Abstract
Biodegradable polymeric nanoparticles are currently being explored as a nonviral gene delivery system; however, many obstacles impede the translation of these nanomaterials. For example, nanoparticles delivered systemically are inherently prone to adsorbing serum proteins and agglomerating as a result of their large surface/volume ratio. What is desired is a simple procedure to prepare nanoparticles that may be delivered locally and exhibit minimal toxicity while improving entry into cells for effectively delivering DNA. The objective of this study was to optimize the formulation of poly(D,L-lactide-co-glycolide) (PLG) nanoparticles for gene delivery performance to a model of the pulmonary epithelium. Using a simple solvent diffusion technique, the chemistry of the particle surface was varied by using different coating materials that adsorb to the particle surface during formation. A variety of cationic coating materials were studied and compared to more conventional surfactants used for PLG nanoparticle fabrication. Nanoparticles (~200 nm) efficiently encapsulated plasmids encoding for luciferase (80–90%) and slowly released the same for 2 weeks. In A549 alveolar lung epithelial cells, high levels of gene expression appeared at day 5 for certain positively charged PLG particles and gene expression was maintained for at least 2 weeks. In contrast, PEI gene expression ended at day 5. PLG particles were also significantly less cytotoxic than PEI suggesting the use of these vehicles for localized, sustained gene delivery to the pulmonary epithelium.
Keywords: PLG, gene delivery, polycations, PEI, A549 cells
INTRODUCTION
Biodegradable nanoparticles have received considerable attention in recent years as a possible means of delivering drugs and genes through multiple routes of administration. Various biodegradable polyesters have been used in drug delivery such as poly(D,L-lactide-co-glycolide) (PLG) and poly(lactic acid) (PLA)1,2 as they may control drug release and thus increase the therapeutic benefit, while minimizing side effects.3–6 Many techniques are available for producing nanoparticles from these or other materials including double-emulsion solvent evaporation, interfacial polymerization, solvent diffusion, nanoprecipitation, and salting-out emulsification methods;7 however, PLG may be of special interest since it is FDA approved.
Pharmaceutical nanoparticles generally range in size from 10 to 1000 nm, and the drug is encapsulated or attached to the nanoparticle matrix.8 The nanometer size ranges of such systems offer certain distinct advantages for drug delivery. Due to their size, nanoparticles can penetrate into tissues through fine capillaries, cross the fenestrations present in the endothelial lining (e.g., liver), and are generally taken up efficiently by many types of cells.9 Also, by modulating polymer chemistry or molecular weight, one can control the release of a therapeutic agent from nanoparticles to achieve a desired drug level in the target tissue for a required duration to optimize therapeutic efficacy. Furthermore, nanoparticles may be delivered to target sites either by localized delivery using a catheter-based approach with a minimally invasive procedure10 or by conjugating a bio-specific ligand, which could direct them to the target tissue or organ.11
Indeed, biodegradable PLG nanoparticles are extensively investigated for drug delivery12–15 and, to a limited extent, gene delivery.16,17 Robust and scalable methods are still needed to overcome the difficulties in formulation reproducibility, particle size, and surface chemistry. The size and surface chemistry of nanoparticles have been identified as the most significant factors in determining pharmacokinetics and biodistribution.9,18,19 The particle size and charge of nanoparticles depend upon some preparative variables such as the type and concentration of stabilizer or coating material, biodegradable polymer used, time, and mechanism of shear (e.g., stirring, sonication, or homogenization), diffusion rate of organic solvent, ratio between aqueous and organic phases, etc.7,20 Creating the proper particle size is a key to effective drug delivery. For example, it has been shown that the efficiency of cellular uptake of 100–200 nm size particles was 15- to 250-fold greater in various cells than in larger sized microparticles.21,22
Apart from the size of nanoparticles, surface modification of particles is desired since the surface charge and relative hydrophobicity determine the amount of adsorbed components, mainly proteins, which qualifies the in vivo fate of nanoparticles.23,24 This modification has been achieved by two methods: (1) reactions to coat the particle surface or (2) adsorption of surfactants or other coating materials.6 Some of the widely used surface coating materials possess a hydrophilic segment such as poly(ethylene glycol) (PEG).25–30 Block copolymers such as poloxamine and poloxamer31–36 have been used as well with fewer examples of coatings such as poly(ethylenimine) (PEI).37–40 Previous reports show that the type of coating material has important effects on drug loading into nanoparticles41 and on the cellular uptake of nanoparticles.42,43 Hydrophilic coating materials such as PEG have been effective for increasing the circulation half-life of nanoparticles delivered by IV administration; however, this study is primarily interested in the potential of direct application of nanoparticles to the pulmonary epithelium. Controlling particle size and surface chemistry dramatically affects endocytic uptake and intracellular distribution of the vehicles, which in turn will affect the delivery of therapeutics such as DNA.
In this study, PLG nanoparticles (~200 nm) encapsulating pDNA encoding for firefly luciferase were prepared via the solvent diffusion method in the presence of selected coating materials. Surface modifications using a variety of cationic materials were compared to more conventional surfactants for the ability to transfect cells. Certain cationic PLG nanoparticles enhanced gene expression for at least 14 days in A549 cells (type II pneumocytes), which serve as a model of the alveolar epithelium. Perhaps most interestingly, PLG particles did not exhibit detectable gene expression until day 5 whereas gene expression achieved using PEI ended on the same day. Reports in the field of gene typically assay for gene expression at short times (24–48 h), which has led to the potential of PLG for gene delivery going largely unnoticed. Here, the features of sustained, low-level gene expression and negligible toxicity suggest potential value for localized, PLG-based gene delivery to the pulmonary epithelium.
MATERIALS AND METHODS
Materials
PLG (50:50) with a molecular weight of 20 kDa was purchased from LACTEL (Cupertino, CA) Absorbable Polymers. 2,2,2-Trifluoroethanol and dimethyldioctadecylammonium bromide (DODAB) were obtained from Fisher Scientific (Pittsburgh, PA). 3β-[N-(dimethylaminoethane)carbamoyl]cholesterol (DC-Chol), hexadecyl trimethyl ammonium bromide (Cetrimide), chitosan, and protamine were purchased from Sigma–Aldrich (St. Louis, MO). Pluronic F-127 was obtained from Invitrogen (Carlsbad, CA). Poly(vinyl alcohol) (PVA) and coumarin 6, laser grade, were obtained from Polysciences, Inc. (Warrington, PA). The plasmid DNA encoding firefly luciferase (pGL3, 4.8 kb) was obtained from Promega (San Luis Obispo, CA) and transformed into Escherichia coli DH5αTM (Invitrogen). A single transformed colony picked from an agar plate was cultured in LB Broth Base (Invitrogen) liquid for plasmid DNA preparation. Plasmid DNA was purified with Plasmid Giga Kit 5 (Qiagen, Valencia, CA) following the manufacturer’s instructions. All pDNA had purity levels of 1.8 or greater by UV/Vis (A260/A280). A549 cells were obtained from the American Type Culture Collection (ATCC, Manassa, VA). The cell culture medium (Ham’s F-12 nutrient mixture, Kaighn’s modified with L-glutamine) was purchased through Fisher Scientific. Fetal bovine serum (FBS) was purchased from Hyclone Thermo Scientific (Waltham, MA). Penicillin–streptomycin was purchased from MB Biomedical, LLC (Solon, OH). Trypsin–EDTA was purchased through Gibco® Invitrogen (Carlsbad, CA). MTS reagent [tetrazolium compound; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was purchased from Promega.
Encapsulation of pDNA in PLG Nanoparticles
PLG nanoparticles were prepared using the solvent diffusion method. In brief, aqueous solution (0.2 mL) containing pGL3 (100–200 μg) in Tris–EDTA (TE) buffer (pH 8) was mixed with 1 mL of PLG (50:50, lactide-to-glycolide ratio, 20 kDa) solution (15 mg/mL) in 2,2,2-trifluoroethanol (TFE).44 The resultant mixture was added dropwise through a syringe pump (17.5 mL/h) to an aqueous solution (10 mL) containing various coating materials stirring at 100–250 rpm at room temperature. The formed nanoparticles were dialyzed using a 1,000,000 Da MWCO Dialysis Cassette (Spectrum Laboratories, Inc., Rancho Oominguez, CA) for 6–16 h against distilled deionized water (DI) to remove excess coating materials and unentrapped pGL3. The mean particle size and zeta potential of the PLG nanoparticles encapsulating pGL3 were measured using a ZetaPALS dynamic light scattering instrument (Brookhaven Instruments Corporation, Holtsville, NY). Zeta potential was determined in 1 mM KCl solution.
Plasmid DNA Analysis
The integrity of pGL3 extracted from PLG nanoparticles was studied. Plasmid DNA was extracted from lyophilized particles using a TFE and TE buffer (TFE–TE) extraction method. Briefly, PLG nanoparticles (20 mg) were dissolved in 150 μL of TFE into which 850 μL of TE buffer was added and the tube was rotated for 1 h at room temperature. The aqueous layer was removed after centrifugation at 10,000 rpm for 10 min. The pGL3 from the extraction was analyzed for its integrity using UV spectrophotometry and agarose gel electrophoresis.
Release and Stability Studies of PLG Nanoparticles
To study the pGL3 release and the change in particle size and zeta potential of incubated nanoparticles over a period of time, some preparations of PLG nanoparticles of different coating materials; PVAm, chitosan, cetrimide, PVA, and Pluronic F-127 were used. Each batch was suspended in 7 mL of PBS and the suspension was split into seven tubes. The tubes were maintained in a shaker incubator (150 rpm) at 37°C. At predetermined time points, the tubes were centrifuged at 12,000 rpm for 8 min and the nanoparticles were then washed twice with double-distilled water and resuspended in water. Study of the size stability of PLG nanoparticles in cell culture media in the absence and presence of 10% FBS was achieved by suspended 200 μL of fresh sample in 800 μL media, and the suspension was incubated at 37°C for 5 and 12 h. The particles size and zeta potential of each sample were measured using the ZetaPALS dynamic light scattering system. On the other hand, for the release study the tubes were centrifuged and then the supernatant was collected into a new tube after a period of time and the nanoparticles were resuspended in fresh PBS and placed back in the incubator. Plasmid DNA concentration was measured in the supernatant using UV spectrophotometry.
Determination of pDNA Loading and Encapsulation Efficiency
An accurately weighed amount of nanoparticles (20 mg), freeze-dried without lyoprotectant, was dissolved in 150 μL of TFE into which 850 μL of TE buffer was added and the tube was rotated for 1–2 h at room temperature. The amount of DNA extracted was assayed using UV spectroscopy at 260 nm. The DNA loading and the encapsulation efficiency were calculated in the following manner:
The pDNA loading studies were performed in triplicate.
Cell Culture
Culturing of human epithelial lung cell line A549 was performed according to the protocol given by the ATCC. A549 cells were grown in F-12K supplemented with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin at 37°C in a humidified air atmosphere containing 5% CO2.
In Vitro Cell Transfection Studies
A549 cells were trypsinized, counted, and diluted to a concentration of approximately 80,000 cells/mL. Then 0.1 mL of that dilution was added to each well of a 96-well plate, and the cells were incubated in a humid 5% CO2 incubator at 37°C for 24 h. Immediately before transfection, the cells were washed once with PBS, and a 100 μL of sample (15% of fresh PLG nanoparticles (1.5 mg/mL, 273 ng of DNA) to 85% of serum-free cell culture media) was added to each well. Cells were incubated with the PLG nanoparticles for 5 h. The media containing nanoparticles were then removed and 100 μL of fresh serum media was added followed by further incubation. A luciferase expression assay was performed using the Luciferase Assay System from Promega following the manufacturer’s recommended protocol. The light units were normalized against protein concentration in the same cell extracts, which were measured using Coomassie PlusTM Protein Assay (Thermo Scientific, Waltham, MA). The transfection results were expressed as relative light units (RLU) per mg of cellular protein.
Assessment of Cytotoxicity (MTS Assay)
Cytotoxicity of lyophilized PLG nanoparticles with 5% (w/v) mannitol was determined by the CellTiter 96® Aqueous Cell Proliferation Assay (Promega). A549 cells were grown as described in the transfection experiments. Cells were treated with the samples for 24 h. The media were then removed and replaced with a mixture of 100 μL of fresh culture medium and 20 μL of MTS reagent solution. The cells were incubated for 3 h at 37°C in a 5% CO2 incubator. The absorbance of each well was then measured at 490 nm using a microtiter plate reader (SpectraMax, M25, Molecular Devices Corp., Sunnyvale, CA) to determine cell viability.
Cell Uptake Studies
A549 cells were seeded and incubated for 1 h with PLG nanoparticles encapsulating coumarin 6 (Polysciences, Inc.), using a solvent diffusion method, in 96-well plates as described above for transfection studies. Cells were then washed thrice with ice-cold PBS and solubilized (80 μL/well) with 0.5% Triton X-100 in 0.2 M NaOH for 30 min. Cell-associated nanoparticles were quantified by analyzing the cell lysate using a fluorescence plate reader (SpectraMax, M25, Molecular Devices Corp., λex 458 nm, λem 505 nm). The fluorescent intensity was normalized against protein concentration in the same cells lysate, which were measured using a BCA protein assay kit (Pierce Biotechnology).
Statistical Analysis
Statistical evaluation of comparing the significance of the difference in expression between the means of two groups was performed using the t-test, a value of p < 0.05 was accepted as significant.
RESULTS
Preparation and Characterization of PLG Nanoparticles
Different coating materials were used for the preparation of PLG/DNA nanoparticles by the solvent diffusion method. Transmission electron micrographs of PLG nanoparticles showed that the nanoparticles were spherical and discrete particles without agglomeration and that they were smooth in surface morphology (Fig. 1). The average particle size of the PLG nanospheres encapsulating DNA was ~200 nm with relatively narrow polydispersity regardless of coating material. A variety of zeta potentials were observed with the different types of coating materials. The values ranged from 32 to 40 mV for cationic surface modifiers and from −11 to −24 mV for weakly anionic surfactants (Tab. 1).
Figure 1.
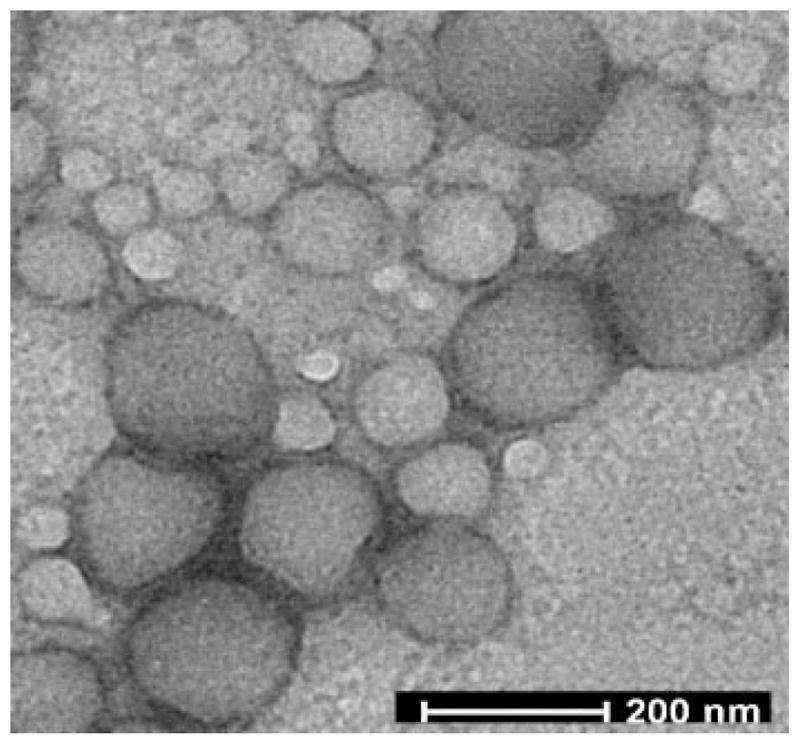
Transmission electron micrograph (TEM) of PLG nanoparticles.
Stability and Encapsulation Efficiency of DNA
Maximum extraction of pGL3 from the PLG/TFE phase into the aqueous phase was achieved within 60 min. The plasmid DNA structure was determined by agarose gel electrophoresis. The distinct bands present on the gel showed that the encapsulated DNA retained its structural integrity regardless of coating material when compared with the original DNA (Fig. 2A). Aqueous extracts from various nanoparticles were analyzed by UV spectrophotometry to determine DNA loading. The efficiency of pGL3 encapsulation in the PLG nanoparticles did not significantly depend on the type of cationic coating materials. Various cationic coating materials exhibited encapsulation efficiencies as high as ~88% (Tab. 1). Nanoparticles made using weakly anionic coating materials showed lower efficiencies for the encapsulation of DNA, reaching only ~79%. These results suggested that the presence of cationic coating materials could improve the entrapment of the anionic DNA molecule within the PLG nanoparticles. It is also possible that retention of the DNA was improved via adsorption of any released DNA by the cationic coating materials on the surface of nanoparticles.
Figure 2.
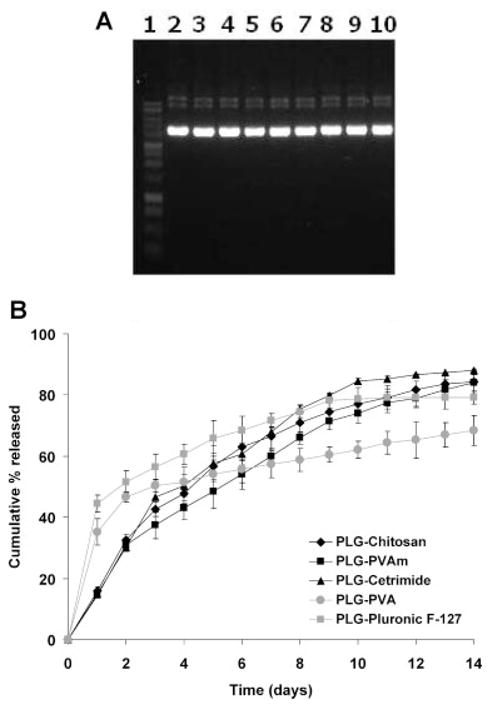
(A) Gel electrophoresis of plasmid DNA extracted from PLG nanoparticles: (1) marker, (2) naked pDNA, (3) PLG–PVA, (4) PLG–PluronicF-127, (5) PLG–PVAm, (6) PLG–chitosan, (7) PLG–DC-Chol, (8) PLG–cetrimide, (9) PLG–protamine, and (10) PLG–DODAB nanoparticles. (B) Release profile of pDNA from PLG nanoparticles in PBS at 37°C. Data are presented as mean ± SD (n = 3).
In Vitro Release and Stability Studies of PLG Nanoparticles
In vitro profiles of plasmid DNA release were compared for five different PLG coating materials: PVAm, chitosan, cetrimide, PVA, and Pluronic F-127 in PBS at 37°C. Irrespective of the type of coating material, the DNA was released in a sustained manner for 14 days (Fig. 2B). Using cationic coating materials significantly decreased the initial burst release of the DNA from the nanoparticles. At the end of the experiment, more than 84% of the total DNA encapsulated in the cationic PLG nanoparticles was released.
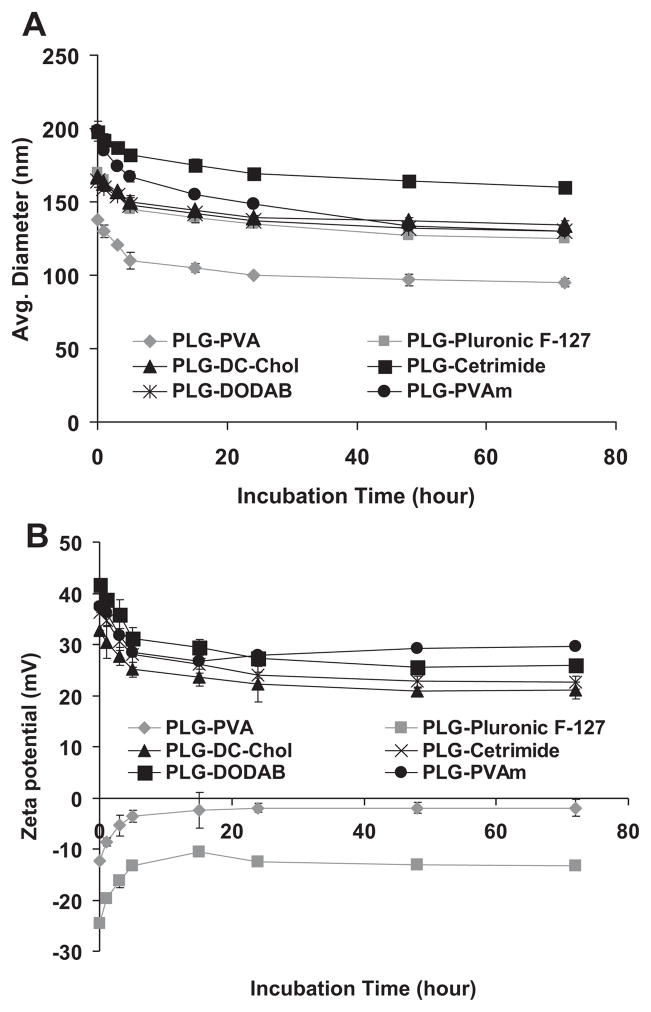
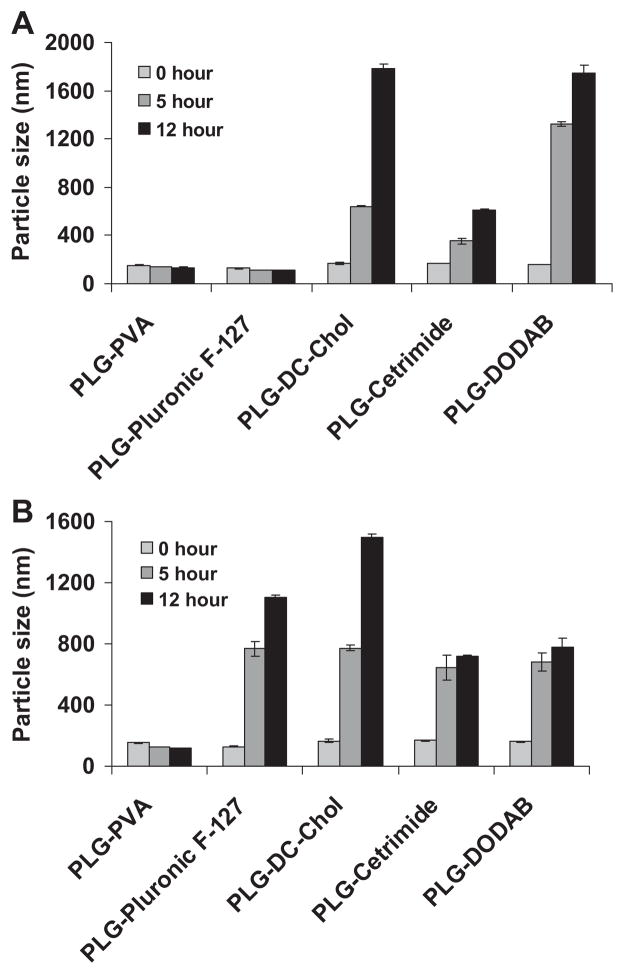
Apart from the release study, the stability of the size and the zeta potential of PVAm, DC-Chol, cetrimide, DODAB, PVA, and Pluronic F-127-coated PLG nanoparticles were investigated as a function of time. Zeta potential and size of gene delivery vehicles have been reported to be important factors in facilitating gene transfer in cells.45,46 The endocytotic uptake of particles is clearly affected by their size and tends to increase with decreasing diameters and increasing zeta potential.47,48 The PLG nanoparticles remained stable in PBS over a period of 3 days and retained their size and surface charge. Exemplary data are shown in Figure 3A and B. The initial change in the particle size and the zeta potential over the first 5 h of this study may be attributable to a reduction in agglomeration as the resuspended nanoparticle/mannitol powders slowly hydrated and dispersed. On the other hand, PLG nanoparticles coated with PVA and Pluronic F-127 showed good stability in serum-free culture media over a period of 5 and 12 h. PLG nanoparticles with cationic coating materials showed some agglomeration. In the presence of 10% FBS, only PLG–PVA nanoparticles retained their size (Fig. 4A and B). Precipitation was not noted in any of the samples over the course of 5 h, which was an analogous time to the transfection experiments. The colloidal stability of the PLG particles over this time suggested that any differences in gene transfection were not attributable to particles precipitating onto the cell monolayers.
Figure 3.
(A) Size of PLG nanoparticles coated with various materials as a function of time. (B) Zeta potential of PLG nanoparticles coated with various materials as a function of time. Data are presented as mean ± SD (n = 3).
Figure 4.
Stability of PLG nanoparticles coated with various materials over time in (A) the absence and (B) presence of 10% FBS. Data are presented as mean ± SD (n = 3).
Effect of PLG Nanoparticles on Cell Viability
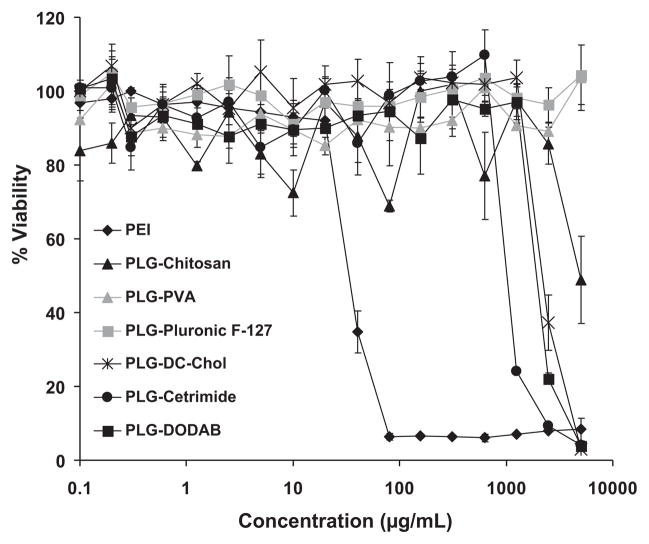
The effect of the PLG nanoparticles on the viability of A549 cells was studied by incubating the cells up to 5 mg/mL of lyophilized PLG/DNA nanoparticles with 5% (w/v) mannitol. Minimal cytotoxic effects were evident and cells maintained a high viability (Fig. 5) even for cationic coating materials (IC50 ~ 1190–2450 μg/mL). In contrast, branched PEI complexes showed significant cytotoxicity at low concentration (IC50 ~ 35 μg/mL).
Figure 5.
Cell viability of PLG nanoparticles in A549 cells in comparison with branched PEI (25k). Data are presented as mean ± SD (n = 3).
Nanoparticle Uptake by A549 Cells
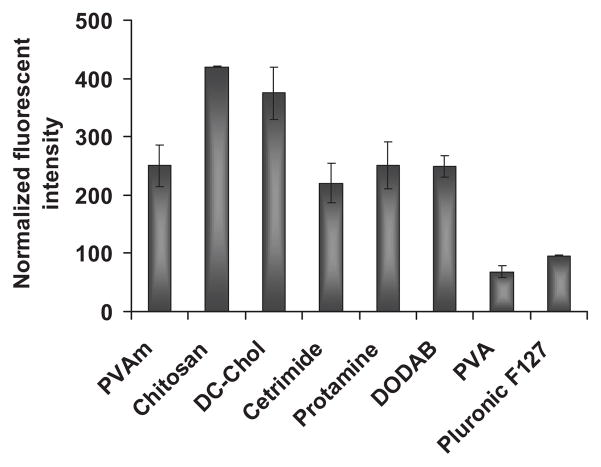
PLG nanoparticles coated with different materials were used to study cellular uptake by A549 lung epithelial cells. In these studies, PLG nanoparticles were stained with the fluorescent dye, coumarin 6. All coating materials showed uptake of nanoparticles within 1 h of incubation but with different magnitudes (Fig. 6). The nanoparticles formulated from PLG with cationic coatings, especially chitosan, showed high levels of nanoparticle uptake compared to weakly anionic coatings as expected. No correlation of the magnitude of zeta potential and nanoparticle uptake was observed. These results support previous research indicating that the nanoparticle surface can be modified to provide enhanced uptake by cells.25 To exclude the possibility of nonspecific staining of cells by coumarin 6, control experiments were performed. PLG–coumarin 6 nanoparticles were shaken in serum-free cell culture media for 3 h and centrifuged. The supernatant was incubated with A549 cells to check for any leakage of dye from nanoparticles, which might stain cells. No fluorescence was detected in the cells.
Figure 6.
Cellular uptake of PLG nanoparticles coated with various materials. Cationic coating materials enhanced the level of nanoparticle uptake compared to anionic coatings. Data are presented as mean ± SD (n = 3).
In Vitro Transfection Efficiency of PLG Nanoparticles
To assess the feasibility of PLG nanoparticles for gene delivery, in vitro transfection was performed using the human lung carcinoma cell line A549. The luciferase-encoding plasmid pGL3 was used as a reporter. On day 2 after transfection, PLG nanoparticles did not show gene expression. In comparison, branched PEI showed excellent transfection efficiency on day 2 (Fig. 7). Gene expression mediated by certain cationic PLG nanoparticles suddenly appeared on day 5, whereas the gene expression of branched PEI showed a marked decrease during the same time frame. The enhanced gene expression from certain cationic PLG nanoparticles was sustained for at least 14 days. The transfection efficiency of PLG with PVAm, DC-Chol, cetrimide, and DODAB coatings was almost similar to the transfection efficiency of branched PEI at day 5 but tended to increase at days 7 and 14. On the other hand, no expression was observed for PLG nanoparticles coated with chitosan, protamine, and Pluronic F-127 despite the fact that these coating materials showed relatively efficient cellular uptake. These results support the findings reported in the literature that nanoparticle uptake is not indicative of gene expression.49 Importantly, however, transfection performance may be the result of the fact that the type of coating materials can ultimately affect uptake pathways and nanoparticle fate within the cell, which remains to be investigated.
Figure 7.
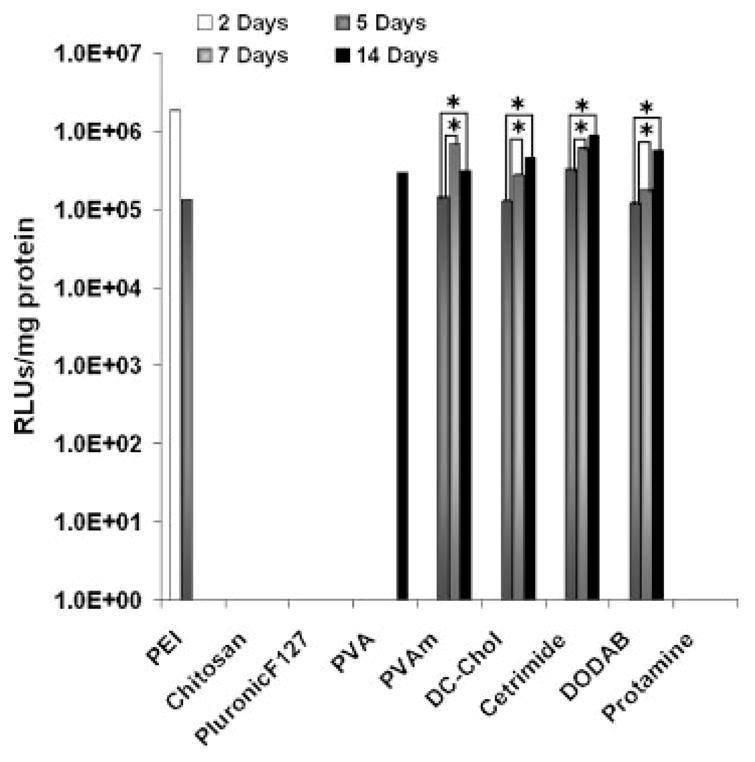
Transfection efficiency of PLG nanoparticles in A549 cells varied with different coating materials. Most PLG nanoparticles exhibited long-term expression that tended to increase over time. PEI complexes N/P 10 showed higher gene expression that dropped to negligible levels by day 5. Expression of day 14 was significantly higher than day 7 in A549 cells. Data are presented as mean ± SD (n = 3), *p < 0.001 when compared to day 5 for the same sample.
DISCUSSION
Nanoparticles of PLG have been widely studied for their use in drug delivery.50 Among nonviral vehicles, these particulates may offer a nontoxic and efficient carrier for sustained intracellular delivery of genes. However, PLG nanoparticles encapsulating DNA need to be carefully designed to achieve efficient gene delivery. In the present study, PLG/DNA nanoparticles were fabricated using a solvent diffusion method. The protocol was developed to avoid shear and centrifugation. These processes may not only affect the integrity of DNA encapsulated in nanoparticles but may also diminish DNA encapsulation efficiency. In addition, the nanoparticle stability may be compromised by centrifugation procedures, which can lead to irreversible agglomeration. The methods reported here resulted in high encapsulation efficiencies (~88%) and retention of DNA structure as indicated by gel electrophoresis of the pDNA extracted from various PLG nanoparticles.
Positively charged nanoparticles have been shown to bind to the negatively charged cell surface, and thereby facilitate their uptake.51 Higher levels of cellular uptake of PLG nanoparticles were achieved using cationic coating materials compared to the weakly anionic PVA and Pluronic F-127 coatings. Unlike PEI, PLG nanoparticles degrade by hydrolysis and were expected to sustain delivery of DNA; however, a cationic coating material was desired to improve cellular uptake of the particles. In the present study, the release of DNA from PLG nanoparticles was sustained for approximately 14 days and release was found to be more constant for nanoparticles with cationic coatings. After observing the slow release of DNA, gene transfection levels were studied for 2 weeks in A549 cells.
The level of gene expression in the A549 lung epithelial cells was higher in the branched PEI/DNA complexes than in the PLG nanoparticles for the initial 4 days, but the trend was reversed after day 5. This was perhaps a result of the sustained DNA release from the PLG nanoparticles. Thus, modified PLG/DNA nanoparticles appeared to be superior to PEI/DNA complexes for the long-term expression of DNA. Typical gene transfection studies reported in the literature assess gene expression at 24 or 48 h, which may explain the low level of previous reports on PLG nanoparticles for gene delivery. At this point, it is difficult to speculate if the differences in gene expression observed for different cationic PLG nanoparticles were caused by endosomal escape, differences in the release of DNA within cells, or other factors. Perhaps most importantly, modified PLG nanoparticles showed little to no cytotoxicity after 24 h incubation with A549 cells compared to that of branched PEI complexes.
CONCLUSION
In this study, PLG nanoparticles coated with different surface modifiers were examined for the ability to transfect A549 lung epithelial cells. A series of physicochemical studies demonstrated that the PLG nanoparticles coated with cationic materials increased DNA encapsulation efficiency and enhanced uptake by A549 lung epithelial cells. PLG nanoparticles exhibited excellent particle size and surface charge stability during 3 days incubation in PBS at 37°C; however, some increase in particle size was noted in media with serum at longer incubation times (>5 h). Gene expression was sustained for at least 14 days for several PLG nanoparticle formulations and gene expression levels tended to increase over the duration of the study. Conversely, gene expression levels for branched PEI/DNA complexes showed high initial gene expression that dropped to negligible levels by day 5. In vitro cytotoxicity studies showed that cationic PLG nanoparticles exhibited much lower cytotoxicity than branched PEI (25 kDa). Taken cumulatively, these data suggest that cationic PLG nanoparticles may be an effective vehicle for delivering DNA to the pulmonary epithelium.
Table 1.
Characteristics of PLG Nanoparticles Coated With Cationic and Anionic Materials
| PVAm | Chitosan | DC-Chol | Cetrimide | Protamine | DODAB | PVA | Pluronic F-127 | |
|---|---|---|---|---|---|---|---|---|
| % (w/v)a | 0.03 | 0.03 | 0.02 | 0.1 | 0.02 | 0.02 | 2.0 | 0.1 |
| Size ± SD (nm) | 196 ± 4 | 201 ± 1 | 167 ± 1 | 197 ± 2 | 204 ± 3 | 163 ± 4 | 137 ± 2 | 169 ± 2 |
| Polydispersity | 0.145 | 0.168 | 0.068 | 0.145 | 0.022 | 0.074 | 0.099 | 0.122 |
| Zeta potential ± SD (mV) | +37.5 ± 0.8 | +36.8 ± 0.4 | +33.2 ± 0.7 | +35.4 ± 1.6 | +17.7 ± 1.4 | +40.9 ± 0.9 | −11.0 ± 2.0 | −23.2 ± 1.1 |
| pDNA loading (%) | 1.08 | 1.06 | 1.10 | 1.14 | 1.09 | 1.12 | 1.00 | 1.03 |
| Encapsulation efficiency (%) | 83.1 ± 1.2 | 81.5 ± 2.3 | 84.6 ± 0.7 | 87.7 ± 0.5 | 83.8 ± 1.2 | 86.2 ± 0.2 | 77.0 ± 1.1 | 79.2 ± 0.5 |
Weight/volume percent (the concentration of the coating materials). Values are representative of three experiments (mean ± SD).
Acknowledgments
We would like to acknowledge the support for this work from the Coulter Foundation, the Higuchi Biosciences Center, and the Cystic Fibrosis Foundation as well as additional lab funding from the American Heart Association, the NIH (R03AR054035, P20 RR016443, and T32 GM08359-11), and the Department of Defense. In addition, we acknowledge the support of the NSF (CHE 0719464). We also thank Prof. C. Russ Middaugh for the use of laboratory equipment and the Microscopy Lab for assistance with electron microscopy. SB would like to acknowledge support from the NIH grant number R21023397.” and “ND would like to acknowledge support from the NIH grant number R03AI080280.
References
- 1.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 2.Langer R. Tissue engineering: A new field and its challenges. Pharm Res. 1997;14:840–841. doi: 10.1023/a:1012131329148. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 4.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 5.Kreuter J. Colloidal drug delivery systems. vii. New York: M. Dekker; 1994. p. 353. [Google Scholar]
- 6.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16:61–73. [Google Scholar]
- 8.Labhasetwar V. Nanoparticles for drug delivery. Pharm News. 1997;4:28–31. [Google Scholar]
- 9.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 10.Song C, Labhasetwar V, Cui X, Underwood T, Levy RJ. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: Results with an acute dog model. J Control Release. 1998;54:201–211. doi: 10.1016/s0168-3659(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 11.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 12.Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr CM, Hoffman M, Maincent P. Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm. 1999;184:97–105. doi: 10.1016/s0378-5173(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 13.Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Maincent P, Kawashima Y, Lehr CM. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299:775–781. [PubMed] [Google Scholar]
- 14.Lemoine D, Francois C, Kedzierewicz F, Preat V, Hoffman M, Maincent P. Stability study of nanoparticles of poly(e-caprolactone), poly(d, l-lactide) and poly(d, l-lactide-co-glycolide) Biomaterials. 1996;17:2191–2197. doi: 10.1016/0142-9612(96)00049-x. [DOI] [PubMed] [Google Scholar]
- 15.Schachter DM, Kohn J. A synthetic polymer matrix for the delayed or pulsatile release of water-soluble peptides. J Control Release. 2002;78:143–153. doi: 10.1016/s0168-3659(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 16.Cohen-Sacks H, Najajreh Y, Tchaikovski V, Gao G, Elazer V, Dahan R, Gati I, Kanaan M, Waltenberger J, Golomb G. Novel PDGFβR antisense encapsulated in polymeric nanospheres for the treatment of restenosis. Gene Ther. 2002;9:607–616. doi: 10.1038/sj.gt.3301830. [DOI] [PubMed] [Google Scholar]
- 17.Panyam J, Zhou W, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly (dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 18.Grayson ACR, Cima MJ, Langer R. Size and temperature effects on poly (lactic-co-glycolic acid) degradation and microreservoir device performance. Biomaterials. 2005;26:2137–2145. doi: 10.1016/j.biomaterials.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 20.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int J Pharm. 2005;290:137–144. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 22.Zauner W, Farrow NA, Haines AMR. In vitro uptake of polystyrene microspheres: Effect of particle size, cell line and cell density. J Control Release. 2001;71:39–51. doi: 10.1016/s0168-3659(00)00358-8. [DOI] [PubMed] [Google Scholar]
- 23.Müller RH, Wallis KH. Surface modification of i.v. injectable biodegradable nanoparticles with poloxamer polymers and poloxamine 908. Int J Pharm. 1993;89:25–31. [Google Scholar]
- 24.Van Oss CJ. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]
- 25.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 26.Hinrichs WLJ, Mancenido FA, Sanders NN, Braeckmans K, De Smedt SC, Demeester J, Frijlink HW. The choice of a suitable oligosaccharide to prevent aggregation of PEGylated nanoparticles during freeze thawing and freeze drying. Int J Pharm. 2006;311:237–244. doi: 10.1016/j.ijpharm.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Moffatt S, Cristiano RJ. Uptake characteristics of NGR-coupled stealth PEI/pDNA nanoparticles loaded with PLGA-PEG-PLGA tri-block copolymer for targeted delivery to human monocyte-derived dendritic cells. Int J Pharm. 2006;321:143–154. doi: 10.1016/j.ijpharm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Quellec P, Gref R, Dellacherie E, Sommer F, Tran MD, Alonso MJ. Protein encapsulation within poly (ethylene glycol)-coated nanospheres II. Controlled release properties. Journal of biomedical materials research. 1999;47(3):388–395. doi: 10.1002/(sici)1097-4636(19991205)47:3<388::aid-jbm14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Vila A, Sanchez A, Evora C, Soriano I, McCallion O, Alonso MJ. PLA-PEG particles as nasal protein carriers: The influence of the particle size. Int J Pharm. 2005;292:43–52. doi: 10.1016/j.ijpharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 31.Csaba N, Caamano P, Sanchez A, Dominguez F, Alonso MJ. PLGA:poloxamer and PLGA:poloxamine blend nanoparticles: New carriers for gene delivery. Biomacromolecules. 2005;6:271–278. doi: 10.1021/bm049577p. [DOI] [PubMed] [Google Scholar]
- 32.Illum L, Davis SS. The organ uptake of intravenously administered colloidal particles can be altered using a non-ionic surfactant (Poloxamer 338) FEBS Lett. 1984;167:79–82. doi: 10.1016/0014-5793(84)80836-4. [DOI] [PubMed] [Google Scholar]
- 33.Illum SL, Davis SS. Effect of the nonionic surfactant poloxamer 338 on the fate and deposition of polystyrene microspheres following intravenous administration. J Pharm Sci. 1983;72:1086–1089. doi: 10.1002/jps.2600720933. [DOI] [PubMed] [Google Scholar]
- 34.Moghimi SM, Gray TA. Single dose of IV-injected poloxamine coated long-circulating particle triggers macrophage clearance of subsequent doses in rats. Clin Sci. 1997;93:371–379. doi: 10.1042/cs0930371. [DOI] [PubMed] [Google Scholar]
- 35.Pitard B, Bello-Roufai M, Lambert O, Richard P, Desigaux L, Fernandes S, Lanctin C, Pollard H, Zeghal M, Rescan PY. Negatively charged self-assembling DNA/poloxamine nanospheres for in vivo gene transfer. Nucleic Acids Res. 2004;32:e159. doi: 10.1093/nar/gnh153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48. [Google Scholar]
- 37.Munier S, Messai I, Delair T, Verrier B, Ataman-Onal Y. Cationic PLA nanoparticles for DNA delivery: Comparison of three surface polycations for DNA binding, protection and transfection properties. Colloids Surf B Biointerfaces. 2005;43:163–173. doi: 10.1016/j.colsurfb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: Influence of ligands, polymer size, and PEGy-lation on internalization and gene expression. AAPS J. 2001;3:43–53. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiyaboonchai W, Woiszwillo J, Middaugh CR. Formulation and characterization of DNA–polyethylenimine–dextran sulfate nanoparticles. Eur J Pharm Sci. 2003;19:191–202. doi: 10.1016/s0928-0987(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 40.Tiyaboonchai W, Woiszwillo J, Sims RC, Middaugh CR. Insulin containing polyethylenimine–dextran sulfate nanoparticles. Int J Pharm. 2003;255:139–151. doi: 10.1016/s0378-5173(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 41.Egea MA, Gamisans F, Valero J, Garcia ME, Garcia ML. Entrapment of cisplatin into biodegradable polyalkylcyanoacrylate nanoparticles. Farmaco. 1994;49:211–217. [PubMed] [Google Scholar]
- 42.Kamruzzaman Selim KM, Ha YS, Kim SJ, Chang Y, Kim TJ, Ho Lee G, Kang IK. Surface modification of magnetite nanoparticles using lactobionic acid and their interaction with hepatocytes. Biomaterials. 2007;28:710–716. doi: 10.1016/j.biomaterials.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Yin Win K, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 44.Hirosue S, Müller BG, Mulligan RC, Langer R. Plasmid DNA encapsulation and release from solvent diffusion nanospheres. J Control Release. 2001;70:231–242. doi: 10.1016/s0168-3659(00)00353-9. [DOI] [PubMed] [Google Scholar]
- 45.Fischer D, von Harpe A, Kissel T. Polyethylenimine: polymer structure influences the physicochemical and biological effects of plasmid/PEI complexes. Biomaterials. 2000:195–211. [Google Scholar]
- 46.Kircheis R, Blessing T, Brunner S, Wightman L, Wagner E. Tumor targeting with surface-shielded ligand–polycation DNA complexes. J Control Release. 2001;72:165–170. doi: 10.1016/s0168-3659(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 47.Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Fundamentals of pulmonary drug delivery. Respir Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 48.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 49.Lühmann T, Rimann M, Bittermann AG, Hall H. Cellular uptake and intracellular pathways of PLL-g-PEG-DNA nanoparticles. Bioconjug Chem. 2008;19:1907–1916. doi: 10.1021/bc800206r. [DOI] [PubMed] [Google Scholar]
- 50.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(d, l-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92:203–217. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]