (C) nestin-positive cells in polyps (400x). Quantification of eNOS (B) was done based on its intensity of cytoplasmic brown staining, and microvessel numbers were quantified by measuring nestin-positive cells (D) in five randomly selected fields at 400x magnification, and represent mean ± SEM of six animals. *, P<0.001 versus control. Polyps from distal portion of small intestine from each group were also analyzed by immunoblotting for eNOS and nestin levels. Values of band intensity adjusted with β-actin. Sb, silibinin.
These resultssuggest that silibinin possibly targets neoangiogenesis through down-regulation of HIF-1α, VEGF, eNOS and nestin expression for its angiopreventive effects during spontaneous intestinal tumorigenesis. This antiangiogenic effect of silibinin was specific and limited to polyps, since we did not observe any considerable changes in the expression of these angiogenic factors in crypt-villus regions in small intestine of control and silibinin-treated APCmin/+ or wild-type C57BL/6J mice (data not shown). In these controls, we did not observe any changes in other molecular analyses described below, and therefore, these results are not mentioned hereafter.
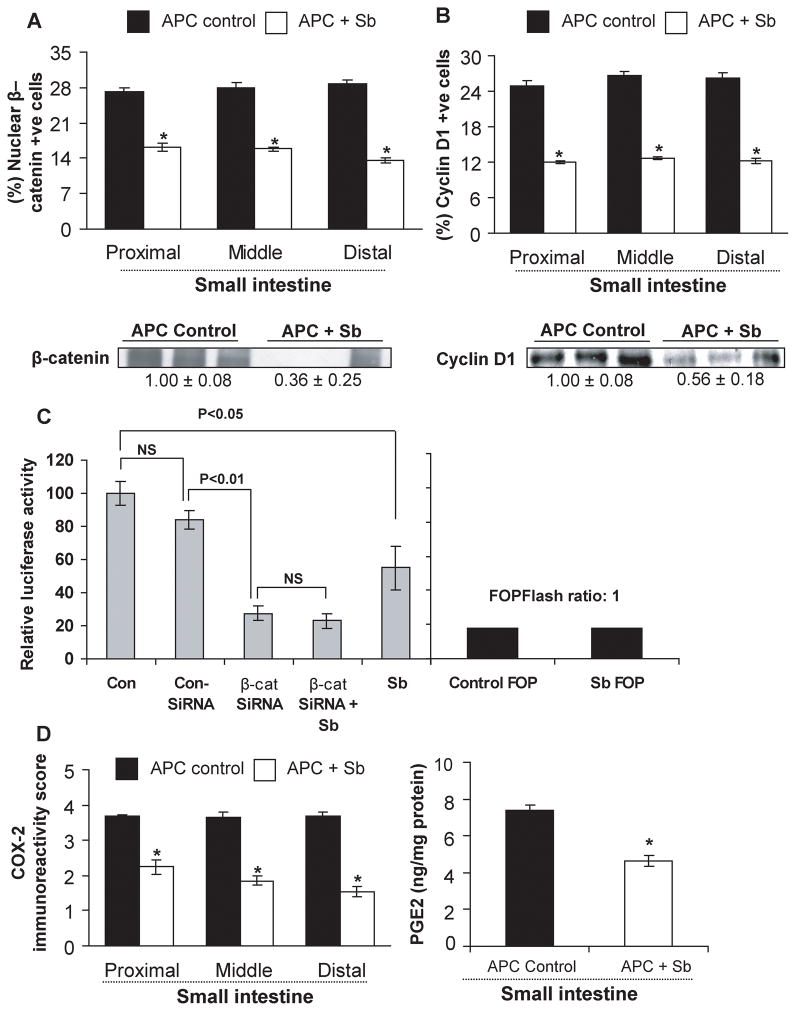
Silibinin feeding modulates β-catenin levels and transcriptional activity and cyclin D1 expression
Alterations in β-catenin pathway due to loss of APC function is implicated in CRC initiation and progression (33). To assess silibinin effect on β-catenin pathway, expression of β-catenin and one of its down-stream transcriptional targets cyclin D1 was analyzed by IHC, which showed strong staining for both nuclear β-catenin and cyclin D1-positive cells in polyps from APCmin/+ mice control but their decreased levels following silibinin treatment (IHC staining data not shown). Quantification of the staining showed that silibinin decreases nuclear β-catenin positive cells by 40% (P<0.001), 43% (P<0.001) and 53% (P<0.001) (Fig. 5A), and cyclin D1-positive cells by 52% (P<0.001), 53% (P<0.001) and 54% (P<0.001) (Fig. 5B) in polyps from proximal, middle and distal portions of small intestine, respectively. Immunoblot analysis further confirmed these results showing that silibinin treatment decreases β-catenin and cyclin D1 expression by 64% (P<0.05) and 44% (P<0.05) in intestinal polyps, respectively (Fig. 5A and 5B). To further substantiate our findings that silibinin targets β-catenin in its efficacy, we also studied silibinin effect on β-catenin mediated transcriptional activity using HT29 cells, where 100 μM silibinin concentration reduced TOPFlash reporter activity by 45% compared to DMSO controls; however, silibinin did not show any effect in decreasing reporter activity when the expression of β-catenin was reduced by siRNA transfection of HT29 cells (Fig 5C).
Figure 5.
Silibinin feeding decreases β-catenin, cyclin D1 and COX-2 expression and PGE2 levels selectively in small intestinal polyps of APCmin/+ mice and inhibits β-catenin mediated transcriptional activity in vitro. Small intestinal segments or polyps were processed for IHC analyses or estimation for (A) nuclear β-catenin, (B) cyclin D1 and (D) COX-2 and PGE2 levels as detailed in Methods. Data represent mean ± SEM of six animals. *, P<0.001 versus control. Polyps from distal portion of small intestine from each group were also analyzed by immunoblotting for β-catenin and cyclin D1 levels. Values of band intensity adjusted with β-actin. C, TOP/FOPFlash reporter activity was measured using dual luciferase assay kit from Promega as described in detail in Methods. Data shown represents mean ± SEM of three independent observations. Sb, silibinin.
Silibinin feeding decreases COX-2 expression and PGE2 levels in small intestinal polyps of APCmin/+ mice
A positive correlation between COX-2 and β-catenin pathway is reported during CRC development (34). COX-2 is a key enzyme in biosynthetic pathway by which arachidonic acid is converted into five structurally-related prostaglandins including PGE2 (35). Since both COX-2 and PGE2 are involved in CRC development (36), we next studied silibinin effect on COX-2 expression by IHC and PGE2 levels by ELISA. Our results showed strong COX-2 immunoreactivity in small intestinal polyps of APCmin/+ mice, which decreased strongly by silibinin treatment (IHC staining data not shown). Quantification of immunostained cells showed that silibinin decreases COX-2 immunoreactivity by 39% (P<0.001) in proximal, 47% (P<0.001) in middle, and 57% (P<0.001) in distal portions of small intestinal polyps (Fig. 5D). PGE2 levels in small intestinal polyps were elevated (8-fold) as compared with those in wild-type small intestinal tissue (data not shown). Silibinin treatment decreased PGE2 levels in small intestinal polyps by 37% (P<0.001) (Fig. 5D). These results indicate that COX-2 could be a potential molecular target for the chemopreventive effects of silibinin against polyp growth.
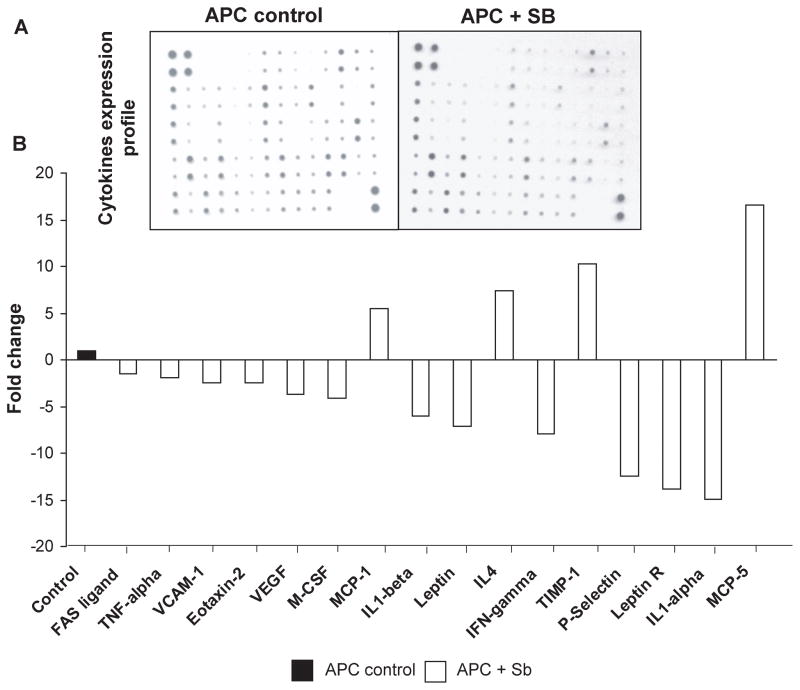
Silibinin treatment modulates cytokine profile in intestinal polyps of APCmin/+ mice
Cytokines are diverse family of secreted proteins and act as immunomodulating agents (37). To examine silibinin effect on various cytokines, we used inflammatory cytokine antibody array to screen intestinal polyps from both control and silibinin-fed APCmin/+ mice (Fig. 6A). We found that among 62 cytokines tested, silibinin decreased the expression levels of 12 cytokines and increased the expression levels of 4 cytokines in small intestinal polyps as compared with controls (Fig. 6B). Specifically, silibinin decreased the levels of Fas ligand (1.5-fold), tumor necrosis factor (TNF)-α (1.9-fold), vascular endothelial adhesion molecule (VCAM)-1 (2.4-fold), eotoxin-2 (2.4-fold), VEGF (3.7-fold), macrophage colony stimulating factor (M-CSF) (4.1-fold), interleukin (IL)-1α (14.9-fold) and β (6.0-fold), leptin (7.1-fold), interferon-γ (IFNγ) (7.9-fold), P-selectin (12.5-fold) and leptin receptor (13.9-fold). However, the levels of monocyte chemo-attractant protein (MCP)-1 (5.4-fold) and MCP-5 (16.6-fold), IL4 (7.3-fold) and tissue inhibitor of metalloproteinases-(TIMP)-1 (10.3-fold) were increased in intestinal polyps from silibinin-fed compared to control APCmin/+ mice. These results indicate that together with its angiopreventive effects, silibinin might also act as an immunomodulating agent, which could further strengthen its chemopreventive efficacy.
Figure 6.
Silibinin feeding modulates cytokines profile in intestinal polyps of APCmin/+ mice. Lysates from small intestinal polyps were analyzed for various cytokines by cytokine antibody array kit. A, Representative cytokines array blots from control and silibinin-treated APCmin/+ mice polyps. B, Densitometric analysis data of cytokines levels from three samples in each group are shown as fold changes by silibinin treatment to that of control.
Discussion
This is the first study demonstrating chemopreventive efficacy of long-term silibinin feeding on spontaneous intestinal tumorigenesis in APCmin/+ mice, a genetically predisposed animal model of human FAP. The key findings of this study are: (a) silibinin significantly reduced the number as well as growth of intestinal polyps, and prominently decreased the incidence of larger colonic polyps in APCmin/+ mice without any adverse health effects; (b) chemopreventive effect of silibinin was associated with a decrease in proliferation and an increase in apoptosis indices in polyps; (c) silibinin inhibited neoangiogenesis as evidenced by reduced HIF-1α, VEGF and eNOS immunostaining and a decrease in nestin-positive microvessels in polyps; and (d) silibinin decreased β-catenin levels and COX-2 pathways, and modulated various cytokines in intestinal polyps in favor of its chemopreventive efficacy. These results together with our earlier findings in CRC xenograft (18) and AOM-induced ACF (19) models strongly support the chemopreventive efficacy of silibinin in three different pre-clinical animals models of CRC, highlighting their translational significance against human CRC.
Chemopreventive effect of silibinin observed in the present study was accompanied by its anti-proliferative, pro-apoptotic and angiopreventive mechanisms. Excessive cell proliferation and insufficient apoptosis are often associated with CRC development and progression (38), and the agents modulating them in neoplastic cells have immense potential in CRC chemoprevention and therapeutic intervention (1). In our study, silibinin inhibited cell proliferation and induced apoptosis as evidenced by decreased PCNA- and increased CC3-positive cells and increased cleaved PARP levels, respectively, in polyps from silibinin-fed animals. This anti-proliferative and pro-apoptotic efficacy of silibinin in small intestinal polyps of APCmin/+ mice could be the underlying mechanisms for the strong decrease in the number as well as size of polyps. Importantly, these effects of silibinin were specific and limited to polyps, and not observed in normal crypt-villus regions in small intestine of control and silibinin-treated APCmin/+ as well as in wild-type C57BL/6J mice.
One of the most important observations in present study was that silibinin strikingly decreased the number of polyps which were larger in size (>3 mm in middle and distal portions of small intestine, and 2–3 mm and >3 mm in colon), suggesting that silibinin exerts strong antiangiogenic activity that possibly inhibited the progression of smaller polyps in to larger one. Neoangiogenesis is triggered when tumors grow beyond a minimal size (~2 mm) and agents that inhibit angiogenesis have significant implications for their clinical uses (26). Overexpression of HIF-1α, VEGF and eNOS is well implicated in stimulating neoangiogenesis (39). Hypoxia and VEGF signaling play a key role in ‘angiogenic switch’ (27, 30), and HIF-1α induction under hypoxic conditions directly activates a number of pro-angiogenic factors, including VEGF (28). Positive correlations between HIF-1α and VEGF expression, and VEGF and eNOS have also been documented (40). Overexpression of eNOS is reported during both carcinogenesis and neoangiogenesis even though it is constitutively expressed in endothelial cells (29). Consistent with these reports, overexpression of HIF-1α, VEGF and eNOS as well as increased newly formed microvessel density were observed in intestinal polyps of control APCmin/+ mice which were significantly decreased by silibinin treatment. Angiopreventive effect of silibinin observed in present study corroborates with previous reports showing silibinin inhibits angiogenesis in various animal models of carcinogenesis (14, 15, 17, 18). Collectively, these anti-angiogenic mechanisms of silibinin possibly played an important role in the observed suppression of vascular growth of polyps in APCmin/+ mice.
Aberrant β-catenin pathway is involved in CRC development (41), and a direct correlation between β-catenin signaling and regulation of angiogenesis is shown in CRC (33). In the present study, APCmin/+ mice showed increased level of β-catenin together with increased expression of cyclin D1, COX-2 and PGE2 in polyps which were significantly decreased by silibinin treatment. Overexpression of cyclin D1, a critical oncogene, is directly associated with increased proliferative index in CRC and results in more aggressive cancer phenotype (42). Thus, down-regulation of β-catenin and cyclin D 1 in polyps from silibinin-treated APCmin/+ mice also support their role in anti-proliferative mechanism of silibinin against CRC. Since over expression of COX-2 and increased PGE2 levels induce endothelial cell proliferation by releasing various angiogenic factors (35, 36), the inhibition of COX-2 and PGE2 by silibinin could be an additional mechanism for its angiopreventive effects. Moreover, VEGF and HIF-1α are also down-stream targets of β-catenin (43, 44) indicating that down-regulation of β-catenin signaling by silibinin could be an important mechanism for its anti-proliferative as well as anti-angiogenic effect in intestinal polyps of APCmin/+ mice.
Various cytokines are also associated with growth and development of CRC which could be modulated by COX-2, PGE2, and proangiogenic factors; some of them are also regulated by β-catenin pathway (45, 46). Tumor promoting roles of Fas ligand, TNF-α, VCAM, eotaxin, M-CSF, leptin and its receptor, IFN-γ, IL1α and β, and P-selectin during cancer development are well documented (37). Therefore, we also examined silibinin effect on the cytokines profile in polyps using cytokine array analysis, which showed that silibinin decreases the levels of all above mentioned tumor promoting cytokines in polyps of APCmin/+ mice. Conversely, silibinin increased MCP-1 and -5 factors that activate tumoricidal activity of macrophages in vivo. TIMP-1 and IL4 are known anti-inflammatory cytokines (47–49) which were also increased by silibinin treatment. Together, these results suggest that immunomodulating effects of silibinin could be one of its chemopreventive mechanisms; however, additional studies are needed in future to support it.
In summary, our results clearly demonstrate in vivo anti-proliferative, pro-apoptotic, anti-angiogenic and anti-inflammatory effects of silibinin in polyps, which collectively contribute to its strong chemopreventive efficacy against spontaneous intestinal tumorigenesis in APCmin/+ mice. Together with our previous reports on silibinin efficacy against CRC in other pre-clinical models, present findings underscore the possibility that silibinin would be an effective agent in CRC prevention trials for patients with FAP.
Acknowledgments
Grant support: NCI RO1 grant CA112304.
References
- 1.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–38. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 3.Hegde MR, Roa BB. Detecting mutations in the APC gene in familial adenomatous polyposis (FAP) Curr Protoc Hum Genet. 2006;Chapter 10(Unit 10):8. doi: 10.1002/0471142905.hg1008s50. [DOI] [PubMed] [Google Scholar]
- 4.Church JM, McGannon E, Hull-Boiner S, et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35:1170–3. doi: 10.1007/BF02251971. [DOI] [PubMed] [Google Scholar]
- 5.Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–62. doi: 10.1007/BF02047300. [DOI] [PubMed] [Google Scholar]
- 6.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 7.Preston SL, Leedham SJ, Oukrif D, et al. The development of duodenal microadenomas in FAP patients: the human correlate of the Min mouse. J Pathol. 2008;214:294–301. doi: 10.1002/path.2294. [DOI] [PubMed] [Google Scholar]
- 8.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 9.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (Silybum marianum) Integr Cancer Ther. 2007;6:104–9. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 10.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–89. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal C, Singh RP, Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–82. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 12.Kaur M, Agarwal R. Silymarin and epithelial cancer chemoprevention: how close we are to bedside? Toxicol Appl Pharmacol. 2007;224:350–9. doi: 10.1016/j.taap.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–79. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14:7773–80. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14:300–8. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 17.Tyagi A, Singh RP, Ramasamy K, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–50. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 19.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res (Phila Pa) 2008;1:376–84. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20:2101–8. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 21.Giacomelli S, Gallo D, Apollonio P, et al. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70:1447–59. doi: 10.1016/s0024-3205(01)01511-9. [DOI] [PubMed] [Google Scholar]
- 22.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 23.Shen G, Khor TO, Hu R, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–44. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 24.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–60. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 26.Tosetti F, Ferrari N, De Flora S, Albini A. Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed S, McDonald PJ, Northover JM, Guenther T. Angiogenesis and hypoxic factors in colorectal cancer. Pathol Res Pract. 2008;204:501–10. doi: 10.1016/j.prp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol. 2003;36:120–7. doi: 10.5483/bmbrep.2003.36.1.120. [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Chen S, Ye C, Zhu S. Nitric oxide synthase expression in human bladder cancer and its relation to angiogenesis. Urol Res. 2003;31:232–5. doi: 10.1007/s00240-003-0302-9. [DOI] [PubMed] [Google Scholar]
- 30.Crawford Y, Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335:261–9. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 31.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111 ( Pt 14):1951–61. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- 32.Teranishi N, Naito Z, Ishiwata T, et al. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593–603. [PubMed] [Google Scholar]
- 33.Gavert N, Ben-Ze’ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–8. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan FG, DuBois RN. Connecting COX-2 and Wnt in cancer. Cancer Cell. 2006;9:6–8. doi: 10.1016/j.ccr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Greenhough A, Smartt HJ, Moore AE, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 36.Eisinger AL, Prescott SM, Jones DA, Stafforini DM. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007;82:147–54. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–80. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 38.Seong J, Chung EJ, Kim H, et al. Assessment of biomarkers in paired primary and recurrent colorectal adenocarcinomas. Int J Radiat Oncol Biol Phys. 1999;45:1167–73. doi: 10.1016/s0360-3016(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 39.Milkiewicz M, Ispanovic E, Doyle JL, Haas TL. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38:333–57. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Erdamar S, Bagci P, Oz B, Dirican A. Correlation of endothelial nitric oxide synthase and vascular endothelial growth factor expression with malignancy in patients with astrocytic tumors. J BUON. 2006;11:213–6. [PubMed] [Google Scholar]
- 41.Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 42.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 43.Pishvaian MJ, Byers SW. Biomarkers of WNT signaling. Cancer Biomark. 2007;3:263–74. doi: 10.3233/cbm-2007-34-510. [DOI] [PubMed] [Google Scholar]
- 44.Giles RH, Lolkema MP, Snijckers CM, et al. Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene. 2006;25:3065–70. doi: 10.1038/sj.onc.1209330. [DOI] [PubMed] [Google Scholar]
- 45.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 46.Neufeld G, Kessler O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 2006;25:373–85. doi: 10.1007/s10555-006-9011-5. [DOI] [PubMed] [Google Scholar]
- 47.Bosco MC, Puppo M, Blengio F, et al. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–49. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 48.Cruz-Munoz W, Khokha R. The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit Rev Clin Lab Sci. 2008;45:291–338. doi: 10.1080/10408360801973244. [DOI] [PubMed] [Google Scholar]
- 49.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]