Abstract
NMDA receptors are found in neurons both at synapses and in extrasynaptic locations. Extrasynaptic locations are poorly characterized. Here we used preembedding immunoperoxidase and postembedding immunogold electron microscopy and fluorescence light microscopy to characterize extrasynaptic NMDA receptor locations in dissociated hippocampal neurons in vitro and in the adult and postnatal hippocampus in vivo. We found that extrasynaptic NMDA receptors on neurons in vivo and in vitro were usually concentrated at points of contact with adjacent processes, which were mainly axons, axon terminals, or glia. Many of these contacts were shown to contain adhesion factors such as cadherin and catenin. We also found associations of extrasynaptic NMDA receptors with the MAGUKs, PSD-95 and SAP102. Developmental differences were also observed. At postnatal day 2 in vivo, extrasynaptic NMDA receptors could often be found at sites with distinct densities whereas dense material was seen only rarely at sites of extrasynaptic NMDA receptors in the adult hippocampus in vivo. This difference probably indicates that many sites of extrasynaptic NMDA receptors in early postnatal ages represent synapse formation or possibly sites for synapse elimination. At all ages, as suggested in both in vivo and in vitro studies, extrasynaptic NMDA receptors on dendrites or the sides of spines may form complexes with other proteins, in many cases, at stable associations with adjacent cell processes. These associations may facilitate unique functions for extrasynaptic NMDA receptors.
Keywords: PSD-95, SAP102, cadherin, catenin, NR2A, NR2B
Extrasynaptic NMDA receptors (NMDARs) are common on neurons but are little understood compared to synaptic NMDARs. In hippocampal neuronal cultures, physiological studies showed that ~ 75% of NMDARs are extrasynaptic at about 1 WIV (= week or weeks in vitro; Rosenmund et al., 1995; Tovar and Westbrook, 1999, 2002) but this decreases with the published values ranging from ~ 20% to 50% at 2 WIV (Ivanov et al., 2006). Immunocytochemical studies indicate that about 80-90% of NMDARs are extrasynaptic at 1 WIV. These comprise both NR2A- and NR2B-containing receptors. At 1 WIV, the majority of NR2A-containing NMDARs are extrasynaptic (~79%) but this decreases to ~ 37% at 2 WIV. The abundance of extrasynaptic NR2B-containing NMDARs however, remains high (~ 83 - 92%) throughout this time period (Groc et al., 2004, 2006, 2009). In acute hippocampal slices, ~ 36% of NMDARs are extrasynaptic at P14-21 (postnatal days 14-21; Harris and Pettit, 2007). Harris and Pettit (2007) also reported similar levels of functional NR2B-containing NMDARs in synaptic and extrasynaptic sites. This is in contrast to the dissociated neuronal cell culture studies of Groc et al. (2004, 2006 and 2009) and also to physiological studies that suggested that extrasynaptic NMDARs are mainly NR2B-containing (e.g., Tovar and Westbrook, 1999; Kim et al., 2005).
While the presence of abundant extrasynaptic NMDARs is well accepted, little is known about their distribution. Some synapses have preferential localization of NMDARs in perisynaptic areas (Pérez-Otaño et al., 2006; Zhang and Diamond, 2006) or at specific sites for endocytosis and probably, exocytosis (Blanpied et al., 2002; Petralia et al., 2003; Racz et al., 2004; see also Washbourne et al., 2004). They may also be found pre-synaptically (Jourdain et al., 2007). In addition, attachment plaques in cerebellar granule layer glomeruli contain NMDARs co-distributed with PSD-95 (Petralia et al., 2002). Allison et al. (1998) suggested that extrasynaptic NMDARs associate during development with PSD-95, but this was not corroborated with EM (electron microscopy or electron microscope).
In the present study, we used a combination of LM (light microscopy or light microscope) and EM immunocytochemical techniques to identify the location of extrasynaptic NMDARs and associated proteins including the MAGUKs, PSD-95 and SAP102. Studies were performed in brain sections and primary neuron cultures from hippocampus supplemented with studies of recombinant NMDARs expressed in primary cultures of hippocampal neurons. We describe here how extrasynaptic NMDAR clusters in early postnatal animals are associated commonly with discrete densities that probably represent stages in synapse formation and maturation. Most examples of extrasynaptic NMDAR sites in adults were not associated with discrete densities. For all ages, we also found that these membrane regions of extrasynaptic NMDARs usually are contact points with adjacent processes, typically axon terminals or shafts, or glia. They were found to contain adhesion factors that presumably contribute to stabilization of the contact.
EXPERIMENTAL PROCEDURES
Antibodies
Most antibodies used in this study have been previously described (Sans et al., 2000; Petralia et al., 2002, 2005, and Yi et al., 2007). These included 1) rabbit polyclonal antibodies: SAP102 (gift of Johannes Hell), flag (Sigma, St. Louis, MO), synapsin (Chemicon, Temecula, CA), NR2A and NR2B (Groc et al., 2006); 2) mouse monoclonal antibodies: PSD-95/93 (MA1-046; Affinity BioReagents, Golden, CO), β-catenin, N-cadherin, and PSD-95 (BD Biosciences, Lexington, KY), pan-cadherin (Sigma), SAP102 (NeuroMab, Davis, CA), bassoon (Stressgen, Victoria, BC, Canada), neuroligin (Synaptic Systems, Göttingen, Germany), tau and glial fibrillary acidic protein (GFAP); Chemicon), SNAP-25 (synaptosomal-associated protein; Covance, Dedham, MA), myc (ATCC, Manassas, VA), NR1 (clone 54.2; directed against amino acids 660-811), NR1 (directed against NR1 amino acids 341-561 ; a gift of B. Wolfe); 3) a guinea pig polyclonal antibody: VGLUT1 (vesicular glutamate transporter; Chemicon), and a rabbit monoclonal antibody: NR1 (C2 portion of C terminus; Chemicon). For MAGUK labeling, it was necessary to use different antibodies for the LM and EM studies. For EM immunogold studies, a mouse monoclonal PSD-95 (BD Biosciences) and rabbit polyclonal SAP102 (provided by Johannes Hell) were used (Sans et al., 2000). For LM, a PSD-95/93 (Affinity Bioreagents, Golden, CO; Sans et al., 2000) and a mouse SAP102 (NeuroMab, Davis, CA; Al-Hallaq et al., 2007) were used. Secondary antibodies used in this study included Alexa Fluor antibodies (Invitrogen/Molecular Probes, Eugene, Oregon), Vectastain immunoperoxidase kits (Vector Laboratories, Burlingame, CA), and BBInternational immunogold (Ted Pella, Redding, CA).
EM preparation and analysis of brain sections
Two methods of brain tissue preparation for EM study were used: preembedding immunoperoxidase and postembedding immunogold. All animal procedures were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 85-23) under NIDCD protocol #1167-07. Preembedding EM immunoperoxidase/DAB labeling was based on established methods (Petralia et al., 1994a,b; Petralia and Wenthold, 1999; Tomita et al., 2003). Briefly, male Sprague-Dawley rats were anesthetized with a mixture of ketamine and xylazine and perfused with 4% paraformaldehyde. Brain sections were cut at 50 μm thickness in PBS and kept for 24 hr in 30% sucrose in PBS, frozen using dry ice and an acetone bath and the sections stored at −80°C. Sections were thawed and washed in PBS, incubated in 10% normal goat serum, placed in the primary antibody overnight at 4°C and processed for immunoperoxidase labeling using Vectastain kit and ImpacDAB (Vector Laboratories). Positive labeling was identified either by a granular reaction product or by the relative density of the membrane labeling compared to the density of the surrounding unlabeled membranes and cytoplasm.
In some cases, silver/gold toning was performed after the DAB step (Sassoe-Pognetto et al., 1994; Fletcher et al., 2000). For this, sections were washed in cacodylate buffer, incubated for 10 min at 60°C in 0.2% silver nitrate/0.2% sodium borate/2.6% hexamethylenetetramine, rinsed in water and incubated in 0.05% gold chloride for 2 min, rinsed and incubated in 3% sodium thiosulfate for 2 min.
For immunoperoxidase labeling, some sections were prepared for LM only; others from the same animals (and labeled simultaneously) were prepared further for EM, i.e., sections were washed in cacodylate buffer, fixed in 2% glutaraldehyde, then in 1% osmium tetroxide, dehydrated in alcohols and propylene oxide and finally embedded in epon. Thin sections were cut on a Leica Ultracut ultramicrotome (Vienna, Austria) and examined with a JEOL JSM-1010 EM (Peabody, MA) and AMT digital camera (Danvers, MA).
For all EM methods, images were stored in their original formats and final images for figures were prepared in Adobe Photoshop; levels and brightness/contrast of images were minimally adjusted evenly over the entire micrograph. For semi-quantification of the silver/gold-toned immunoperoxidase, random samples were taken of labeled spines and dendrites from up to five micrometers from the section surface from two adult animals. Controls for both variations of the immunoperoxidase method lacked the primary antibody.
For postembedding immunogold, established methods were used (Petralia and Wenthold, 1999; Petralia et al., 1999; 2002, 2003, 2005; Sans et al., 2000; Yi et al., 2007; Park et al., 2009). Briefly, rats were perfused with 4% paraformaldehyde plus 0.5% glutaraldehyde, and sections were cut at 350 μm, cryoprotected and frozen in a Leica EM CPC (Vienna, Austria), and further processed and embedded in Lowicryl HM-20 resin using a Leica AFS freeze-substitution instrument. Thin sections were incubated in 0.1% sodium borohydride plus 50 mM glycine in Tris-buffered saline plus 0.1% Triton X-100 (TBST), followed by 10% normal goat serum (NGS) in TBST, and primary antibody in 1% NGS/TBST overnight, followed by immunogold labeling using either F(ab’)2 fragments or whole antibodies bound to gold conjugates (Ted Pella, Redding, CA) in 1% NGS in TBST plus 0.5% polyethylene glycol (20,000 MW). Finally, sections were stained with uranyl acetate and lead citrate.
For double labeling, the two primary and respective secondary antibodies were applied together. For the main studies with the NR1 rabbit monoclonal antibody, we used 10 nm gold alone, or either 5 or 15 nm gold in combination with a mouse antibody directed against another protein with its gold secondary antibody. Controls lacked the primary antibody.
Quantitative analyses of immunogold labeling were carried out by two different methods. One quantitative analysis of extrasynaptic NR1 employed only the subset of the studies with 5 nm gold, all done with the identical procedure; the analyzed sample included all extrasynaptic gold particles found in a random sample area studied by visual searching on the microscope, in order to provide data on relative distribution. Another quantitative analysis used a random sample of spine synapses and dendrite profiles, to provide data on the average numbers of extrasynaptic gold particles (5 nm) on synapse spine and dendrite profiles.
The NR1 rabbit monoclonal primary antibody was used at a low concentration (1/1000-1/2000) for the immunoperoxidase method and at a high concentration for immunogold (1/50-1/200). The pattern of extrasynaptic labeling was similar using the two methods.
EM preparation of cultured neurons
Two major methods were also employed for the EM study of cultured hippocampal neurons prepared according to Yi et al. (2007). Labeling was carried out on native receptors at 2-3 WIV; for transfections, neurons were transfected using LipoFectamine2000 (Invitrogen) four days prior to labeling.
For preembedding EM immunoperoxidase labeling, procedures were modified from established methods (Eshhar et al., 1993; Petralia and Wenthold, 1999). A method was developed to correlate LM and EM immunoperoxidase/DAB labeling patterns (Seabold et al., 2008). It was possible to correlate individual puncta seen using LM with labeled regions seen using EM through serial sections. In practice, this was very difficult and time consuming, and only representative examples of the correlation were prepared and analyzed. In the main studies of live surface labeling, live neurons were incubated in primary antibody in neurobasal medium at room temperature for 45 minutes, then the glass coverslips were fixed in 4% paraformaldehyde and labeled essentially as described above. In some cases, coverslips were incubated in 0.3% H2O2 in PBS for 5 minutes prior to incubation in NGS. After embedding in epon, and photographing for LM, the glass coverslip was dissolved with hydrofluoric acid prior to cutting thin sections for EM. For postembedding immunogold, the method was similar to that used for postembedding immunogold of whole brain. After fixation, cultures were scraped off the coverslips and the cells were centrifuged to form a pellet. Then, the procedure described above for postembedding immunogold of fixed brain sections was followed.
LM preparations for neuronal cultures
For LM immunofluorescence, established methods were used (Yi et al., 2007). For live surface labeling of NMDA receptors, NR2A and NR2B rabbit antibodies were applied as above; for intracellular labeling, cells were permeabilized using 0.1% Triton X-100 prior to antibody incubation. Double and triple labeled coverslips were examined in a Zeiss LSM510 scanning confocal microscope (Thornwood, NY) using a 63x plan-apochromat (1.4 NA) objective and 2x zoom. For image capture of the triple colocalization studies with the anti-NR2A and NR2B antibodies, most settings were kept uniform, but the percent of laser power and amplitude gain were varied to optimize gray level spread. While this is not good for quantification of intensity among micrographs, it optimizes the accuracy of colocalization. Stacks of optical slices were taken through dendrites and examined, but quantitative analysis was done on fused stacks to ensure inclusion of all colocalization. Brightness and contrast of the 3 channels were adjusted separately (evenly over the entire image) in Adobe Photoshop to minimize background labeling. Puncta overlap was identified manually (at least one pixel overlap). Each dendrite section examined was ~20 μm. For thick dendrites near a neuron soma, results were combined from 3 experiments (5 neurons and 15 dendrite sections per experiment) for both NR2A and NR2B labeling in association with VGLUT and five proteins: PSD-95/93, SAP102, pan-cadherin, N-cadherin, or β-catenin.
For these proximal dendrites, three 20 μm dendrite sections were measured from each micrograph. Most of these were from the secondary dendrites, but we included some dendrite sections from primary dendrites, depending on the quality of each individual micrograph. For thinner dendrites that were found in micrographs taken more distal from a neuron soma, results were combined from 3 experiments (5 neurons and 15 dendrite sections per experiment) for both NR2A and NR2B in association with VGLUT and PSD-95/93 or SAP102, and from 1 experiment (3 neurons and 9 dendrite sections per experiment) for both NR2A and NR2B in association with VGLUT and pan-cadherin or N-cadherin. The thinner, distal dendrites were taken from micrographs that were acquired well away from the proximal zone as described above. The total structures examined in this study, including all proximal and distal dendrites, were 666 dendrite sections from 222 neurons (nearly 20,000 puncta examined manually). We also utilized a subset of these samples in the Volocity program (Perkin Elmer/Improvision) to analyze synaptic and extrasynaptic puncta size of native and transfected NMDARs and separately, to study differences in intensity of synaptic and extrasynaptic puncta of PSD-95/93 versus SAP102. For the latter study of intensity (based on a 4095 gray level scale for 12 bit images), we used only the ratio of values within each micrograph because the percent of laser power and amplitude gain had been varied to optimize gray level spread for colocalization studies, as explained above. Ten dendrite segments from 10 neurons each for PSD-95/93 and SAP102 were analyzed. Five dendrites were examined from the NR2A and NR2B labeled groups and these were combined into 10 since there was no significant difference between them. Volocity measures structural associations based on volume (i.e., voxels) and thus it can use the information available in a confocal stack to provide a more accurate representation of labeling distribution and colocalization compared to pixel-based methods of analysis. We also used Volocity to compare NR2A (native or flag-NR2A) or NR2B (native or flag-NR2B) surface puncta size on dendrites. As above, synaptic puncta overlapped with VGLUT labeling. For these experiments, dendrite regions from 5 NR2A and 5 NR2B labeled neurons were combined for N's of 10 each for 2 or 3 WIV for transfected (colabeled for PSD-95/93) or 3 WIV native NMDAR subunits (colabeled for PSD-95/93 or SAP102). For each, NR2A and NR2B data were combined because there was no significant difference between them. Note also that, as an additional check for the associations of the MAGUKs with extrasynaptic NMDARs, preliminary comparisons of labeling of the transfected and native receptors using a Volocity analysis program also showed substantial overlap of these proteins with the extrasynaptic receptors (data not shown).
The Leica TSC Stimulated Emission Depletion Microscope (STED) was used for a preliminary study using Atto 647 rabbit secondary antibody from Active Motif for live NR2A labeling, along with labeling for VGLUT and SAP102 as described above. NR2A/647 was examined with STED at resolution below the diffraction limit of LM. This was performed by Geoff Daniels and Jochen Sieber from Leica.
RESULTS
Immunofluorescence studies of extrasynaptic NMDARs in vitro
We first studied the organization of extrasynaptic NMDARs in cultures of hippocampal neurons at the LM level. We compared live surface labeling of native receptors using anti-NR2A and NR2B rabbit antibodies in cultures of hippocampal neurons at 3 WIV, with live surface labeling of transfected neurons at 2-3 WIV using anti-FLAG antibodies to detect flag-NR2A and flag-NR2B. In each case, in parallel immunolabeling was carried out with an antibody raised in guinea pigs against the presynaptic marker, VGLUT, and also antibodies raised in mice and directed against candidate proteins that are thought to associate with NMDA receptors, i.e. anti-PSD-95/93, SAP102, pan-cadherin, N-cadherin, and catenin antibodies.
For the native NMDA receptors, VGLUT immunolabeling indicated that ~ half of the puncta for both NR2A and NR2B were localized extrasynaptically (Fig. 1,2). PSD-95/93 and SAP102 antibodies all showed substantial overlap (~ one third) with the extrasynaptic puncta of NR2A and NR2B labeling with no significant differences seen between NR2A and NR2B (Fig. 1D). However, while both PSD-95/93 and SAP102 labeling indicated that these proteins are found outside synapses, the relative intensity of synaptic PSD-95/93 was greater than that observed at extrasynaptic sites whereas there was less difference in intensity between synaptic and extrasynaptic puncta of SAP102 (Fig. 1E). This suggests that PSD-95/93 is more concentrated at synapses while SAP102 is more widespread.
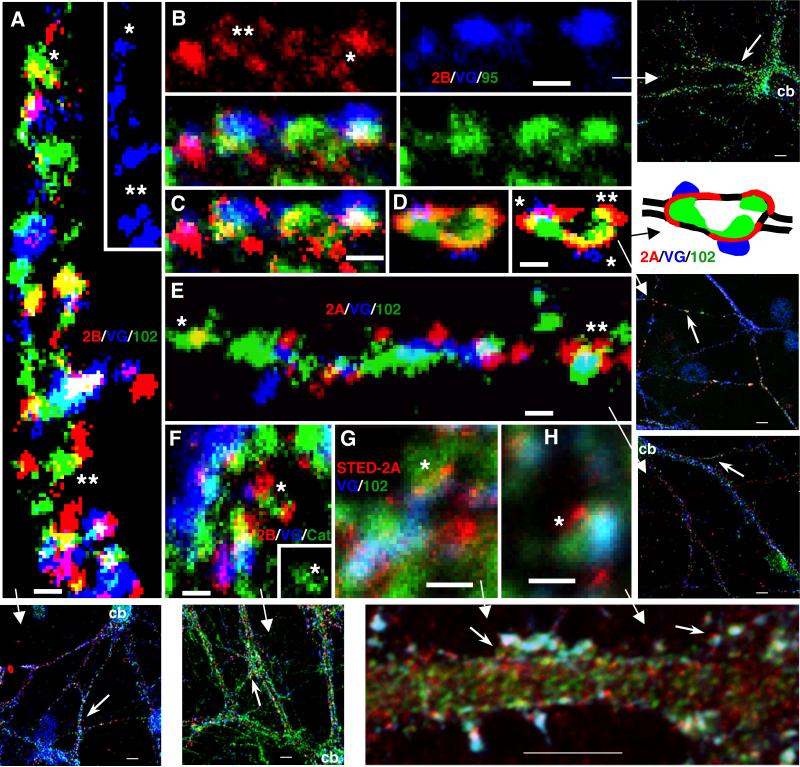
Figure 1.
Triple immunofluorescence labeling of hippocampal cultures using live surface labeling of NR2A or NR2B (555; red), followed by labeling for the presynaptic marker VGLUT (647; blue), and a third protein (488; green) including PSD-95/93, SAP102 (Neuromab), pan-cadherin (P-cad), N-cadherin (N-cad), or catenin. A. NR2A/VGLUT/PSD-95/93; B. NR2B/VGLUT/N-cadherin; C. NR2B/VGLUT/pancadherin. A and B are examples of thick dendrites near the cell soma (shown in upper right) and C shows a thinner dendrite that is more distal to the soma. A and C are fused stacks of confocal sections. B is a single confocal section; note how the cadherin-labeled glial processes seem to enwrap the dendrites directly (even evident in a single section). D1-3. Portion (based on averages of data from neurons) of extrasynaptic NR2s that colocalized with other proteins; NR2A is shown in blue and NR2B in red. There were no significant differences between NR2A and NR2B for any protein. There were no significant differences between proximal (thick) and distal (thin) dendrites except for NR2B with PSD-95/93 (D3; asterisks; p=0.004). For statistical analyses in D1 and D3, N=15 neurons per bar; for D2, N=15 neurons per bar for PSD-95 and SAP102 and 3 neurons per bar for pan-cadherin and N-cadherin. E. Ratio of synaptic to extrasynaptic puncta intensity for PSD-95/93 and SAP102 using Volocity (intensity based on 4095 gray level scale for 12 bit images). Note that extrasynaptic puncta of PSD-95/93 are much less intense than synaptic puncta, as compared to SAP102 (p=6.74×10−6). F. Comparison of synaptic (Sy) and extrasynaptic (Ex) puncta size (μm3) in transfected 2 WIV and 3 WIV NMDARs and in native 3 WIV NMDARs (colabeled with antibodies to PSD-95/93 (95) or SAP102 (102) as indicated; data combined for NR2A and NR2B-see methods). Note that synaptic puncta are larger than extrasynaptic puncta (p<0.01). Scale bars are 5 micrometers.
Figure 2.
High magnifications of parts of micrographs (additionally processed for brightness and contrast) from triple immunofluorescence labeling of hippocampal cultures with 1) live surface labeling of NR2A (red; 555 for D,E; 647 [STED] for G,H) or NR2B (red-555; A-C,F), 2) the presynaptic marker VGLUT (blue; 647 for A,D-H; 488 for B,C), and 3) a third protein (green; 488 for A,D-H; 647 for B,C) including PSD-95/93 (B,C), SAP102 (Neuromab; A,D,E,G,H), or catenin (F). A. NR2B/SAP102: note colocalization in areas devoid of VGLUT labeled terminals (shown also alone in the inset)-some appear to be real associations (*) while others may be coincidental (**). B,C. NR2B/PSD-95/93: NR2B forms in a perisynaptic ring (**) around synaptic PSD-95/93 and the terminal, and forms a ring of 3 puncta around an extrasynaptic punctum of PSD-95/93 (*); C is the same image, with the NR2B labeling shown in higher contrast. D. NR2A/SAP102: this is an enlarged region found along a thin, distal dendrite. Note how NR2A labeling is spread in the perisynaptic regions surrounding 2 synapses (*)-SAP102 forms around the enlargement in conjunction with both the synaptic and extrasynaptic (**) NR2A; the right image is a high contrast version of the left one. In the diagram, the outline of the thin dendrite is shown as black lines. E. NR2A/SAP102: NR2A forms a partial perisynaptic ring around synaptic SAP102 (**); in another example (*), the colocalization of NR2A with an elongate punctum of SAP102 may be coincidental (see text). F. NR2B/catenin: 2 NR2B puncta, one synaptic and one extrasynaptic, associate with an elongate punctum of catenin (*; seen alone in the inset). G,H. NR2A[STED]/SAP102: synaptic or perisynaptic puncta (*) of NR2A can be seen at slightly higher resolution than in the confocal images. Scale bars are 500 nm.
Antibodies against cadherin and catenin showed an interesting pattern of light to moderate labeling in the dendrites and dense labeling in glial processes, which could be seen to enwrap the dendrites. Dendritic labeling, as for PSD-95 and SAP102, showed substantial overlap (~ one third) with the extrasynaptic puncta of native NR2A and NR2B labeling with no significant differences seen between NR2A and NR2B.
We also compared the patterns of co-distribution of scaffolding and adhesion proteins with extrasynaptic native NR2A and NR2B in the proximal dendrites with those found at a distance from the cell body. Proximal dendrites that are near the cell body tend to be thick (<105 μm from the soma; average 1.9 μm in diameter) whereas more distal ones are generally thinner (>105 μm from the soma; average 0.9 μm in diameter). This comparison was important because using LM for visualization, it is difficult to distinguish between proteins that are attached directly to the cell surface from those that are situated at various levels below the plasma membrane in the cell cytoplasm. This difficulty is greater for thicker dendrites even when using confocal sections. The portion of extrasynaptic NR2B that overlapped with PSD-95/93 was significantly higher (Fig. 1D3) in thicker versus thinner dendrites. No other significant differences between thick, proximal versus thin, distal dendrites were evident. This was in contrast to proteins colocalizing with synaptic NR2s. For thin, distal dendrites, it was more common for SAP102 or pan-cadherin to colocalize with NR2A than with NR2B (Fig. S1). In comparisons of NR2A-containing synapses on thick, proximal versus thin, distal dendrites, colocalization of NR2A with PSD-95/93 was more common in thick, proximal dendrites, and colocalization of NR2A with SAP102 was more common in thin, distal dendrites (Fig. S1). On thick, proximal dendrites, colocalization of NR2B with PSD-95/93 was more common compared to co-distribution in distal dendrites (Fig. S1). Other associations were not significant (Fig. S1).
Punctal size was always significantly larger for synaptic compared to extrasynaptic NR2A and NR2B puncta (Fig. 1F). We did not see any significant difference in punctal size between native and transfected NMDARs, although we did find that transfected NMDAR puncta were larger for both synaptic and extrasynaptic receptors at 2 WIV compared to 3 WIV (data not shown). Native NMDARs at 2 WIV were not studied in detail. Other aspects of transfected NMDAR distribution were not examined in detail with LM in this study, although overall patterns of labeling were similar to native.
We also examined some triple-labeled sections in greater detail to investigate possible morphological evidence for the association of native extrasynaptic NMDARs with PSD-95/93, SAP102, cadherin and catenin. Typically, native NR2A- or NR2B-labeled synaptic puncta were large and showed substantial overlap with the puncta produced by VGLUT (Fig. 2). However, in some cases, smaller puncta were resolved. These could form a partial ring around a VGLUT punctum. This suggests that these represent perisynaptic locations or possibly, small groups of receptors near the inside border of the synaptic active zone. Similarly, examples were found of partial rings of small puncta for NR2A or NR2B where there was no overlap with a VGLUT punctum, i.e., extrasynaptic NMDARs. Note in the example in Fig. 2B and Fig. 2C how these extrasynaptic red puncta surround a punctum of PSD-95/93. This supports the idea that there is some direct association between NMDAR and PSD-95/93 puncta. These NMDAR labeled puncta were at the minimum size that can be resolved with LM. They are probably real structures since they were seen with 4-frame averaging and fusion of the confocal stack. However, they would be better characterized if resolution was improved. Thus, a preliminary study using the Leica STED microscope was carried out. This gave superresolution for one color in the x and y axes. We used the superresolution for live surface labeling of NR2A receptors co-labeled with VGLUT and SAP102 (the latter 2 were at standard confocal resolution only). Now, numerous fine puncta as small as about 100 nm, including some that may be perisynaptic around presynaptic terminals were observed (Fig. 2G, H; compare with perisynaptic SAP102 immunogold labeling in Fig. S2F).
Thus, the in vitro LM investigation revealed that extrasynaptic NMDARs show some close associations with the MAGUKs, PSD-95 and SAP102, as well as with the adhesion proteins, cadherin and catenin.
EM immunoperoxidase studies of live surface labeling for NMDARs in vitro
The LM in vitro studies were extended to the EM level to identify the specific structures associated with surface extrasynaptic NMDARs. In order to study the definitive distribution of extrasynaptic surface NMDARs in cultures of hippocampal pyramidal neurons, we developed a method for correlating the LM and EM localizations of immunoperoxidase labeling using live, surface labeling of native or transfected NR2A and NR2B-containing receptors (Figs. 3, 4). This involved firstly at the LM level, the identification and photographing of labeled neurons (Fig. 3A) followed by sectioning of the labeled neuron for EM studies. Visualization was via the regular procedure and with silver/gold toning (see Methods).
Figure 3.
LM/EM correlation of immunoperoxidase/DAB live surface labeling of a hippocampus culture 2 WIV, transfected with myc-NR2B and labeled with myc antibody. Extrasynaptic sites of labeling are indicated by arrowheads, p=presynaptic terminal, asterisk=postsynaptic membrane/density, sp=spine, and d=dendrite shaft; note also vesicle clusters (v) and microtubules (small arrows) in axons. The light micrograph in A shows the neuron cell body and major dendrites. The very small letters in A (b-h) indicate the positions of the EM micrographs in B-H. Note especially the labeled contacts between axons and the cell body (E,H) or dendrites (C,G). Some contacts appear to be early synaptic contacts (arrowheads: C-bottom and G-2nd from top) while others are definitive synapses (asterisks in B,F,D). In some cases, endosomes (e) can be seen just subjacent to a labeled contact, as in the top example in E. Scale bar is 10 μm in A, 500 nm in C,E, and G, and 100 nm in B,D,F, and H.
Figure 4.
Examples of EM immunoperoxidase/DAB live surface labeling of a hippocampus culture 3 WIV, labeled with NR2A (A,B) or NR2B (D-F) antibody or control lacking the primary antibody (C; same experiment as for NR2A (A,B)). Extrasynaptic sites of labeling are indicated by arrowheads, p=presynaptic terminal, asterisk=postsynaptic membrane/density, sp=spine, and d=dendrite shaft. The labeled small process to the right of the spine in D is probably a filopodium or a small spine. Note as in figure 3 the labeled contacts between a large dendrite and presumptive axons running parallel to it (B,E,F; with examples of microtubules labeled with very small arrows). Scale bar is 100 nm in A-D,F and 2 micrometers in E.
Generally, the distribution of the immunoperoxidase/DAB reaction product was seen in patches on the extrasynaptic surface of dendrites and spines. When a patch was at a point of contact with another process, the reaction product was associated with the membranes of both the process and the dendrite or spine surface. This is expected since the chromagen reaction is associated with labeling of extracellular epitopes. Thus it is localized within the narrow cleft between processes. The neuron in Fig. 3 shows a hippocampal pyramidal neuron that was transfected with the myc-NR2B clone and live surface labeled with anti-myc antibodies at 2 WIV. Typically, the main dendrites of a large neuron in culture were covered with bundles of thinner processes that mostly run parallel to the length of the dendrite. These processes included axons, dendrites and glial processes. The most common were axons. At the EM level, these axons could be identified definitively by following them along their length to areas with large numbers of synaptic vesicles (Fig. 3C, H). Other presumptive axons were identified by their straight, non-tapering profile and the fact that they are filled with microtubules (Figs. 3E, H, 4B, E, F). Distinctive examples of labeling between presumptive axons and dendrites are shown in Fig. 3C, G; examples with the soma are shown in Fig. 3E, H, and at spine synapses in Fig. 3B, F. In all these examples, as described for the LM studies, some labeling extended into the perisynaptic region. Synapse identification was not always definitive and it may be that some apparent associations represent early synaptic contacts. In some cases, endosomes were seen just subjacent to the surface labeling (e.g. Fig. 3E). Analysis of 65 extrasynaptic labeled regions (as noted above, each of these regions would be equivalent to a punctum identified with LM) on the dendrites and cell body of this neuron (taken from several serial sections) showed that more than half of the extrasynaptic labeled regions were adjacent to axons or presumptive axon profiles. Most other profiles could not be identified. These extrasynaptic labeled regions ranged in length from 51 - 768 nm (mean = 251 ± 18 nm; Table 1). Synaptic or possible synaptic labeled regions ranged from 128 - 384 nm (mean = 221 ± 32 nm). In an analysis of 35 labeled regions along a single dendrite profile in a 2 WIV culture transfected with the flag-NR2A clone and live surface labeled with anti-flag antibodies, ~25 % of the extrasynaptic regions were between the dendrite and adjacent axons. Approximately 50% of the regions were between at least 1 axon and another process in the bundle surrounding the dendrite (data not shown). For this single dendrite profile, the extrasynaptic, labeled surface regions ranged from 50-560 nm (mean = 227 ± 19 nm; Table 1).
Table 1.
Width of labeled region of extrasynaptic NMDARs using immunogold localization of NR1 in CA1 stratum radiatum of hippocampus, or DAB/immunoperoxidase of hippocampal neuronal cultures
| Experiment* | Average in nm (N=) | Range in nm | Combined averages |
|---|---|---|---|
| Gold particles | 25 (77) | 5-93 | |
| Flag-NR2A-in vitro | 227 (35) | 50-560 | |
| Myc-NR2B-in vitro | 251 (65) | 51-768 | Transf. 2A+2B=243# |
| NR2A-in vitro | 198 (50) | 51-538 | |
| NR2B-in vitro | 186 (69) | 51-602 | Native 2A+2B=191# |
Gold particle clusters of 2-6 5 nm gold particles were counted from 13 experimental groups on 2 animals. Live surface NR2A or NR2B labeling was counted from 2 experiments.
p=0.00127 for transfected NR2 subunits versus native NR2 subunits.
For labeling of native NMDARs in cultures, 2 studies were completed (Fig. 4). For these, we did not concentrate on a single labeled neuron for the LM and EM correlation as for the exogenous NMDARs since the native receptor labeling was widespread in cultured neurons. Anti-NR2A (Fig. 4A, B) and anti-NR2B antibodies (Fig. 4D - F) usually produced distinctive labeling at extrasynaptic sites. These included the side of spines (Fig. 4A, D) and sites along the dendritic shaft (Fig. 4B, E, F). The extrasynaptic labeled regions ranged from 51 - 538 nm in length (mean = 198 ± 12 nm; N = 50) and 51 - 602 nm (mean = 186 nm ± 11; N = 69) for NR2A and NR2B, respectively. These mean lengths are larger than those found with immunogold labeling (Table 1; see also, in brain sections, below). This is probably due to the greater sensitivity of immunoperoxidase labeling (Petralia, 1997; Petralia and Wenthold, 1998; further references in the supplemental text) as well as some lateral diffusion of the DAB reaction product. No significant differences were found for the two measurements of extrasynaptic puncta between exogenous flag-NR2A and myc-NR2B and between the NR2A and NR2B native receptors. There was however a significant difference between the combined totals of exogenous (flag-NR2A+myc-NR2B; mean = 243 ± 13 nm; N = 100) versus native (NR2A+NR2B; mean = 191 ± 8 nm; N=119) extrasynaptic NR2 regions (p = 0.00127). By comparison, identified synaptic labeled regions for native receptors ranged from 115 to 369 nm (mean = 184 ± 20 nm; N=13) and 128 to 333 nm (mean = 223 ± 13 nm; N=23) for NR2A and NR2B, respectively. Thus, the average size of synaptic and extrasynaptic sites were similar. The greater range of the extrasynaptic labeling may reflect the lack of restriction of an extrasynaptic region to a limiting area such as that defined by a PSD.
In summary, in vitro EM analysis revealed that extrasynaptic NMDARs are on sides of spines and along dendrite shafts. The vast majority of these sites are at contact points with processes adjacent to the neuron the most common of which are axons and axon terminals.
Electron microscope localization of NR1 in brain sections
Distribution of cell surface proteins in neuronal cells in culture is not necessarily representative of their distribution in vivo in the brain itself. We examined therefore the distribution of NMDA receptors in sections from the CA1 stratum radiatum of the hippocampus of 3 P37 (Fig. 5), 2 P10, and 2 P2 (Fig. 7) rats using post-embedding immunogold methods with an antibody directed against the NR1 C2 C-terminal exon. NMDA receptor distribution was also determined in 3 P35 rats using pre-embedding immunoperoxidase/DAB methods and the anti-NR1 C2 antibody (Fig. 6). This replicates the methodology that was used for the in vitro labeling
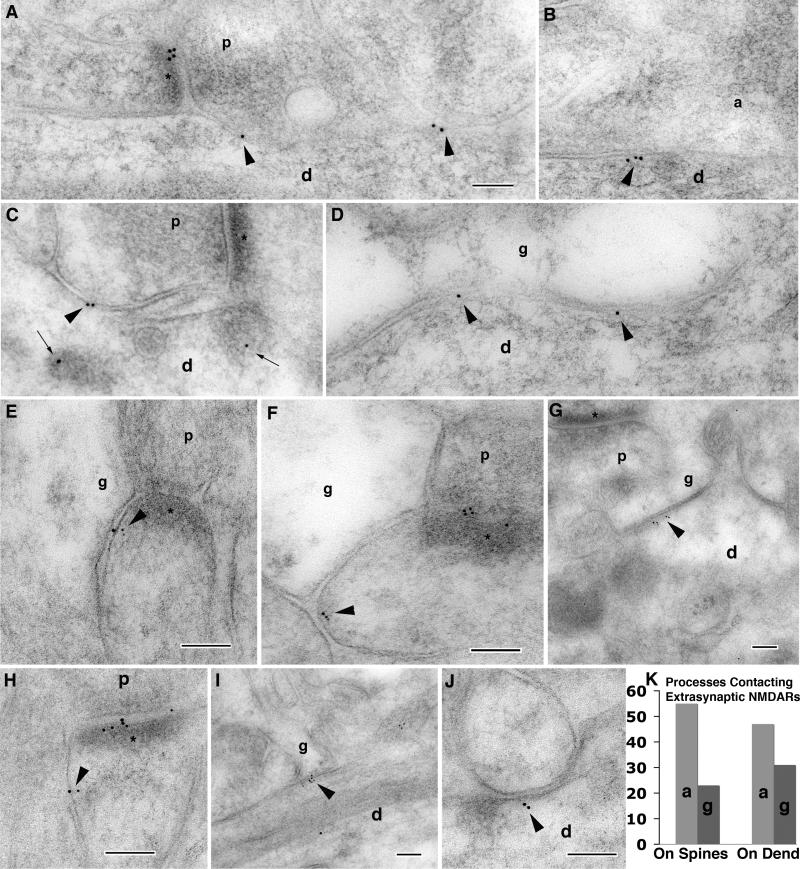
Figure 5.
Immunogold labeling with NR1 antibody in the adult hippocampus CA1 stratum radiatum (10 nm gold in A-D and 5 nm gold in E-J). Extrasynaptic or perisynaptic sites of labeling are indicated by arrowheads; p=presynaptic terminal, asterisk=PSD, d=dendrite shaft, a=axon terminal, and g=presumptive glial process. In A and C, the axon terminals contacting the gold labeling on the dendrite are presynaptic terminals of adjacent spine synapses. In C, the gold-labeled structures (small arrows) in the cytoplasm are associated with an endosomal complex that is out of the micrograph to the right. Note also the slightly dense material present at extrasynaptic sites in F and G. K. Analysis of 146 extrasynaptic sites of 5 nm immunogold labeling (263 gold particles: 1-6 per site) on postsynaptic spines or on dendrites that were associated with axon/axon terminal-like (a) or glia-like (g) processes (see text). Scale bars are 100 nm in A for A-D, and in E-J.
Figure 7.
Immunogold labeling with NR1 antibody in the P2 hippocampus CA1 stratum radiatum (5 nm gold). Extrasynaptic or perisynaptic sites of labeling are indicated by arrowheads; p=presynaptic terminal, asterisk=PSD, and d=dendrite shaft. NR1-labeled extrasynaptic densities are seen in A-F, and labeled clathrin-coated pits and associated endocytic structures are seen in G and H. Densities in A and C are relatively light and probably are contacted by axon growth cones (these contain mainly diffuse material and some large vesiculate structures [portions not shown in figure]), while those in B and E represent “bare densities” (see text). In D, a labeled dense region appears to be anchored by a bundle of filaments roughly similar in size to the gold particles, and thus probably representing actin filaments. Compare to the abundant filaments in the spine in F; in this example, the oblique PSD is labeled, but labeling also is present in an oblique density on a contacting dendrite. The synapse in G is probably on a short protrusion on a dendrite shaft (portion not shown in figure). Scale bars are 100 nm, in A for A-E,H, in F for F and inset (in G), and in G for G only.
Figure 6.
EM immunoperoxidase/DAB labeling with NR1 antibody in the adult hippocampus CA1 stratum radiatum; those in E-H were processed further with silver/gold toning. Extrasynaptic or perisynaptic sites of labeling are indicated by arrowheads; p=presynaptic terminal, asterisk=PSD, d=dendrite shaft, and e=labeled endosomes/endocytic vesicles and other tubulovesicular structures. Note the distinctive axon terminals (a) that contact extrasynaptic or perisynaptic sites of labeling in A,B,C,H. Presumptive glial processes (g) contact these sites in D and F. Note also the nearly identical pattern of labeling on the side of the spine in D and E using different methods in different animals. Scale bar is 100 nm in A for A,D, in B for B,C,E,F, and in H for G,H.
Postembedding immunogold labeling for NR1 in the adult hippocampus showed abundant labeling with 5, 10, or 15 nm gold secondary antibodies in the PSDs with less labeling at extrasynaptic sites. Extrasynaptic immunogold labeling in dendrites (Fig. 5A-D, G, I, J) and spines (Fig. 5E, F, H) was usually adjacent to other cell processes. Often, these could be identified as axons/axon terminals (Fig. 5A - C) or presumptive glia (Fig. 5D – G and I; defined in Methods; also see the supplemental text concerning the glial marker, GFAP). In most cases, there was no obvious density present under the membrane in these areas. Occasionally, a faint density was evident when compared to adjacent PSDs (Fig. 5F, G).
The size and distribution of extrasynaptic immunogold labeling for NR1 was quantified in this adult tissue (Fig. 5K; Table 1). A total of 146 extrasynaptic sites of immunogold labeling (5 nm gold particles; 263 particles) on dendrites (68 sites) and postsynaptic spines (78 sites) in the CA1 stratum radiatum of 2 P37 rats were analyzed. Of the sites on spines, 35/78 were within 100 nm of the PSD (i.e., they were perisynaptic). Nearly all of the extrasynaptic sites were in close contact with an adjacent process. Using the criteria to define axon/axon terminal-like or glia-like described earlier, for extrasynaptic/perisynaptic contacts on postsynaptic spines, 55% and 23% were adjacent to axon/axon terminal-like and glia-like processes, respectively compared to 47% and 31% for contacts on dendrites (Fig. 5K). The remaining associated processes included postsynaptic spines, dendrites or were not identified. For those sites that contained more than 1 gold particle (i.e., N = 77 sites with 2-6 particles), the gold particles were found along an average length of 24.6 nm of the cell membrane (range: 5.3 - 92.7 nm; Table 1). Thus, all of the patches seen suggest that the extrasynaptic sites are smaller than synapses, which when identified by morphology in the CA1 stratum radiatum are about 195 nm in length (Tao-Cheng et al., 2007).
Gold labeling for NR1 in a random sample of synapses from the CA1 stratum radiatum was also studied. It was noted that the spread of gold labeling in a synapse covered only a portion of the width of the synapse. The density of labeling was 0.99 ± 0.08 gold particles per postsynaptic membrane profile (N = 281 synapse profiles from two P37 rats with no significant difference between animals). This value is similar to that reported previously for NR1 in synapses (Petralia et al., 1999). These synapse profiles also had 0.33 gold particles per extrasynaptic membrane (0.22 ± 0.04 for perisynaptic plus 0.11 ± 0.03 for the remainder of the extrasynaptic membrane). A sample of mediumsized dendritic profiles in the same sample area (N = 26 profiles from 2 animals, average dimensions 1.5 × 4.8 μm) had up to 6 gold particles on the extrasynaptic membrane (mean = 2.08 ± 0.37 gold particles per profile). While immunogold labeling is not a good measure of the absolute quantity of receptors due to its insensitivity (Petralia et al., 1999), this suggests that a portion of NMDARs is found outside the postsynaptic membrane on spines and dendrite.
For the immunoperoxidase labeling experiments, the distribution of NR1 was examined first with LM. This showed a wide distribution of labeling throughout the brain as described previously using a polyclonal antibody made to the identical region of NR1 (data not shown; Petralia et al., 1994b). At the EM level, the DAB reaction product was localized to subregions of the dendrites and spines (Fig. 6). For NR1 labeling of postsynaptic spines, many spines showed only light labeling in the PSD but dense labeling in the membrane on the side of the spine (Fig. 6B-F; the examples shown are from 3 animals), indicating that NR1 is localized to the perisynaptic/extrasynaptic areas. Examples of dense labeling in the PSD are not shown (except for moderately dense labeling in Fig. 6B), but have been published previously for NR1 and NR2 antibodies using immunoperoxidase/DAB (Petralia et al., 1994a,b, 1996). These papers also included some examples of perisynaptic or extrasynaptic labeling on spines, although they were not described in the text (see also Aoki et al., 1994). Note particularly in Fig. 6D and 6E, the nearly identical shape and position of the labeled area from 2 different animals using 2 different methods. Similar patches of labeling could be found in discrete areas of the dendrite surface membrane (Fig. 6A, G, H; examples shown are from 3 animals). The typical widths of these patches of labeling on dendrites and spines were ~ 100 - 200 nm (Fig. 6); the smallest were ~30 nm. In most cases, labeled patches of membrane were contacted directly by an adjacent process. Most contacts appeared to be with axon terminals (Fig. 6A, B, C, H) or glial processes (Fig. 6D, F; as described for immunogold above). In dendrites, some surface membrane patches of labeling were associated with labeling in adjacent tubulovesicular structures within the dendrite (Fig. 6G, H; examples shown are from 2 animals). These may be endosomal structures (Washbourne et al., 2004; Wang et al., 2008) or other organelles (such as modified components of the endoplasmic reticulum, Golgi, or trans-Golgi network) involved in trafficking of NMDARs in dendrites (Sytnyk et al., 2004; Jeyifous et al., 2009).
We also performed semi-quantification of labeling from sections from two animals, using the silver/gold toning immunoperoxidase method. About thirteen percent of labeled spine synapses (N = 62) showed silver/gold labeling throughout the spine, while ~37% showed preferential labeling of the extrasynaptic surface and ~50% showed preferential labeling of the extrasynaptic surface plus the postsynaptic membrane. This indicates that at least one third of labeled spine synapses have extrasynaptic labeling that is not a simple diffusion artifact and thus it probably represents extrasynaptic NMDARs. This is consistent with our immunogold study described above. Also in this material, 79% of labeled dendrite profiles (N =42) showed some labeling on the dendrite surface. All the labeled dendrites showed labeling in endosomes and presumptive endosomal structures.
In contrast to the adult, regions of extrasynaptic NR1 labeling often had associated densities in the hippocampus at P2 (Fig. 7A - F). Similar densities have been described previously, labeled with NR1 (polyclonal), NR2A/B, PSD-95, SAP102 and SynGAP antibodies (Sans et al., 2000; Petralia et al., 2003). Also, similar structures have been described using stronger fixative regimes (Fiala et al., 1998). Many of these regions of extrasynaptic NR1 labeling that have associated densities are not contacted directly by another process, particularly those that have fairly dense densities (Fig. 7B, E). These are called “bare densities” and they are possibly remnants of former synapses (Sans et al., 2000). Other regions of extrasynaptic NR1 labeling that have associated densities have light densities and these may represent new contacts with axonal growth cones that will develop into synapses (Fig. 7A,C). Surface labeling with the NR1 antibody was associated with clathrin-coated pits and associated endocytic structures at P2 (Fig. 7G, H) as noted previously using another NR1 and NR2A/B antibodies (Petralia et al., 2003). Other labeled regions of extrasynaptic NR1 were similar to those described in the adult, but overall processes were more difficult to identify due to their immature state, and less of the surface of processes was in contact with other processes at this age. Thus a detailed classification of these contacts was not practical for this study (note: we also carried out some labeling at an intermediate age between P2 and adult, i.e. P10 using immunogold; see supplemental text).
Thus, localization of extrasynaptic NMDARs in vivo resembles that seen in vitro with close associations of these sites with adjacent processes, particularly axons and axon terminals, but also with glia and other processes such as dendrites or postsynaptic spines. This distribution was corroborated by two methods, immunogold for greater specificity of localization and immunoperoxidase for greater sensitivity. The study also revealed that early postnatal ages showed evidence of distinct dense material at many of the extrasynaptic NMDAR sites compared to adults.
EM studies of proteins associated with NMDARs
This study so far has revealed the specific localizations of extrasynaptic NMDARs bothin vitro and in vivo. In this section, we extend our earlier in vitro LM studies to investigate the association of extrasynaptic NMDARs with the scaffolds PSD-95/93, SAP102 and the adhesion proteins, pan-cadherin, N-cadherin, and catenin at the EM level. Double-labeling immunogold and single labeling immunoperoxidase methods were both used.
Catenin showed distinctive labeling patterns consistent with its known distribution (Fig. 8). Immunogold double labeling revealed that catenin was co-distributed with extrasynaptic NMDA receptors (Fig. 8A-F; n = 2 animals). Similar distributions were found in cultured hippocampal neurons at 3 WIV (Fig. 8G, H). Labeling patterns appeared to be similar using anti-N-cadherin and anti-pan-cadherin antibodies, although the labeling was not as strong or definitive as observed with antibodies directed against catenin (for synapses see Petralia et al., 2005). This localization represents the association of NMDARs near cadherin/catenin bridges that are used for adhesion at contact points.
Figure 8.
Immunogold double labeling with NR1 antibody (5 nm) and β-catenin (15 nm), in the adult hippocampus (A-F) or in hippocampal culture (G,H; 3 WIV). Extrasynaptic or perisynaptic sites of labeling are indicated by arrowheads; p=presynaptic terminal, asterisk=PSD, and d=dendrite shaft. In B, double labeling is seen between the neck of one spine and the perisynaptic region of another, more oblique spine. In D and E, the processes adjacent to the double-labeled sites on dendrites are axon terminals with clusters of vesicles. The process that contacts the dendrite in G appears to be an axon (contains some synaptic-like vesicles in portion not shown); note how the membranes come closer together at the point of double labeling. Scale bars are 100 nm in A for A,B,D-H), and in C for C only.
The anti-PSD-95 antibody that works well for EM immunogold labeling results in a distribution of immunoreactivity that is highly localized to PSDs in the adult hippocampus (Sans et al., 2000; Yi et al., 2007). Immunoreactivity is also prevalent in distinctive densities, such as in “bare densities” in the P2 hippocampus and in extrasynaptic attachment plaques found in glomeruli in the adult cerebellum (Petralia et al., 2002). Consequently, here little anti-PSD-95 immunoreactivity was found on the extrasynaptic regions of neurons in vivo or in vitro (which show little or no density as noted above), although occasional examples were seen (Fig. S2A-C). The SAP102 antibody that works well for EM immunogold labeling is an antibody raised in rabbits; thus double labeling with 2 rabbit antibodies is problematic. However, some double labeling for SAP102 and NR1 subunits was carried out using an NR1 mouse monoclonal antibody (Fig. S2D, E). Double labeling for SAP102 and PSD-95 also was carried out (Fig. S2F). These studies revealed some distinctive extrasynaptic sites of single labeling for SAP102 (Fig. S2D,F) that were similar to extrasynaptic sites seen for NR1 labeling. In addition, we did preembedding immunoperoxidase/DAB of the ABR PSD-95/93 or the Neuromab SAP102 (that were used for immunofluorescence) in brain sections of 2 adult rats (Fig. S3). At the LM level, neuronal labeling for either antibody was distinctive in the hippocampus and many other regions of the brain. Control sections were unlabeled (data not shown). In the pyramidal neurons of the hippocampus, labeling in apical dendrites was moderate for PSD-95/93 and dense for SAP102. Both antibodies also appeared to label the mossy fibers of the stratum lucidum. With EM, labeling in the hippocampus (CA1stratum radiatum; CA3 stratum lucidum) for PSD-95/93 was particularly abundant in postsynaptic spines (Fig. S3A). Labeling was also seen in dendrites and in some axon terminals with some labeling at extrasynaptic sites (Fig. S3B). For SAP102, compared to PSD-95/93, labeling density and frequency were higher in dendrites and lower in spines (Fig. S3C-E). SAP102 dendrite labeling was often concentrated in patches associated with transport vesicles where the labeling frequently extended to the adjacent dendrite cell membrane. It was also seen at distinct extrasynaptic sites (Fig. S3E). Similarly to PSD-95/93, labeling was found in some axon terminals. Overall, the immunoperoxidase/DAB data were consistent with immunofluorescence data that showed that SAP102 labeling is more prevalent in dendrites than PSD-95/93 labeling.
Thus, in this section, we showed that sites with extrasynaptic NMDARs can be associated with adhesion proteins. This is probably relevant especially since these sites are usually at contact points with adjacent processes. MAGUKs such as SAP102 and PSD-95 can associate with extrasynaptic NMDARs. This probably corresponds to the colocalizations of extrasynaptic NMDARs and MAGUKs shown with in vitro LM studies described above.
DISCUSSION
In this study, we combined LM and EM studies in brain and in vitro to show that extrasynaptic NMDARs are localized to discrete locations along dendrites and postsynaptic spines. In most cases, extrasynaptic NMDARs were found accumulated at points in close contact with adjacent cell processes including mainly axons/axon terminals or glia. This is consistent with previous studies showing extrasynaptic membrane localizations of immunogold for NMDARs (Valtschanoff et al., 1999), which includes NMDARs on extrasynaptic dendrite membrane areas apposed to astrocyte processes (Kharazia and Weinberg, 1999; Bezzi et al., 2004). With EM, in adults these locations seldom show a distinctive PSD-like structure. At P2, numerous extrasynaptic contacts show a distinct PSD-like structure and these structures probably include both early contacts that will mature eventually into definitive synapses, and “bare densities” that may be remnants of former synapses. Extrasynaptic contacts were associated with adhesion proteins. This was apparent for both EM and LM studies. In addition, with LM, extrasynaptic NR2A or NR2B containing NMDARs colocalized with the MAGUKs, PSD-95 and SAP102 (Fig. 9).
Figure 9.

Diagram illustrating the synaptic and extrasynaptic distributions of NMDARs and associated scaffolding and adhesion proteins, and especially the associations of extrasynaptic NMDARs with adjacent cell processes (see text for details).
Identification of extrasynaptic NMDAR sites
Identification of extrasynaptic NMDAR sites is not straight-forward and it is subject to possible artifacts using either EM or LM. With EM of the adult or developing brain, there is usually no question that the identified sites are extrasynaptic since these sites lack a synaptic cleft, vesicles near a presynaptic active zone and a PSD. But, sometimes it is difficult to ascertain if the extrasynaptic site is perisynaptic rather than at a greater distance from a synapse since, in order to include a large sample population, we look at single profiles of any area, rather than a 3D reconstruction of the adjacent areas. For LM of hippocampal cultures, where synaptic sites are defined by overlap with the presynaptic marker, VGLUT, detection of definitive extrasynaptic NMDAR sites is more problematic because of the inherent low resolution of LM. LM studies in vitro in the current work indicate that synaptic and extrasynaptic NMDARs are about equally common. This is consistent overall with other studies (see Introduction). However, it is not clear if absence of VGLUT overlap means that there are no vesicles in adjacent processes or that there are just a few vesicles. In the latter case, e.g., sites of ectopic release, VGLUT may not label above background (see below for further discussion). Similarly, early synaptic contacts in the hippocampal neuronal cultures corresponding to those described in the P2 brain here, may also not label sufficiently with VGLUT. These may constitute a subset of our defined extrasynaptic NMDAR sites seen in vitro. Another point to consider is that the VGLUT antibody that was used here was made to VGLUT1. It should label the vast majority of glutamatergic synapses especially since we studied mature hippocampal cultures. It is possible however that a few of our identified extrasynaptic sites were actually synapses containing only VGLUT2 or VGLUT3 (Wojcik et al., 2004; De Gois et al., 2005). The common associations of synaptic vesicle-filled structures with extrasynaptic NMDARs, as described here with both in vivo and in vitro EM, suggests that a subset of the sites that we defined as synaptic with LM may be extrasynaptic sites of NMDARs associated with adjacent axon terminal structures.
There was no distinctive difference in distribution of NR2A and NR2B at extrasynaptic sites. In contrast, a number of studies have indicated that NR2B-containing NMDARs are more abundant in extrasynaptic sites. However, consistent with our findings, Harris and Petit (2007) found in acute P14-21 hippocampal slices that synaptic and extrasynaptic sites have similar levels of functional NR2B-containing NMDARs. Nevertheless, even the same extrasynaptic and synaptic NMDARs may be associated with different sets of proteins, leading to differences in response and plasticity of function (e.g., van Zundert et al., 2004; Kim et al., 2005; Hardingham, 2006; Newpher and Ehlers, 2009).
The size of extrasynaptic NMDAR sites
The width of extrasynaptic sites seems to vary more than that of synapses. At the EM level, it may be underestimated by the immunogold labeling but overestimated by the immunoperoxidase method. The smallest identified labeled sites with EM had a single 5 nm gold particle or an immunoperoxidase spread of 30-50 nm, which may represent a single NMDAR molecule. The methods however are not accurate enough to determine this. Since postembedding immunogold labeling can detect only a fraction of the total population of NMDARs (Petralia et al., 1999), it is more likely that a single gold particle represents one NMDAR in a small cluster. Immunoperoxidase is a more sensitive method than the immunogold. Thus the observed 30-50 nm spread could be accurate or it may be due to a bleeding artifact from the labeling of a single NMDAR. The 30-50 nm spread could be 4 NMDARs, since NMDARs are about 20 nm in diameter (e.g., Chen et al., 2008) and assuming a roughly round cluster area seen in profile. Larger spreads of immunoperoxidase staining could represent labeling of larger NMDAR clusters that form along the contact with the adjoining process. An effect on NMDAR surface distribution by adjacent processes may be expected simply due to the large size of the NMDAR, which projects about 10 nm above the cell membrane, i.e., roughly the distance to the adjacent cell membrane in many places. Fluorescence data agreed with the immunogold labeling in that a consistent pattern of larger synaptic regions compared to extrasynaptic regions was found.
It was notable that we observed with EM that transfected NMDAR subunits produce larger extrasynaptic puncta compared to native NMDARs. This is consistent with Groc et al. (2007) who suggested that transfected NMDARs behave differently to native surface NMDARs. They observed that at 10-15 DIV, transfected NR2B shows increased surface trafficking compared to native NR2B (Groc et al., 2007).
Proteins associated with extrasynaptic NMDARs
The lack of a distinctive density structure at most adult extrasynaptic NMDAR localizations suggests that there is no extensive, organized scaffold as is present in the PSD (Blanpied et al., 2008). Nevertheless, proteins could associate with the NMDARs in these extrasynaptic sites. The PDZ protein, GIPC, may be preferentially localized to extrasynaptic sites (Yi et al., 2007). Other studies have suggested that extrasynaptic NMDARs may (Allison et al., 1998) or may not (Gerrow et al., 2006) associate with extrasynaptic PSD-95. In fact Gerrow et al. (2006) reported extrasynaptic complexes of PSD-95, GKAP and shank, and some of these complexes also contained neuroligin 1. A number of studies have suggested that trafficking of NMDARs to the surface may involve associations with SAP102 (Sans et al., 2003, 2005; Washbourne et al., 2004). There is previous definitive EM immunogold evidence of associations of extrasynaptic NMDARs with PSD-95 and SAP102 in specialized structures noted above such as the “bare densities” of P2 hippocampus (Sans et al., 2000; Petralia et al., 2003) and the attachment plaques of the adult cerebellum (Petralia et al., 2002). In the present study we were not able to find substantial direct co-distributions of extrasynaptic NMDARs with PSD-95 and SAP102 in the adult hippocampus using EM immunogold. This was largely due to methodological limitations such as steric hindrance and labeling efficiency differences for different gold sizes (Merighi, 1992). However, the EM immunogold studies do indicate that SAP102 may be present in extrasynaptic sites that are similar to those containing NMDARs (see also Aoki et al., 2001). This is consistent with the fluorescence LM results herein. But, fluorescence LM studies are limited by the relatively low resolution of LM. We cannot therefore exclude that most or all of the extrasynaptic colocalizations of NMDARs with MAGUKs, represent MAGUKs in the cytoplasm below the surface NMDARs but too far away to be in direct contact (see Fig. S2E; compare this to the left NR2A/SAP102 punctum in Fig. 2E; also see the EM immunoperoxidase/DAB data for SAP102-Fig. S3C-E). But we do at least observe some examples with fluorescence where the close co-distribution of labeling of NMDARs and MAGUKs suggests that there is a real association between the two. In these cases, however it is still uncertain whether it represents a direct binding of the MAGUK to NMDARs or alternatively, the presence of MAGUKs associated with endosomes or other organelles that are adjacent to the site. PSD-95 has been reported to traffic with tubulovesicular structures (El-Husseini et al., 2000). The putative proximity of NMDARs to the surface with other proteins in adjacent endosomes or other organelles implies a functional association (e.g., Sytnyk et al., 2004; Wang et al., 2008).
Extrasynaptic NR2A and NR2B labeling is associated to a similar degree with PSD-95/93 and SAP102. This may reflect biochemical findings showing that NR2A and NR2B-containing NMDARs associate similarly with PSD-95, SAP102 and PSD-93 (Al-Hallaq et al., 2007). For PSD-95/93, our LM studies revealed that there is less association with extrasynaptic NR2B in thin vs. thick dendrites (Fig. 1D3; maybe for NR2A also but not significant). The simplest explanation for this is that much of the PSD-95/93 labeling is not directly associated with the extrasynaptic NR2s so that less apparent overlap is visualized in thin dendrites. Future studies that can detect the close proximity of these proteins using FRET (fluorescence resonance energy transfer) or FLIM (fluorescence lifetime imaging microscopy; e.g., Lee et al., 2009) may help resolve this question.
Our study suggests that extrasynaptic NMDARs may collect at or near sites of adhesion between the neuron and an adjacent process during development and in adults. This may be part of the mechanism for the formation of excitatory synapses as indicated by the role of the cell adhesion protein, NCAM, in the assembly and remodeling of the postsynaptic signaling complex (Sytnyk et al., 2006). Numerous adhesion proteins can be localized between neurons and adjacent processes (Shapiro et al., 2007; Brose, 2009). One of the most abundant complexes of adhesion proteins contains cadherin plus catenin, which form at many kinds of intercellular adhesions. They are important in development and function of, and interactions between, neurons and glia (Schnädelbach et al., 2000; Yu and Malenka, 2003; Petralia et al., 2005; Rubio et al., 2005; Kanemaru et al., 2007; Jones et al., 2008; Tai et al., 2008; Tran et al., 2008; Xie et al., 2008). They are also involved in functional associations with both NMDARs and AMPARs (Okabe et al., 2003; Saglietti et al., 2007; Silverman et al., 2007; Tai et al, 2007, 2008). Interestingly, the adhesion protein, ephrinB2 (adhesion via linkage to an ephB receptor on the opposite membrane) stabilizes AMPA receptors at cell membranes via mutual interaction with the PDZ protein GRIP. It was suggested that this may control the preferential supply of extrasynaptic AMPA receptors (Essmann et al., 2008). A number of adhesion proteins have been shown to associate directly or indirectly with NMDARs via extracellular N-terminal domains (Dalva et al., 2000; Woo et al., 2006, 2009). The prevalence of axons and axon terminals as processes associated with extrasynaptic NMDARs in our study could be due to interactions of nectin with cadherin-catenin complexes that favor this kind of interneurite affinity (Togashi et al., 2006).
In contrast to the extrasynaptic NMDAR sites in the CA1 stratum radiatum of the hippocampus, as described in the current study, extrasynaptic NMDARs are abundant in structures with very dense and distinctive densities; these are the attachment plaques that connect granule cell dendrites in mossy terminal glomeruli in the cerebellum (Petralia et al., 2002). These structures have abundant cadherins (Rose et al., 1995; Bahjaoui-Bouhaddi et al., 1997) but also have abundant PSD-95 (Petralia et al., 2002) as well as SAP102 (R.S. Petralia, Y.X Wang, and R.J. Wenthold, unpublished observations), suggesting that the NMDARs are held in a scaffold structure similar to that found in synaptic PSDs. Extrasynaptic NMDAR sites in the adult CA1 region of the hippocampus can be contrasted with those seen at P2 as described here. At this age, additional extrasynaptic locations of NMDARs show distinctive structures resembling PSDs. These probably represent a sequence of events in synaptogenesis, starting with early contacts between dendrites and axon growth cones, followed by the earliest distinctive synaptic contacts, then maturing synapses and finally, in some cases, synapse elimination (Sans et al., 2000; Petralia et al., 2003, 2005). This last stage may be represented by “bare densities” (Sans et al., 2000; Petralia et al., 2003). The latter structures have NMDARs, PSD-95, SAP102, and SynGAP, organized in a density that is as developed as the PSDs of distinctive synapses in the neuropil at this age (Sans et al., 2000).
Function of extrasynaptic NMDARs
Our study shows that extrasynaptic NMDARs are positioned in a variety of locations and arrangements characterized by close association with different kinds of adjacent processes and located at different distances from the nearest synaptic active zones (Fig. 9). Presumably the function of these extrasynaptic NMDARs is related to these factors. A number of studies suggest that extrasynaptic NMDARs function differently from synaptic and this may depend on the receptor type as well as that of associated proteins (Kim et al., 2005, Hardingham et al., 2006; Newpher and Ehlers, 2009). Functional extrasynaptic NMDARs presumably must be activated by glutamate spillover or ectopic release (unless some of them function purely as mechanoreceptors as reported by Paoletti and Ascher, 1994; Cahusac et al., 2005). In some cases, such as the NMDARs of the attachment plaques of cerebellar mossy terminal/granule cell glomeruli, the glomerular structure seems to be designed to favor spillover, since glial processes are excluded from the central region of mossy terminal/dendrite synapses (Mitchell and Silver, 2000; Petralia et al., 2002; Rossi et al., 2002, Szapiro and Barbour, 2009). Spillover may also work well where the extrasynaptic glutamate receptors are on a membrane in close proximity to a glutamatergic synaptic cleft (Rusakov and Kullmann, 1998; Alonso-Nanclares et al., 2004; Merchán-Pérez et al., 2009). Spillover is especially important when considering the perisynaptic zone, which as noted above, is a ring ~100 nm out from the postsynaptic membrane. This can be a specialized area with its own set of proteins that include some NMDARs as well as some other types of glutamate receptors and associated proteins (e.g., discussed in Petralia et al., 2005). In some cases, a preferential perisynaptic localization may represent NMDARs that are in a position to be internalized (e.g., Pérez-Otaño et al., 2006) or these may be sites of exocytosis (review by Petralia et al., 2009). Alternatively, receptors in the perisynaptic zone are ideally situated to respond during facilitated evoked glutamate release. They thus constitute a mechanism to provide a different postsynaptic response than that evoked by a minimal release of neurotransmitter that would be limited more to the active zone (reviewed by Köhr, 2006). Thus, in some neurons, neuronal and glial transporters and other factors may limit spillover to the perisynaptic zone. In these cases, the perisynaptic zone may be functionally different from the more distant extrasynaptic zone (Köhr, 2006), where NMDARs would be activated by glutamate spillover only from adjacent active zones or by ectopic release of glutamate from non-active zone sites.
Ectopic release of glutamate has been described for various types of extrasynaptic contacts: 1) from glia onto extrasynaptic NMDARs in the hippocampus (postsynaptic--Fellin et al., 2004, Bergersen and Gundersen, 2009; presynaptic--Jourdain et al., 2007); 2) from the extrasynaptic membrane of presynaptic terminals onto AMPARs on cerebellar Bergmann glia (Matsui et al., 2005); and 3) from white matter axons, often with abundant vesicles, onto glial AMPARs and NMDARs (Kukley et al., 2007, Ziskin et al., 2007) (see also review by Szapiro and Barbour, 2009). In the present study, we were not able to identify specific examples of ectopic vesicular release, but vesicular release profiles would be difficult to find and identify in our material, even at a synapse. Nevertheless, in spite of the difficulty in identifying many of the adjacent processes, it is clear that axons, axon terminals with synaptic vesicles, and glia are common processes found opposite extrasynaptic NMDARs and any of these could be sites of direct release of low levels of glutamate. Interestingly, even at the synapse, spontaneous miniature synaptic events can activate a different population of NMDARs from that activated by evoked glutamate release (Atasoy et al., 2008; Sutton and Schuman, 2009) and those activated by the former may control specific functions, perhaps via discrete signaling pathways (McKinney et al., 1999; Sutton et al., 2007; Sutton and Schuman, 2009).
Supplementary Material
Acknowledgments
We thank Nicole Thompson for help formatting the references, and Dr. Inna A. Belyantseva for helpful comments on the text. Research was supported by the NIDCD Intramural Research Program. A.Z. was funded (in part) by ORWH-FAES NIH.
LIST OF ABBREVIATIONS
- AMPAR
AMPA receptor
- DAB
3,3'-Diaminobenzidine
- DIV
days in vitro
- EM
electron microscope or microscopy
- FLIM
fluorescence lifetime imaging microscopy
- FRET
fluorescence resonance energy transfer
- LM
light microscope or microscopy
- MAGUK
membrane associated guanylate kinase
- NGS
normal goat serum
- NMDAR
NMDA receptor
- PBS
phosphate-buffered saline
- PSD
postsynaptic density
- Px
x postnatal days
- STED
stimulated emission depletion microscope
- TBST
tris-buffered saline plus 0.1% Triton X-100
- WIV
weeks in vitro
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Nanclares L, Minelli A, Melone M, Edwards RH, Defelipe J, Conti F. Perisomatic glutamatergic axon terminals: a novel feature of cortical synaptology revealed by vesicular glutamate transporter 1 immunostaining. Neuroscience. 2004;123:547–556. doi: 10.1016/j.neuroscience.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. doi: 10.1002/syn.1047. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahjaoui-Bouhaddi M, Padilla F, Nicolet M, Cifuentes-Diaz C, Fellmann D, Mege RM. Localized deposition of M-cadherin in the glomeruli of the granular layer during the postnatal development of mouse cerebellum. J Comp Neurol. 1997;378:180–195. [PubMed] [Google Scholar]
- Bergersen LaVG. Morphological evidence for vesicular glutamate release from astrocytes. . Neuroscience. 2009;158:260–265. doi: 10.1016/j.neuroscience.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Bezzi PVG, Galbete JL, Seifert G, Steinhauser C, Pilati E, Voltera A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Kerr JM, Ehlers MD. Structural plasticity with preserved topology in the postsynaptic protein network. Proc Natl Acad Sci U S A. 2008;105:12587–12592. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Brose N. Synaptogenic proteins and synaptic organizers: “many hands make light work”. Neuron. 2009;61:650–652. doi: 10.1016/j.neuron.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Cahusac PM, Senok SS, Hitchcock IS, Genever PG, Baumann KI. Are unconventional NMDA receptors involved in slowly adapting type I mechanoreceptor responses? Neuroscience. 2005;133:763–773. doi: 10.1016/j.neuroscience.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar N, Petralia RS, Winters CA, Niedzielski AS, Wenthold RJ. The segregation and expression of glutamate receptor subunits in cultured hippocampal neurons. Neuroscience. 1993;57:943–964. doi: 10.1016/0306-4522(93)90040-m. [DOI] [PubMed] [Google Scholar]
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 2008;11:1035–1043. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- Fellin TOP, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocyte glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Groc L, Bard L, Choquet D. Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives. Neuroscience. 2009;158:4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J Neurosci. 2007;27:12433–12437. doi: 10.1523/JNEUROSCI.3349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE. 2B synaptic or extrasynaptic determines signalling from the NMDA receptor. J Physiol. 2006;572:614–615. doi: 10.1113/jphysiol.2006.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de Silva T, Montgomery JM, Garner CC, Green WN. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci. 2009;12:1011–1019. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KJ, Korb E, Kundel MA, Kochanek AR, Kabraji S, McEvoy M, Shin CY, Wells DG. CPEB1 regulates beta-catenin mRNA translation and cell migration in astrocytes. Glia. 2008;56:1401–1413. doi: 10.1002/glia.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, Okubo Y, Hirose K, Iino M. Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J Neurosci. 2007;27:8957–8966. doi: 10.1523/JNEUROSCI.2276-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Köhr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Merchán-Pérez A, Rodriguez JR, Ribak CE, DeFelipe J. Proximity of excitatory and inhibitory axon terminals adjacent to pyramidal cell bodies provides a putative basis for nonsynaptic interactions. Proc Natl Acad Sci U S A. 2009;106:9878–9883. doi: 10.1073/pnas.0900330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A. Post-embedding electron microscopic immunocytochemistry. In: Priestley J. P. a. J., editor. Electron Microscopic Immunocytochemistry: Principles and Practice. Oxford; New York: 1992. pp. 51–87. [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Okabe T, Nakamura T, Nishimura YN, Kohu K, Ohwada S, Morishita Y, Akiyama T. RICS, a novel GTPase-activating protein for Cdc42 and Rac1, is involved in the beta-catenin-N-cadherin and N-methyl-D-aspartate receptor signaling. J Biol Chem. 2003;278:9920–9927. doi: 10.1074/jbc.M208872200. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9:611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS. Immunocytochemical localization of ionotropic glutamate receptors in neural circuits. In: Monaghan DT, Wenthold RJ, editors. The Ionotropic Glutamate Receptors. Humana Press; Totowa, NJ: 1997. pp. 219–263. [Google Scholar]
- Petralia RS. Al-Hallaq RA. Wenthold RJ. Trafficking and targeting of NMDA receptors. In: VanDongen AM, editor. Biology of the NMDA Receptor. CRC/Taylor and Francis; Boca Raton, FL: 2009. pp. 149–200. [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci. 1994a;14:6102–6120. doi: 10.1523/JNEUROSCI.14-10-06102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. Eur J Neurosci. 2002;15:583–587. doi: 10.1046/j.1460-9568.2002.01896.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Internalization at glutamatergic synapses during development. Eur J Neurosci. 2003;18:3207–3217. doi: 10.1111/j.1460-9568.2003.03074.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. J Comp Neurol. 1996;372:356–383. doi: 10.1002/(SICI)1096-9861(19960826)372:3<356::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Glutamate receptor antibodies: Production and immunocytochemistry. In: Ariano MA, editor. Receptor Localization Laboratory Methods and Procedures. Wiley-Liss; New York: 1998. pp. 46–74. [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. Methods Mol Biol. 1999;128:73–92. doi: 10.1385/1-59259-683-5:73. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994b;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Rose O, Grund C, Reinhardt S, Starzinski-Powitz A, Franke WW. Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc Natl Acad Sci U S A. 1995;92:6022–6026. doi: 10.1073/pnas.92.13.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–2795. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, Sola E, Taglietti V, Borchardt T, Steigerwald F, Utvik JK, Ottersen OP, Kohr G, D'Angelo E. NMDA receptor 2 (NR2) C-terminal control of NR open probability regulates synaptic transmission and plasticity at a cerebellar synapse. J Neurosci. 2002;22:9687–9697. doi: 10.1523/JNEUROSCI.22-22-09687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Curcio C, Chauvet N, Bruses JL. Assembly of the N-cadherin complex during synapse formation involves uncoupling of p120-catenin and association with presenilin 1. Mol Cell Neurosci. 2005;30:611–623. [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Sans N, Wang PY, Du Q, Petralia RS, Wang YX, Nakka S, Blumer JB, Macara IG, Wenthold RJ. mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat Cell Biol. 2005;7:1179–1190. doi: 10.1038/ncb1325. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Wassle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha 1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnadelbach O, Blaschuk OW, Symonds M, Gour BJ, Doherty P, Fawcett JW. N-cadherin influences migration of oligodendrocytes on astrocyte monolayers. Mol Cell Neurosci. 2000;15:288–302. doi: 10.1006/mcne.1999.0819. [DOI] [PubMed] [Google Scholar]
- Seabold GK, Wang PY, Chang K, Wang CY, Wang YX, Petralia RS, Wenthold RJ. The SALM family of adhesion-like molecules forms heteromeric and homomeric complexes. J Biol Chem. 2008;283:8395–8405. doi: 10.1074/jbc.M709456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci. 2007;27:8505–8516. doi: 10.1523/JNEUROSCI.1395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Partitioning the synaptic landscape: distinct microdomains for spontaneous and spike-triggered neurotransmission. Sci Signal. 2009;2:pe19. doi: 10.1126/scisignal.265pe19. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns'ka I, Dityatev A, Schachner M. Trans-Golgi network delivery of synaptic proteins in synaptogenesis. J Cell Sci. 2004;117:381–388. doi: 10.1242/jcs.00956. [DOI] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns'ka I, Nikonenko AG, Schachner M. NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J Cell Biol. 2006;174:1071–1085. doi: 10.1083/jcb.200604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Parasynaptic signalling by fast neurotransmitters: the cerebellar cortex. Neuroscience. 2009;162:644–655. doi: 10.1016/j.neuroscience.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Tai CY, Kim SA, Schuman EM. Cadherins and synaptic plasticity. Curr Opin Cell Biol. 2008;20:567–575. doi: 10.1016/j.ceb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. J Comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Tran MD, Wanner IB, Neary JT. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J Neurochem. 2008;105:272–286. doi: 10.1111/j.1471-4159.2008.05214.x. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Burette A, Wenthold RJ, Weinberg RJ. Expression of NR2 receptor subunit in rat somatic sensory cortex: synaptic distribution and colocalization with NR1 and PSD-95. J Comp Neurol. 1999;410:599–611. [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr., Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Kim E. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol Cell Neurosci. 2009;42:1–10. doi: 10.1016/j.mcn.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, Huganir RL, Penzes P. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Petralia RS, Fu Z, Swanwick CC, Wang YX, Prybylowski K, Sans N, Vicini S, Wenthold RJ. The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J Neurosci. 2007;27:11663–11675. doi: 10.1523/JNEUROSCI.3252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.