Abstract
The tumor microenvironment is a key factor in cancer treatment response. Recent work has shown that changes in the tumor vasculature can be achieved by inhibiting tumor cell signaling resulting in enhanced tumor oxygenation. These changes could promote responses to both chemo- and radiation therapy.
Background
The tumor microenvironment (TME) presents both impediments and targets for therapy. Tumor vasculature functions poorly, with variable blood flow through leaky immature vessels, compounded by inadequate lymphatic drainage. These factors and the resultant increased interstitial fluid pressure can impede the delivery of nutrients and oxygen and reduce delivery of chemotherapy to the tumor cells (1). Decreased IFP after paclitaxel treatment has been linked to increased oxygenation in breast cancer patients (2) and low IFP has been associated with better prognosis in cervical cancer patients (3). Functionally impaired tumor vasculature also leads to areas in solid tumors that are nutrient-deprived, acidic and necrotic as well as regions exhibiting both chronic and intermittent hypoxia (4). Hypoxia, poor vascular perfusion and reduced tumor uptake of therapeutic agents contribute to both radio- and chemotherapy treatment failure and select for more aggressive tumors (5).
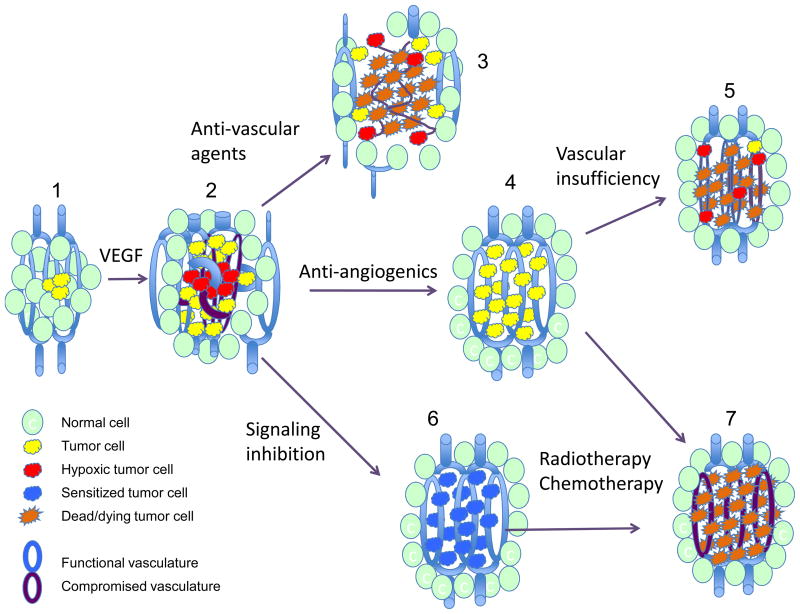
The abnormalities found in tumor vessels are in large part a result of dysregulated angiogenic signaling. This signaling is initiated by tumor cell over-expression of angiogenic factors such as vascular endothelial growth factor (VEGF) that results from both TME and tumor cell oncogenic signaling (6, 7). Dysregulated angiogenic signaling leads to increased vascular permeability and aberrant vessels (Figure 1-2). Once established, the TME itself can act to perpetuate abnormal angiogenesis through hypoxic signaling by hypoxia-inducible factor 1 (HIF-1), a transcription factor that activates expression of dozens of genes including VEGF (8). Therapeutic intervention can also result in VEGF up-regulation through HIF-1 (9). Thus there are multiple levels during tumor development and therapy at which angiogenesis can be targeted, including the altered tumor vasculature itself, angiogenic signaling, and oncogenic signaling (Figure 1-3, -4 and -6).
Figure 1.
Tumor development and response to therapeutic intervention.
(1) Small tumors proliferate without angiogenesis to the point where new vasculature is required. The “angiogenic switch” results in increased angiogenic factor expression and development of abnormal tumor vasculature with increased tortuosity, blind ends and poor vessel maturity (2). The tumor lacks lymphatic drainage and has high interstitial pressure limiting diffusion. Anti-vascular targeting agents such as combretastatin cause rapid tumor vascular endothelial cell death and tumor necrosis (3), but cells in the tumor periphery can survive using adjacent normal vascular supplies. Anti-angiogenic treatment causes a transient enhancement of tumor vasculature function and reduced hypoxia (4), but then lead to tumor vascular insufficiency and recurrence of hypoxia (5). Tumor cell oncogenic signaling inhibition (6) leads to sustained normalization of the tumor vasculature and reduced hypoxia. Combining tumor signaling inhibition or anti-angiogenic therapy with radiation or cytotoxic drug treatment during the period of enhanced oxygenation and vascular perfusion may promote tumor killing (7).
There have been two main pharmacologic approaches developed to target tumor vessels: vascular disruptive agents and anti-angiogenic agents. Vascular disruptive agents such as combrestatin A4 are designed to destroy tumors by preferentially ablating pre-existing tumor vessels (10). However, these agents are limited by the presence of collateral supplies to the tumor periphery from the surrounding normal tissue vasculature. Furthermore, these agents may exacerbate hypoxia.
Another means of altering the TME was proposed in the 1970’s by Judah Folkman, who suggested targeting new vessel growth (angiogenesis) as a strategy to control the growth of cancers (11). Anti-angiogenic agents inhibit the action of factors that stimulate new blood vessel development (12). There are currently a number of anti-angiogenic approaches including anti-VEGF receptor antibodies, VEGF traps and inhibitors of VEGF kinase activity in various stages of development and in clinical trials (reviewed in(13)). Unfortunately, success to date using VEGF blockers as single agents has been limited (14). Possible reasons include development of resistance to angiogenic inhibitors via up-regulation of redundant angiogenic pathways and increased tumor metastatic potential (reviewed in (15)).
Investigations into the combined use of anti-VEGF agents with cytotoxic therapies have yielded more promising results than VEGF-targeting monotherapy. Pre-clinical work has shown that VEGF can be induced in response to radiation and that inhibition of VEGF can increase tumor control after radiation (16). There has been a great deal of interest in studying the combined use of the anti-VEGF monoclonal antibody bevacizumab (Avastin) with other cytotoxic agents. Early success was seen using combined treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (17). Bevacizumab has shown efficacy in combination with conventional chemotherapy in other cancers although the results have generally been modest, with small improvements in overall survival at best and sometimes only in progression-free survival (18, 19). The toxicity of Bevacizumab in combination with radiotherapy has also been a concern (20).
How does anti-VEGF therapy potentiate cell killing in response to cytotoxic therapy? VEGF receptor inhibition reduces endothelial cell proliferation in vitro after irradiation and also reduces microvessel density in irradiated tumors (21). Thus it may sensitize tumor endothelial cells to cell death in response to radiation. However, there may be additional mechanisms at work. Jain and co-workers have shown that blocking VEGF signaling with DC101, a VEGF receptor-2 (VEGFR-2) antibody, decreased interstitial fluid pressure in xenografts in mice by producing a morphologically and functionally “normalized” vascular network (22) Furthermore, DC101 induced a hydrostatic pressure gradient across the vascular wall, which led to deeper penetration of molecules into tumors. Vascular normalization may in fact be a prognostic marker for treatment response (23). This “vascular normalization” is accompanied by a transient reduction in tumor hypoxia and enhanced vascular flow and diffusion (24). The normalization period offers an opportunity for enhanced efficacy of radiation and chemotherapy, but this period is relatively short and followed by vascular insufficiency due to strong and prolonged anti-angiogenic activity using current approaches. In addition, vascular normalization has not been observed in all studies (reviewed in (10)).
An alternative strategy: inhibiting oncogenic signaling in cancer cells
The approach used by Jain and colleagues relies on using agents that directly target VEGF or VEGFR on endothelial cells. Another strategy is to indirectly target VEGF by inhibiting oncogenic signaling in cancer cells, hence decreasing both oncogenic activity and VEGF secretion. Two studies have recently appeared that highlight this approach (25, 26). Both studies arose out of attempts to target tumor cells for sensitization to radiation and cytotoxic drugs. In the course of studying the effects of signaling inhibition on tumor sensitivity in vivo, significant changes in tumor oxygenation were seen (27). These observations led to investigation of the mechanisms for the vascular changes induced by oncogene signaling inhibition (28), and to definition of the important targets for this inhibition in the EGFR to AKT signaling pathway (25, 29). Qayum et al. demonstrated that in cells with activation of EGFR to AKT signaling, inhibition at multiple points in this signaling pathway resulted in prolonged enhancement of vascular function accompanied by decreased hypoxia and enhanced diffusion of small molecules into the tumor stroma. Vessels in treated tumors were less tortuous, and showed markers indicating a more mature phenotype. This was seen in both xenografts and spontaneous tumor models. The study by Cerniglia et al. (26) using the EGFR inhibitor erlotinib reached similar conclusions. They showed that erlotinib led to decreased VEGF secretion by cancer cells. Erlotinib treatment of mice bearing xenografts enhanced vascular functioning in the tumors, as measured by blood flow and also improved oxygenation. Extending these findings, they showed increased delivery of the cytotoxic drug cisplatin to the tumors when mice were pre-treated with erlotinib for 4 days prior to cisplatin injection. This resulted in tumor growth delay that was greater than that seen after the order of the treatments was reversed, i.e. cisplatin followed by erlotinib treatment. Thus, these parallel studies have shown that inhibition of tyrosine kinase receptor signaling through RAS, PI3-Kinase and AKT results in enhanced vascular function (Figure 1-6). As a further consequence, this normalization enhances tumor oxygenation and the delivery of cytotoxic drugs that may promote antitumor activity (Figure 1-7). Preliminary findings indicate that a similar potentiation of tumor radiation response is likely (unpublished observations).
These two studies show that using agents that inhibit oncogenic signaling in tumor cells can lead to vascular changes consistent with vascular normalization. The results are similar to those observed after direct inhibition of VEGF signaling, but demonstrate more prolonged effects on the TME. This raises the as yet unanswered question of whether a lower dose of anti-angiogenic treatment could yield prolonged enhancement of vascular function? Conversely, experiments by Izumi et al (30) and others, using higher doses of signaling inhibitors, have resulted in anti-angiogenic effects. So it is apparent that the application of signaling inhibitors at high doses (such as one might achieve if aiming for a maximally tolerated dose (MTD)) might be counter-productive from the point of view of effects on the TME.
Are there advantages to signaling inhibition over direct anti-angiogenic approaches? Inhibition of oncogenic signaling often increases the intrinsic sensitivity of the cancer cells independent of the TME (31, 32), whereas direct and specific anti-angiogenic therapy does not. This enhanced tumor cell susceptibility to cytotoxic treatment could add to the effects of improved tumor vascular function and counter any advantage to tumor growth imparted by the enhanced oxygen and nutrient supplies during cytotoxic therapy. Secondly, inhibition of oncogenic signaling might have the advantage of a wider dose-response range for vascular effects because of the partial down-regulation of VEGF expression achieved by this approach. For example, both Cerniglia et al. and Qayum et al found that treatment of cancer cells with EGFR inhibitors or nelfinavir resulted in only a partial decrease in VEGF expression (25, 26). Anti-angiogenic agents are designed to work by potently and completely inhibiting VEGF signaling. They therefore have the potential to severely compromise vascular function and drug delivery on the one hand and increase hypoxia and reduce radiation efficacy unless administered at precisely the right doses and times relative to radiation or cytotoxic drug treatments. In clinical practice, such precise timing and dosing may be very difficult to achieve. An alternative approach to the use of anti-angiogenics could be to administer these agents after completion of cytotoxic therapies to inhibit the repair of tumor vascular damage and tumor regrowth.
Clinical implications
These findings may have important clinical implications. Increasing blood flow through the tumor vessels should lead to improved drug delivery. Increased oxygenation during radiotherapy should enhance radiation response. Pre-clinical data support these predictions and offer a rationale as to why the optimal time to start these inhibitors of oncogenic signaling might be a week or so prior to cytotoxic therapy. Furthermore, if changes in the TME are important for the efficacy of subsequent cytotoxic therapy, then the TME could be imaged prior to and after 5–10 days of inhibitor treatment, to determine whether there has been any modulation of the TME and to determine whether this change could predict outcome. One report showing a positive correlation between vascular normalization and treatment outcome has already appeared (23). The technology to monitor these changes exists and could be used to assess tumor vascularity (e.g. DCE MRI, Power Doppler) and/or oxygenation changes (e.g. PET scanning with hypoxia sensitive tracers, EPR oximetry) (33, 34). These techniques may help to answer the question of how long enhanced vascular function persists in human tumors, and address how cytotoxic therapies impact on the vascular changes induced by signaling inhibitors.
It is of interest to note that other approaches to altering tumor vasculature are being reported. The integrin antagonist, cilengitide, has been shown to have similar effects to signaling inhibition on tumor vascular morphology (35). Inhibition of Hedgehog signaling has also been recently shown to enhance delivery of gemcitabine to pancreatic tumors in mice (36). Whether these approaches will be applicable to a wide range of tumor types is not yet known. Thus there may be other options available for achieving enhanced vascular function in tumors should this be shown to be of benefit to therapy. Which of these options will be most effective in enhancing tumor cell killing by cytotoxic therapies remains to be determined. Together, these recent studies further point to the need to examine the effects of signaling inhibition on the TME when evaluating the effects of signaling inhibitors on cancer growth and radio- or chemotherapy treatment responses.
Acknowledgments
The authors were supported by grants from the NIH: CA093638 (A.M.), CA73820 (E.B.) and MRC (E.B.) during the studies described in this report. We wish to thank Drs Helen Stone and Bhadrasian Vikram for helpful comments. We apologize to authors whose work was not cited due to restraints on article length.
Contributor Information
Amit Maity, Email: maity@xrt.upenn.edu.
Eric J. Bernhard, Email: bernhardej@mail.nih.gov.
References
- 1.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvascular Research. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taghian AG, Abi-Raad R, Assaad SI, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. Journal of Clinical Oncology. 2005;23:1951–61. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 3.Fyles A, Milosevic M, Pintilie M, et al. Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiotherapy & Oncology. 2006;80:132–7. doi: 10.1016/j.radonc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 5.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [see comment] [DOI] [PubMed] [Google Scholar]
- 6.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Research. 2001;61:6020–4. [PubMed] [Google Scholar]
- 7.Rak J, Mitsuhashi Y, Sheehan C, et al. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Research. 2000;60:490–8. [PubMed] [Google Scholar]
- 8.Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) Journal of Pathology. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 9.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–41. doi: 10.1016/s1535-6108(04)00115-1. [see comment] [DOI] [PubMed] [Google Scholar]
- 10.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Research. 2006;66:11520–39. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y. Tumor angiogenesis and molecular targets for therapy. Frontiers in Bioscience. 2009;14:3962–73. doi: 10.2741/3504. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Ellis LM. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer Journal. 2008;14:170–7. doi: 10.1097/PPO.0b013e318178d9de. [DOI] [PubMed] [Google Scholar]
- 15.Eikesdal H, Kalluri R. Drug resistance associated with antiangiogenesis therapy. Seminars in Cancer Biology. 2009 doi: 10.1016/j.semcancer.2009.05.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Research. 1999;59:3374–8. [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. Journal of Clinical Oncology. 2006;24:5601–8. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 19.Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Current Treatment Options in Oncology. 2007;8:15–27. doi: 10.1007/s11864-007-0022-4. [DOI] [PubMed] [Google Scholar]
- 20.Spigel D, Hainsworth J, Yardley D, et al. Tracheoesophageal Fistula Formation in Patients With Lung Cancer Treated With Chemoradiation and Bevacizumab. J Clin Oncol. 2009 Nov 9; doi: 10.1200/JCO.2009.24.7353. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Hess C, Vuong V, Hegyi I, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 [correction of ZK222548] combined with ionizing radiation on endothelial cells and tumour growth. British Journal of Cancer. 2001;85:2010–6. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research. 2004;64:3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Research. 2009;69:5296–300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [see comment] [DOI] [PubMed] [Google Scholar]
- 25.Qayum N, Muschel RJ, Im JH, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Research. 2009;69:6347–54. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerniglia G, Pore N, Tsai J, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One. 2009;4:e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen-Jonathan E, Evans SM, Koch CJ, et al. The farnesyltransferase inhibitor L744, 832 reduces hypoxia in tumors expressing activated H-ras. Cancer Research. 2001;61:2289–93. [PubMed] [Google Scholar]
- 28.Maity A, Pore N, Lee J, Solomon D, O’Rourke DM. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Research. 2000;60:5879–86. [PubMed] [Google Scholar]
- 29.Prevo R, Deutsch E, Sampson O, et al. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Research. 2008;68:5915–23. doi: 10.1158/0008-5472.CAN-08-0757. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 31.Kim IA, Bae SS, Fernandes A, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Research. 2005;65:7902–10. doi: 10.1158/0008-5472.CAN-05-0513. [DOI] [PubMed] [Google Scholar]
- 32.Bernhard EJ, Stanbridge EJ, Gupta S, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Research. 2000;60:6597–600. [PubMed] [Google Scholar]
- 33.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. Journal of Nuclear Medicine. 2008;49 (Suppl 2):113S–28S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 34.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. Journal of Nuclear Medicine. 2008;49 (Suppl 2):129S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 35.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS ONE. 2009;4:e4449. doi: 10.1371/journal.pone.0004449. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]