Abstract
Tissue engineering of cartilage, i.e., the in vitro cultivation of cartilage cells on synthetic polymer scaffolds, was studied on the Mir Space Station and on Earth. Specifically, three-dimensional cell-polymer constructs consisting of bovine articular chondrocytes and polyglycolic acid scaffolds were grown in rotating bioreactors, first for 3 months on Earth and then for an additional 4 months on either Mir (10−4–10−6 g) or Earth (1 g). This mission provided a unique opportunity to study the feasibility of long-term cell culture flight experiments and to assess the effects of spaceflight on the growth and function of a model musculoskeletal tissue. Both environments yielded cartilaginous constructs, each weighing between 0.3 and 0.4 g and consisting of viable, differentiated cells that synthesized proteoglycan and type II collagen. Compared with the Earth group, Mir-grown constructs were more spherical, smaller, and mechanically inferior. The same bioreactor system can be used for a variety of controlled microgravity studies of cartilage and other tissues. These results may have implications for human spaceflight, e.g., a Mars mission, and clinical medicine, e.g., improved understanding of the effects of pseudo-weightlessness in prolonged immobilization, hydrotherapy, and intrauterine development.
Keywords: spaceflight, microgravity, chondrocyte, polymer, bioreactor
Exposure of humans to microgravity affects cells and tissues at a variety of levels (1, 2). Musculoskeletal changes (e.g., significant bone and muscle loss) occur even when astronauts exercise regularly, but the mechanisms are not yet understood (3, 4). Tissue engineering, a new field that enables tissue equivalents to be created from isolated cells in combination with biomaterials (5, 6) and bioreactor culture vessels (7), potentially can provide a basis for systematic, controlled in vitro studies of tissue growth and function. We developed a method to create three-dimensional cartilaginous tissue based on cartilage cells (chondrocytes), biodegradable polyglycolic acid (PGA) scaffolds (8), and bioreactors (7, 9). The scaffold induces cell differentiation and degrades at a defined rate, whereas the bioreactor maintains controlled in vitro culture conditions that permit tissue growth and development.
In the present work, we studied tissue engineering in space by using cartilage as a model musculoskeletal tissue. Cartilage was selected because of its resilience and low metabolic requirements (10). Previous microgravity experiments involving mammalian cells focused on the cells themselves in monolayers and lasted for 6–28 days (11). In the present study, three-dimensional engineered cartilage grown for 3 months on Earth followed by 4 months on the Mir Space Station at 10−4–10−6 g (11) was compared with that grown for 7 months on Earth at 1 g (Fig. 1). The objectives were to: (i) examine chondrocyte viability and differentiated function in a long-term flight study, and (ii) assess the effects of the space environment which, in the present experiment and during human spaceflight, includes exposure to microgravity as well as launch and landing, on cartilage growth and function.
Figure 1.
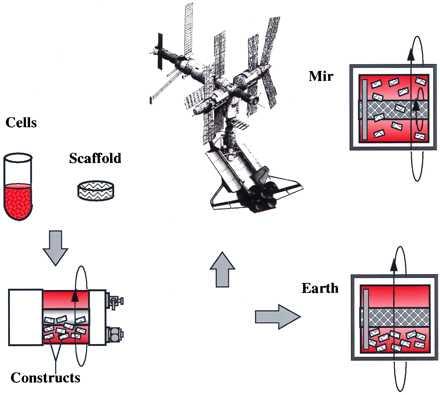
Experimental design. Cartilage cells were seeded onto polymer scaffolds, and the resulting constructs were cultivated in rotating bioreactors first for 3 months on Earth and then for 4 additional months either on the Mir Space Station (10−4–10−6 g) or on Earth (1 g).
MATERIALS AND METHODS
Materials.
Rotating bioreactors used for the first 3 months of cultivation on Earth were from Synthecon (RCCV-110, Houston, TX). The Biotechnology System (BTS) used for the subsequent 4 months of cultivation on either Mir or on Earth (see below) was custom-made according to the specifications of the Biotechnology Center at the National Aeronautics and Space Administration–Johnson Space Center. All other materials were obtained from previously specified sources (9).
Cell-Polymer Construct Preparation on Earth.
Chondrocytes were obtained by enzymatic digestion of full-thickness articular cartilage harvested from the femoropatellar grooves of 2- to 3-week-old bovine calves within 8 hr of slaughter (12). Scaffolds were 5-mm diameter × 2-mm thick discs made of PGA (the same material used to make Dexon absorbable surgical sutures) formed as a 97% porous mesh of 13-μm diameter fibers (8). Culture medium consisted of DMEM with 4.5 g/liter glucose, 10% fetal bovine serum, 10 mM N-2-hydroxy-ethyl piperazine N′-2-ethane sulfonic acid, 0.1 mM nonessential amino acids, 0.4 mM proline, 50 mg/liter ascorbic acid, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.5 μg/ml fungizone. Powdered medium components were sterilized by gamma irradiation (2.5–3.8 MRad) and rehydrated before use. Chondrocytes were seeded onto scaffolds (5 million cells per PGA disc) in spinner flasks stirred at 80 rpm in a 37°C humidified 10% CO2 incubator (13). After 6 days, cell-PGA constructs were transferred into rotating bioreactors (11 constructs per 110-ml vessel) configured as the annular space between a 5.75-cm diameter polycarbonate outer cylinder and a 2-cm diameter hollow inner cylinder covered with a 175-μm thick silicone membrane (14). The entire vessel was rotated as a solid body around its central axis while gas exchange was provided by pumping incubator air through the inner cylinder at 0.7–1.2 liters/min. Culture medium was replaced at a rate of 50% every 3–4 days, and the vessel rotation speed gradually was increased from 15 to 28 rpm over 3 months, to induce mixing by gravitational construct settling (7).
Construct Cultivation on Earth and on Mir.
After 3 months, constructs were transferred into each of two flight-qualified rotating, perfused bioreactors (BTS) for an additional 4 months of cultivation on either the Mir Space Station or on Earth (Fig. 1). Specifically, one BTS containing 10 constructs was transferred to Mir via the U.S. space shuttle STS-79 (9/16/96 launch) and brought back to Earth via STS-81 (1/22/97 landing). A second BTS with 10 constructs served as an otherwise identical study conducted on Earth, at the Johnson Space Center. In the BTS, the bioreactor was configured as the 125-ml annular space between a 5-cm diameter polycarbonate outer cylinder and a 1.5-cm diameter hollow inner cylinder covered with a 50-μm cut-off, 36% open area nylon mesh (Tetko, Kansas City, MO). A flat, thin 3.8-cm diameter disc attached near one end of the inner cylinder served as a viscous pump (15). Fluid entered the bioreactor behind the disc and exited via the mesh on the inner cylinder. In particular, the bioreactor was connected to a recirculation loop that included tubing (C-flex 15, Cole Parmer, Niles, IL), a peristaltic pump (Randolph model 250, Manchaca, TX), and a silicone membrane gas exchanger (Avecor model 0400–2A, Plymouth, MN). A mixture of 10% CO2 and 90% air flowed continuously through the gas exchanger at 0.6 ml/min. The temperature within the BTS was maintained at 37°C.
Medium was recirculated between the bioreactor and the gas exchanger at 4 ml/min for 20 min four times per day, and 50–100 ml of fresh medium was infused into the system approximately once per day. As a result, medium metabolic parameters were maintained within previously established target ranges [e.g., pH between 6.9 and 7.4; partial pressure of oxygen (pO2) between 71 and 127 mmHg] in both groups for the duration of the study, as assessed by using portable cartridges (G3+, I-Stat, Princeton, NJ). Concentration gradients within the bioreactor were minimized by differential rotation of the inner and outer vessel walls in the same direction at 10 and 1 rpm, respectively, in microgravity (ref. 15, and S. Kleis, personal communication), and by the convection associated with gravitational construct settling during solid body rotation of the bioreactor at 28 rpm in unit gravity (7). In the Mir group, gas bubbles were observed in the bioreactor between flight days 40 and 130. The amount of gas stabilized at approximately 20% of the total bioreactor volume, and the bubbles did not come into direct contact with the constructs, as assessed by videography. An equal amount of gas was introduced into the bioreactor in the Earth group, to match the conditions on Mir as closely as possible.
Construct Analysis.
Constructs were assessed at the time of launch (i.e., after 3 months of culture) and after 4 additional months on either Mir or Earth (i.e., after 7 months of culture), and compared with full-thickness natural calf articular cartilage (Table 1). Cell viability was assessed by using trypan blue exclusion and an intracellular esterase assay (Molecular Probes). Samples for light microscopy were fixed in 10% neutral buffered formalin, cross-sectioned, embedded, sectioned (5 μm thick), and stained with safranin-O for glycosaminoglycan (GAG). Samples for transmission electron micrography were fixed in Karnovsky’s reagent (0.1 M sodium cacodylate with 2% paraformaldehyde and 2.5% glutaraldehyde), postfixed in 1% osmium tetroxide with 0.2 M collidine, embedded, and sectioned (70 nm thick). Samples for biochemical analyses were radiolabeled in mixed dishes for 18 hr in medium containing 35SO4 and 3H-proline, frozen, lyophilized, and enzymatically digested (16). Previously reported methods were used to quantitate cellularity (DNA) (17), macromolecular incorporation of radiolabeled tracers (16), GAG (18), type II collagen (19), and total collagen (20). Samples for mechanical studies were cored into 3-mm diameter × 2-mm thick discs, which excluded the upper and lower surfaces (approximately 0.5 mm thick) of both engineered and natural cartilage, placed in a cylindrical chamber, and subjected to confined compression and stress relaxation at increments of 10% strain up to a maximum of 40% strain as previously described (12, 21). At a static offset strain of 30%, sinusoidal strains of 0.5% amplitude were superimposed at frequencies of 0.025–1.0 Hz. Aggregate modulus (i.e., the ratio of incremental stress and incremental strain at equilibrium), dynamic stiffness (i.e., the ratio of the amplitude of the dynamic stress to the amplitude of the dynamic strain), and hydraulic permeability were calculated as described (12, 21).
Table 1.
Comparison of tissue engineered constructs and natural cartilage
| Parameter | Tissue engineered constructs
|
Natural cartilage** | ||
|---|---|---|---|---|
| At launch* | Mir-grown | Earth-grown | ||
| Culture time (months) | 3 | 7 | 7 | 0 |
| Chondrocyte function | ||||
| Sulfate incorporation (ng/μg DNA/day) | 80.8 ± 37.2 (3) | 88.9 ± 8.0 (3) | 83.4 ± 13.4 (3) | 332 ± 36.3 (7) |
| Proline incorporation (ng/μg DNA/day) | 82.0 ± 20.6 (3) | 93.0 ± 3.0 (3) | 87.9 ± 22.7 (3) | 205 ± 46.8 (7) |
| Construct composition | ||||
| Weight (mg wet) | 250 ± 38 (20) | 330 ± 25 (5) | 429 ± 14 (5) | 461 ± 72.8 (7) |
| Water (%) | 89.2 ± 0.89 (3) | 89.2 ± 0.31 (3) | 86.3 ± 0.53 (3) | 82.8 ± 0.93 (7) |
| Cells (% wet weight) | 0.64 ± 0.03 (3) | 0.40 ± 0.01 (3) | 0.46 ± 0.02 (3) | 0.66 ± 0.09 (7) |
| GAG (% wet weight) | 6.03 ± 0.84 (3) | 3.59 ± 0.22 (3) | 8.83 ± 0.93 (3) | 7.05 ± 0.56 (6) |
| Collagen (% wet weight) | 2.70 ± 0.75 (3) | 3.42 ± 0.17 (3) | 3.68 ± 0.27 (3) | 10.7 ± 0.91 (6) |
| Total of above components (% wet weight) | 98.6 ± 0.07 (3) | 96.6 ± 0.68 (3) | 99.3 ± 0.60 (3) | 101 ± 0.46 (6) |
| Type II collagen (% total collagen) | 91.6 ± 19.1 (2) | 78.0 ± 4.1 (4) | 75.3 ± 7.8 (4) | 90.3 ± 17.9 (5) |
| Mechanical behavior | ||||
| Aggregate modulus (MPa) | 0.108 ± 0.047 (2) | 0.313 ± 0.045 (4) | 0.932 ± 0.049 (3) | 0.949 ± 0.021 (3) |
| Hydraulic permeability (×10−15 m4/Ns) | 8.25 ± 1.94 (2) | 6.73 ± 3.02 (4) | 3.72 ± 0.167 (3) | 2.72 ± 0.641 (3) |
| Dynamic stiffness (MPa, 1 Hz) | 2.23 ± 0.12 (2) | 3.80 ± 0.39 (4) | 7.75 ± 0.30 (3) | 16.8 ± 1.14 (3) |
Data represent average ± standard deviation. Parentheses indicate the number of samples analyzed per group.
Statistical significance was assessed using ANOVA (α = 0.05) in conjunction with Tukey’s studentized range test. For each parameter, statistically significant differences between groups (P < 0.05) are denoted by different colors.
Wet weights at 3 months were those of the actual constructs used in 7-month Mir and Earth cultivations; all other parameters in this group were assessed using additional 3-month constructs from the same batch.
Wet weights for natural cartilage were scaled by a factor of five for better comparison with corresponding values measured for Earth-grown tissue engineered constructs.
RESULTS AND DISCUSSION
Cellular Viability Postflight.
Mir-grown constructs assessed 30 hr postflight were comparable to Earth-grown constructs with respect to cell viability and biosynthetic activity. Specifically, constructs from both groups consisted of 95–99% viable cells, as judged by trypan blue exclusion and intracellular esterase activity (Fig. 2), and incorporated radiolabeled tracers into macromolecules at comparable rates (Table 1). The latter may represent a rebound in chondrocyte metabolism in the Mir-grown constructs, which were assayed after return from space, in a manner analogous to the prolonged and time-dependent stimulation of chondrocyte metabolism that was observed in cartilage explants after the release of a compressive force (16).
Figure 2.
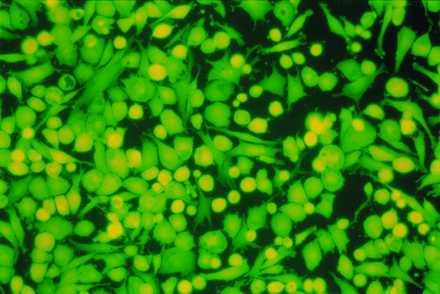
Cell viability postflight. Cells isolated from a 7-month construct from the Mir group after 2 days of monolayer culture. (×200.) Intracellular esterase activity is indicated by green color.
Construct Shape.
Constructs grown on Mir tended to become more spherical, whereas those grown on Earth maintained their initial discoid shape, as assessed histologically (Fig. 3 A and B) and from their respective aspect ratios (i.e., height to width) of 0.72 ± 0.08 and 0.62 ± 0.03 (P < 0.05). These findings might be related to differences in cultivation conditions, i.e., videotapes showed that constructs floated freely in microgravity but settled and collided with the rotating vessel wall at 1 g. In particular, on Mir the constructs were exposed to uniform shear and mass transfer at all surfaces such that the tissue grew equally in all directions, whereas on Earth the settling of discoid constructs tended to align their flat circular areas perpendicular to the direction of motion (22), increasing shear and mass transfer circumferentially such that the tissue grew preferentially in the radial direction.
Figure 3.
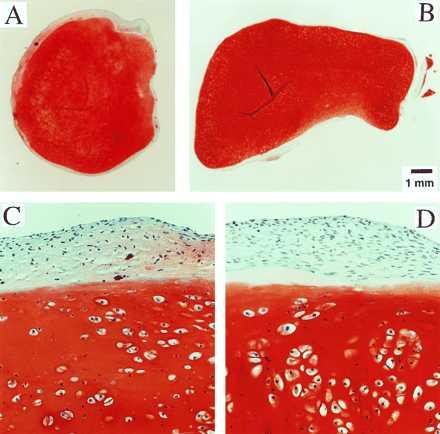
Construct structure. (A and B) Full cross sections of constructs from Mir and Earth groups. (×10.) (C and D) Representative areas at the construct surfaces. (×200.) GAG is stained red with safranin-O.
Construct Structure.
Final samples from Mir and Earth appeared histologically cartilaginous throughout their entire cross sections (5–8 mm thick), with the exception of fibrous outer capsules (0.15–0.45 mm thick), as assessed by using safranin-O stain for GAG (Fig. 3 A–D) and immunohistochemical staining for collagen type II (data not shown). Constructs grown on Earth appeared to have a more organized extracellular matrix with more uniform collagen orientation as compared with constructs grown on Mir (Fig. 4 A and B), but the average collagen fiber diameter was similar in the two groups (22 ± 2 nm) and comparable to that previously reported for developing articular cartilage (23). Randomly oriented collagen in Mir samples would be consistent with previous reports that microgravity disrupts fibrillogenesis (24, 25).
Figure 4.
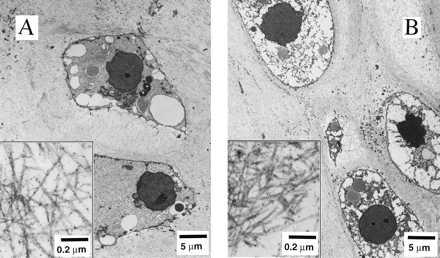
Construct ultrastructure. Transmission electron micrographs of constructs from Mir (A) and Earth (B) groups at magnifications of ×3,500 and ×120,000 (Inset).
Construct Composition.
Constructs at the time of launch and after additional cultivation on Mir and on Earth contained 13 ± 1, 14 ± 0.8 and 19 ± 0.2 million cells, respectively. On Earth, construct wet weights increased 1.7-fold between 3 and 7 months, which could be attributed to increasing amounts of cartilage-specific tissue components (i.e., collagen type II and GAG) (Table 1). In contrast, on Mir construct wet weights increased 1.3-fold over the same time interval, because of deposition of collagen and unspecified components that were not GAG (Table 1). The PGA scaffold represented less than 0.3% of the final construct wet weight, based on previous degradation studies (8). The fraction of the total collagen that was type II decreased, but not significantly, from 92 ± 19% at launch to 78 ± 4% at landing, demonstrating relatively good maintenance of the chondrocytic phenotype. Constructs grown for 7 months on Mir and/or Earth had type II collagen fractions that were comparable to each other and to those previously reported for 8-month cultures of chondrocytes in alginate beads (26).
Construct Function.
Construct mechanical properties improved both on Mir and on Earth resulting in an increase in aggregate modulus, HA, and a decrease in hydraulic permeability, k (Table 1). Dynamic stiffness also increased with culture time and showed the characteristic frequency dependence of natural cartilage (12) (data not shown). Mechanical properties of Mir-grown constructs were inferior to those of Earth-grown constructs. In particular, the aggregate modulus of Earth-grown constructs was indistinguishable from natural calf cartilage and was 3-fold higher than that of Mir-grown constructs (Table 1). These data support the hypothesis that, in radially confined compression, HA is determined mainly by GAG content (12), whereas k and dynamic stiffness are likely to depend on a variety of factors, including collagen content and organization, GAG immobilization, and overall tissue architecture (12, 27).
The finding that constructs in the Earth group had markedly higher wet weights, GAG fractions, and aggregate moduli than constructs in the Mir group (Table 1) may be attributed to the effects of physical forces at unit gravity (i.e., settling-induced hydrodynamic and contact forces) on the growth and development of the engineered tissue. This finding is consistent with previous studies of cartilage explants in which dynamic compression (amplitudes of 1–5% and frequencies of 0.01–1 Hz) increased GAG and protein synthesis rates (16), and cyclic hydrostatic pressure (5 MPa, 0.25 Hz) increased GAG synthesis (28). The envisioned mechanism of mechanotransduction is analogous to that previously described for bone (29), involving four steps: mechanocoupling, biochemical coupling, signal transmission, and effector cell response.
Cells maintained viability and differentiated phenotype over 7 months both on Mir and on Earth. The observed differences between constructs in the two groups might be attributed to differences in construct cultivation conditions (i.e., free floating vs. gravity settling). In particular, spherical shape, relatively low GAG fraction and inferior mechanical properties of Mir-grown constructs might be attributed to the space environment. However, we cannot distinguish between the relative contributions of launch, exposure to microgravity and local environmental factors (e.g., cosmic radiation), and landing, emphasizing the need for the use of an in-flight, 1-g centrifuge for control groups in future flight experiments (30).
The present study is the longest cell culture experiment ever carried out in space and demonstrates the feasibility of microgravity tissue engineering. Final tissue constructs consisted of viable, metabolically active cells and were structurally and functionally cartilaginous, but constructs grown on Mir were smaller and mechanically inferior to those grown on Earth. Our results are consistent with previous reports that musculoskeletal tissues remodel in response to physical forces (31, 32) and are adversely affected by spaceflight (1, 2). The same cell-polymer-bioreactor system could be used for a variety of controlled microgravity studies aimed at improving our fundamental understanding of how gravity affects cell function and tissue development. These results may have implications for human spaceflight, e.g., a Mars mission, and clinical medicine, e.g., improved understanding of the effects of pseudo-weightlessness in prolonged immobilization, hydrotherapy, and intrauterine development.
Acknowledgments
We are grateful to A.J. Grodzinsky, who provided the expertise and facility needed to measure construct mechanical properties; F.J. Schoen, who prepared and, with A.J. Grodzinsky, helped interpret the transmission electron micrography; A.P. Hollander, who developed and performed the quantitative collagen type II assay; S. Eisenberg, S. Kleis, B. Obradovic, and D. Stamenovic for many stimulating discussions; Z. Antonijevic, who helped with the statistical analysis; and J. Barry, R. Padera, H. Shing, and S. Treppo for their technical assistance. This study could not have been accomplished without the dedicated efforts of J. Blaha (Mir), and D. Brown, M. Casteel, J. Craig, R. Garcia, S. Gonda, T. Goodwin, L. McClarty, T. Trinh, J. Washington, and M. Young (Johnson Space Center). The funding for this work was provided by the National Aeronautics and Space Administration Microgravity Research Division and Grant NAG9-836 with additional support from Advanced Tissue Sciences.
ABBREVIATIONS
- GAG
glycosaminoglycan
- PGA
polyglycolic acid
- BTS
Biotechnology System (bioreactor)
Footnotes
A commentary on this article begins on page 13380.
References
- 1.Churchill S E. Fundamentals of Space Life Sciences. Malabar: Krieger; 1997. [Google Scholar]
- 2.Nicogossian A E, Huntoon C L, Pool S L. Space Physiology and Medicine. Philadelphia: Lea and Febiger; 1994. [Google Scholar]
- 3.Cann C E. In: Fundamentals of Space Life Sciences. Churchill S E, editor. Malabar: Krieger; 1997. pp. 83–103. [Google Scholar]
- 4.Edgerton V R, Roy R R. In: Fundamentals of Space Life Sciences. Churchill S E, editor. Malabar: Krieger; 1997. pp. 105–120. [Google Scholar]
- 5.Nerem R M. Ann Biomed Eng. 1991;19:529–545. doi: 10.1007/BF02367396. [DOI] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti J P. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Freed L E, Vunjak-Novakovic G. In: Principles of Tissue Engineering. Lanza R P, Chick W, Langer R, editors. Austin: Landes; 1997. pp. 151–165. [Google Scholar]
- 8.Freed L E, Vunjak-Novakovic G, Biron R J, Eagles D, Lesnoy D, Barlow S K, Langer R. Bio/Technology. 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 9.Freed L E, Vunjak-Novakovic G. In: The Biomedical Engineering Handbook. Bronzino J D, editor. Boca Raton, FL: CRC; 1995. pp. 1788–1806. [Google Scholar]
- 10.Buckwalter J A, Mankin H J. J Bone Joint Surg. 1997;79-A:600–611. [Google Scholar]
- 11.Lewis M L, Hughes-Fulford M. In: Fundamentals of Space Life Sciences. Churchill S E, editor. Malabar: Krieger; 1997. pp. 21–39. [Google Scholar]
- 12.Buschmann M D, Gluzband Y A, Grodzinsky A J, Kimura J H, Hunziker E B. J Orthop Res. 1992;10:745–752. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 13.Vunjak-Novakovic G, Freed L E, Biron R J, Langer R. AIChE J. 1996;42:850–860. [Google Scholar]
- 14.Schwarz R P, Goodwin T J, Wolf D A. J Tissue Cult Methods. 1992;14:51–58. doi: 10.1007/BF01404744. [DOI] [PubMed] [Google Scholar]
- 15.Kleis S J, Schreck S, Nerem R M. Biotech Bioeng. 1990;36:771–777. doi: 10.1002/bit.260360803. [DOI] [PubMed] [Google Scholar]
- 16.Sah R L Y, Kim Y J, Doong J Y H, Grodzinsky A J, Plaas A H K, Sandy J D. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y J, Sah R L, Doong J Y H, Grodzinsky A J. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 18.Farndale R W, Buttle D J, Barrett A J. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 19.Hollander A P, Heathfield T F, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole A R. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woessner J F. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 21.Bonaventure L J, Frank E H, Murray J C, Paguio C G, Moore V L, Lark M W, Sandy J D, Wu J J, Eyre D R, Grodzinsky A J. Arthritis Rheum. 1995;38:173–183. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- 22.Willmarth W W, Hawk N E, Harvey R. Physics Fluids. 1964;7:197–208. [Google Scholar]
- 23.Parry D A D, Craig A S. In: Ultrastructure of the Connective Tissue Matrix. Ruggeri A, Motta P M, editors. Boston: Martinus Nijhoff; 1984. pp. 34–64. [Google Scholar]
- 24.Duke P J, Durnova G, Montufar-Solis D. FASEB J. 1990;4:41–46. doi: 10.1096/fasebj.4.1.2295377. [DOI] [PubMed] [Google Scholar]
- 25.Turner R T, Bell M, Duvall P, Bobyn D, Spector M, Morey H E, Baylink D. Proc Soc Exp Biol Med. 1985;180:544–549. doi: 10.3181/00379727-180-42215. [DOI] [PubMed] [Google Scholar]
- 26.Hauselmann H J, Fernandes R J, Mok S S, Schmid T M, Block J A, Aydelotte M B, Kuettner K E, Thonar E J M. J Cell Sci. 1994;107:17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong C G, Mow V C. J Bone J Surg. 1982;64A:88–94. [PubMed] [Google Scholar]
- 28.Parkkinen J J, Ikonen J, Lammi M J, Laakkonen J, Tammi M, Helminen H J. Arch Biochem Biophys. 1993;300:458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 29.Duncan R L, Turner C H. Calcified Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 30.Van Loon J J, Bervoets D J, Burger E H, Dieudonne S C, Hagen J W, Semeins C M, Doulabi B Z, Veldhuijzen J P. J Bone Miner Res. 1995;10:550–557. doi: 10.1002/jbmr.5650100407. [DOI] [PubMed] [Google Scholar]
- 31.Salter R B. In: Articular Cartilage and Knee Function: Basic Science and Arthroscopy. Ewing J W, editor. New York: Raven; 1990. pp. 335–352. [Google Scholar]
- 32.Thompson D W. On Growth and Form. New York: Cambridge University; 1961. pp. 221–267. [Google Scholar]


