Abstract
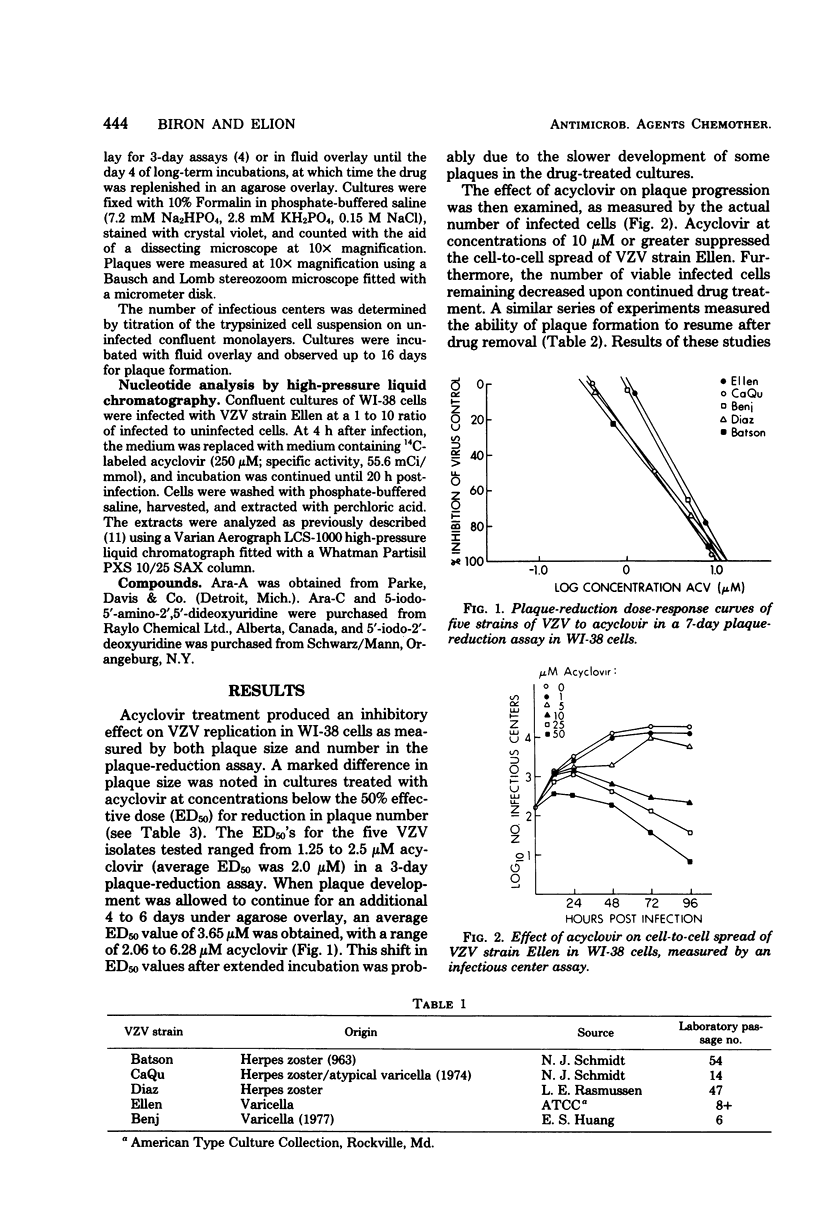
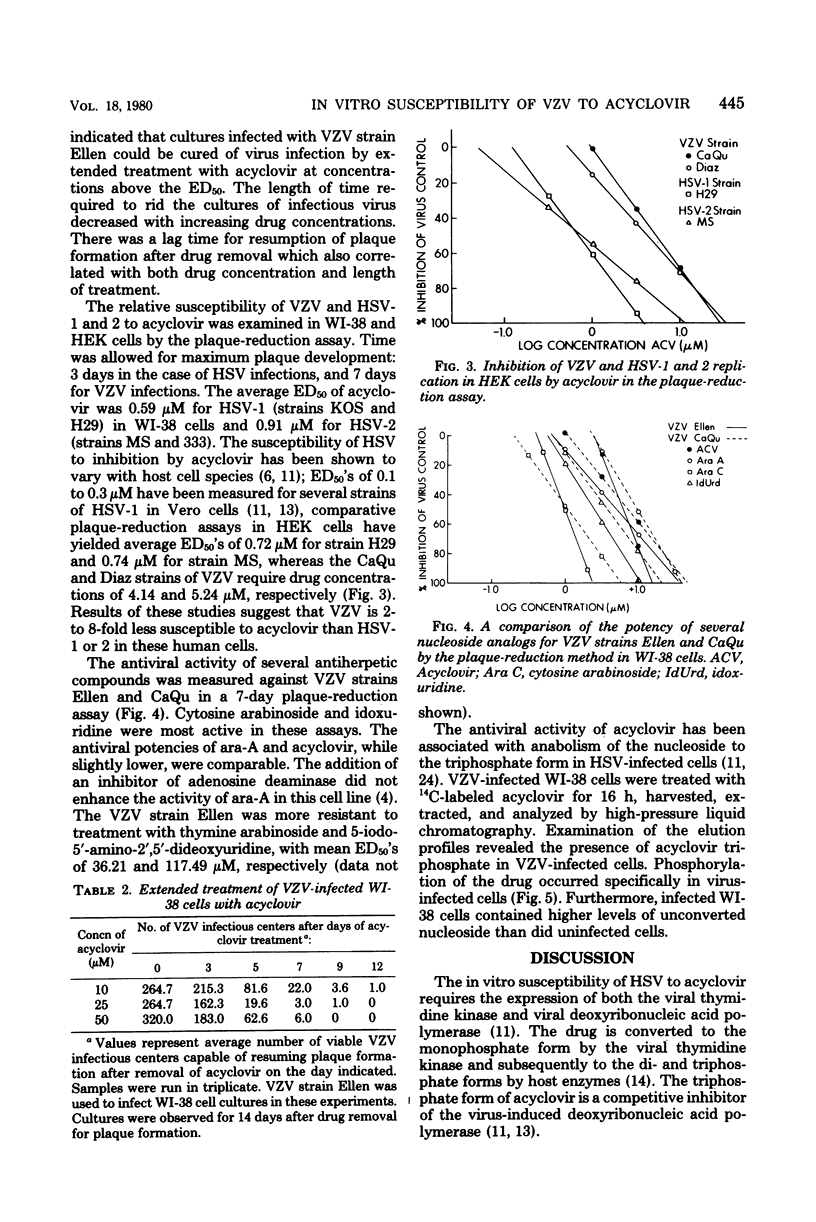
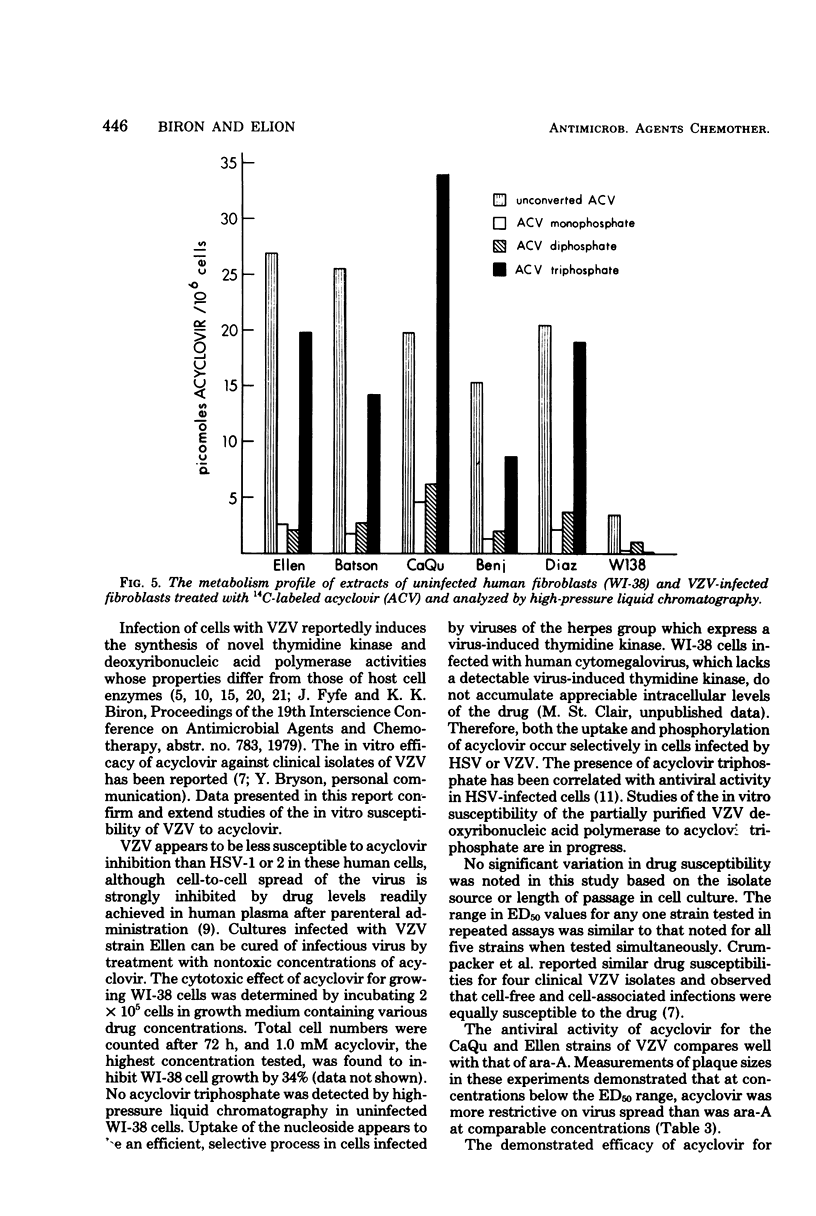
The in vitro susceptibility of five strains of varicella-zoster virus to acyclovir was examined by the plaque-reduction method in human diploid lung cells. The 50% effective doses of acyclovir ranged from 2.06 microM to 6.28 microM in a 7-day assay, with a mean of 3.65 microM. Irreversible inhibition of plaque formtation was achieved by drug doses exceeding the 50% effective dose for plaque reduction but nontoxic to the cells. Studies on the relative in vitro susceptibility of varicella-zoster virus and herpes simplex virus types 1 and 2 to acyclovir suggested that varicella-zoster virus is two- to eightfold less susceptible to the drug. The antiviral potency of acyclovir for varicella-zoster virus in vitro was compared with that of several other nucleoside analogs. Analysis of the metabolism of acyclovir in varicella-zoster virus-infected WI-38 cells revealed that, as with herpes simplex virus types 1 and 2, the formation of the triphosphate forms of the drug is specific to viral infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian F. O., Rabson A. S., Yee C. L., Tralka T. S. Herpesvirus varicellae isolated from human dorsal root ganglia. Arch Pathol. 1974 May;97(5):331–333. [PubMed] [Google Scholar]
- Bauer D. J., Collins P., Tucker W. E., Jr, Macklin A. W. Treatment of experimental herpes simplex keratitis with acycloguanosine. Br J Ophthalmol. 1979 Jun;63(6):429–435. doi: 10.1136/bjo.63.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Connor J. D. In vitro susceptibility of varicella zoster virus to adenine arabinoside and hypoxanthine arabinoside. Antimicrob Agents Chemother. 1976 Mar;9(3):540–543. doi: 10.1128/aac.9.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Tsou T. Y., Hackstadt T., Mallavia L. P. Induction of thymidine kinase and DNase in varicella-zoster virus-infected cells and kinetic properties of the virus-induced thymidine kinase. J Virol. 1979 Jul;31(1):172–177. doi: 10.1128/jvi.31.1.172-177.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. The activity in vitro against herpes virus of 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine), a new antiviral agent. J Antimicrob Chemother. 1979 Jul;5(4):431–436. doi: 10.1093/jac/5.4.431. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. M., VanDersarl J. V., Coltman C. A., Jr Failure of cytarabine in varicella-zoster infections. JAMA. 1973 Apr 2;224(1):122–123. [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Bell S. E., Elion G. B., Nash A. A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979 Apr;15(4):554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Hackstadt T., Mallavia L. P. Deoxypyrimidine nucleoside metabolism in varicella-zoster virus-infected cells. J Virol. 1978 Feb;25(2):510–517. doi: 10.1128/jvi.25.2.510-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. T., Buchanan R. A., Luby J. P., Mikulec D. Treatment of varicella-zoster virus infections with adenine arabinoside. J Infect Dis. 1975 Mar;131(3):225–229. doi: 10.1093/infdis/131.3.225. [DOI] [PubMed] [Google Scholar]
- Joncas J. H. Persistence, reactivation, and cell transformation by human herpeviruses: herpes simplex 1, 2 (HSV-1, HSV-2), cytomegalovirus (CMV), varicella-zoster (VZV), Epstein-Barr virus (EBV). Can J Microbiol. 1979 Mar;25(3):254–260. doi: 10.1139/m79-041. [DOI] [PubMed] [Google Scholar]
- Kaufman H. E., Varnell E. D., Centifanto Y. M., Rheinstrom S. D. Effect of 9-(2-hydroxyethoxymethyl)guanine on herpesvirus-induced keratitis and iritis in rabbits. Antimicrob Agents Chemother. 1978 Dec;14(6):842–845. doi: 10.1128/aac.14.6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. J., Zaia J. A. Immunosuppression and infection-progress. N Engl J Med. 1977 Jun 16;296(24):1406–1408. doi: 10.1056/NEJM197706162962411. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Huang Y. S., Huang E. S. Purification and characterization of varicella-zoster virus-induced DNA polymerase. J Virol. 1978 May;26(2):249–256. doi: 10.1128/jvi.26.2.249-256.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Rapp F. Varicella-zoster virus-induced DNA polymerase. J Gen Virol. 1977 Sep;36(3):515–524. doi: 10.1099/0022-1317-36-3-515. [DOI] [PubMed] [Google Scholar]
- Oxford J. S. Inhibition of herpes virus by a new compound--acyclic guanosine. J Antimicrob Chemother. 1979 Jul;5(4):333–334. doi: 10.1093/jac/5.4.333. [DOI] [PubMed] [Google Scholar]
- Park N. H., Pavan-Langston D., McLean S. L., Albert D. M. Therapy of experimental herpes simplex encephalitis with aciclovir in mice. Antimicrob Agents Chemother. 1979 Jun;15(6):775–779. doi: 10.1128/aac.15.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schimpff S. C., Fortner C. L., Greene W. H., Wiernik P. H. Cytosine arabinoside for localized herpes zoster in patients with cancer: failure in a controlled trial. J Infect Dis. 1974 Dec;130(6):673–676. doi: 10.1093/infdis/130.6.673. [DOI] [PubMed] [Google Scholar]
- Schimpff S., Serpick A., Stoler B., Rumack B., Mellin H., Joseph J. M., Block J. Varicella-Zoster infection in patients with cancer. Ann Intern Med. 1972 Feb;76(2):241–254. doi: 10.7326/0003-4819-76-2-241. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Ch'ien L. T., Dolin R., Galasso G. J., Alford C. A., Jr Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study. N Engl J Med. 1976 May 27;294(22):1193–1199. doi: 10.1056/NEJM197605272942201. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Whitley R. J., Blum M. R., Keeney R. E., Barton N., Cocchetto D. M., Good S., Hemstreet G. P., 3rd, Kirk L. E., Page D. A. Acyclovir kinetics after intravenous infusion. Clin Pharmacol Ther. 1979 Dec;26(6):718–728. doi: 10.1002/cpt1979266718. [DOI] [PubMed] [Google Scholar]



