Abstract
Two important, related pathways are involved in cancer growth. The insulin/insulin-like growth factor-1 (IGF1) signaling pathway, which is activated when nutrients are available, and the adenosine mono-phosphateactivated protein kinase (AMPK) pathway, activated when cells are starved for carbohydrates. Metformin inhibits transcription of key gluconeogenesis genes in the liver, increases glucose uptake in skeletal muscle, and decreases circulating insulin levels. Metformin reduces levels of circulating glucose, increases insulin sensitivity, and reduces insulin resistance associated hyperinsulinemia. At the level of cell signaling, metformin activates AMPK. There are extensive pre-clinical data showing the anticancer effects of metformin in all breast cancer subtypes as well as in cytotoxic therapy-resistant models. These data, and the epidemiological and retrospective data supporting the antineoplastic effects of metformin, provide the rationale to study the role of metformin for breast cancer therapy in a variety of clinical settings.
Keywords: Metformin, AMPK, insulin, breast cancer
Background
Energy signaling and cancer
Cell growth and proliferation is coordinately regulated by multiple signals including growth factors, availability of nutrients and energy (intracellular ATP). The insulin/insulin-like growth factor-1 (IGF1) signaling pathway is activated when nutrients are available, whereas the adenosine mono-phosphate activated protein kinase (AMPK) pathway, a sensor of cellular energy, is activated when cells are starved for energy (1). In mammals, insulin promotes lipid, protein, and glycogen synthesis, whereas AMPK inhibits these biosynthetic pathways. The effect of insulin on protein synthesis is mediated in part by activation of the mammalian target of rapamycin (mTOR) pathway via phosphorylation of tuberous sclerosis complex 2 protein (TSC2, also known as tuberin), whereas activation of AMPK causes phosphorylation of different sites on TSC2 and inhibits mTOR (2,3). Thus, TSC2 integrates insulin and energy signaling to control cell growth and survival. Further, there is direct crosstalk between insulin and energy signaling. In some tissues, such as cardiac muscle, insulin antagonizes activation of AMPK by activating Akt (4). This in turn phosphorylates AMPKα on Ser485/Ser491, which reduces AMPK Thr172 phosphorylation by LKB1, and the resulting activation of AMPK (5). However, in processes that regulate plasma glucose levels, the insulin and AMPK signaling pathways work in the same direction (6). Activation of AMPK plays a role on the ability of muscle contraction to stimulate glucose uptake, and to increase the insulin sensitivity of glucose uptake with exercise (7). Although unclear, the mechanism for this effect may be due to the ability of AMPK to inhibit the mTOR pathway, which is activated by insulin and exerts a feedback regulation on insulin signaling by downregulating IRS1(8–10). In the liver and in adipocytes, insulin and AMPK repress the expression of enzymes of gluconeogenesis (11), and suppress the activation of hormone-sensitive lipase, and lipolysis (12,13), respectively.
IGF signaling has an important role in normal cell growth, but it is also a known mediator of the malignant phenotype. IGF1 receptor ligand binding leads to autophosphorylation of tyrosines at its kinase domain. This induces the phosphorylation of tyrosines and serines to form binding sites for insulin receptor substrates (IRS) and Src and subsequent activation of signaling via the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR and RAS/RAF/mitogen-activated protein kinase (MAPK) pathways (14–16). Regulation of IGF1R occurs at multiple levels including ligand availability (17), and intracellularly through Src, phosphatases, integrins, and the RACK1 scaffolding protein (18). Downstream, IGF1R effectors mTOR complex1 (mTORC1) and S6 kinase, participate on the feedback suppression of the PI3K/AKT signaling (15,18,19). Although IGF1R is not unique in driving tumor cell proliferation, it is necessary for oncogene induced cellular transformation and mediates both the survival and the proliferation signaling required for cell growth which enables transformed cells to form tumors, and to survive detachment (14,20). Further, preclinical studies have shown that IGF1R overexpression may induce tumor formation and metastasis (21,22).
As stated above, mTOR activity is in part regulated by cellular energy levels and nutrients as well as oxygen and growth factors (23). When mTOR is deregulated, it leads to increased cell growth and proliferation. Further, the signaling pathways that control its activity are often deregulated in human cancers. For instance, activating PIK3CA mutations or loss of expression of the tumor suppressor PTEN, both prevalent in breast cancer can lead to uncontrolled mTOR activity (24,25) and subsequent translation of mRNAs that encode for growth factors, apoptosis inhibitors, activators of the cell cycle, and angiogenic factors all of which contribute to tumor formation and growth (26). As a result, the PI3K/Akt/mTOR signaling pathway is a prime target for anticancer therapies (27), and inhibiting the energy pathway through AMPK activation and mTOR inhibition is a potential mechanism to prevent and decrease cancer growth.
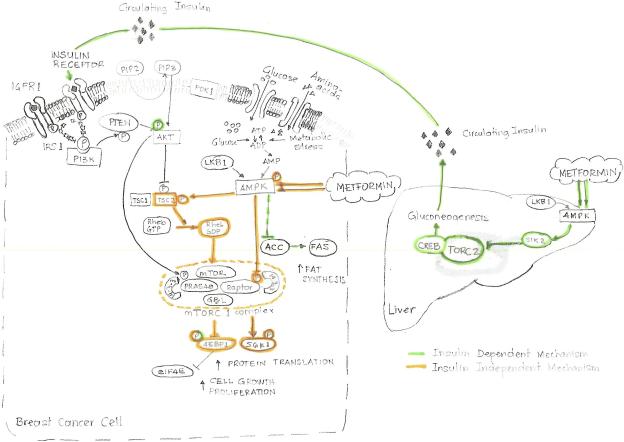
Figure 1 summarizes the biological effects of metformin inside and outside the cancer cell.
Figure 1.
Mechanisms of action of metformin. Metformin appears to exert its cell growth-inhibitory effects through two distinct mechanisms. A direct mechanism that inhibits the mTOR pathway and an indirect mechanism depending on insulin levels. Metformin activates adenosine monophosphate activated protein kinase (AMPK), the cellular energy sensor. Activation of AMPK leads to suppression of many of the processes highly dependent on ATP, such as gluconeogenesis, protein, fatty acid, and cholesterol biosynthesis. It inhibits transcription of gluconeogenesis genes in the liver and increases glucose uptake in skeletal muscle. This appears to reduce the levels of circulating glucose, increase insulin sensitivity, and reduce the hyperinsulinemia associated with insulin resistance.
In the cancer cell, the mammalian target of rapamycin (mTOR) signaling pathway promotes cell growth and proliferation by interplay of two opposing upstream pathways involving the Akt pathway, which signals availability of nutrients, and the AMPK pathway, which signals lack of energy. TOR complex 1 (TORC1), directly regulates cell growth and contains raptor (regulatory associated protein of mTOR) and PRAS40 (proline-rich Akt substrate of 40 kDa), which represses mTOR activity. There are two types of input that activateTORC1: increased availability of amino acids at the cellular level, and activated insulin or the related growth factor IGF1 signaling. They activate phosphatidylinositol-3-kinase (PI3K) which switches on Akt. TORC1 is stimulated by the active, GTP-bound form of the Rheb, and immediately upstream of Rheb is the TSC1:TSC2 heterodimer. TSC2 converts Rheb to its inactive Rheb:GDP form. Activation of Akt causes phosphorylation of TSC2, which is thought to inhibit its GAP activity and stimulates TORC1. On the other hand, Akt also phosphorylates PRAS40 and appears to relieve its inhibitory effect.
AMPK is activated when ATP levels lower switching off the mTOR pathway over the positive effects of amino acids or growth factors via phosphorylation of TSC2 by AMPK which stimulatesits Rheb-GAP activity. Metformin and its analogs also activate AMPK in the absence of TSC2 thought Raptor phosphorylation. This effect appears to be a direct effect on mTOR kinase activity, possibly involving increased binding of 14-3-3 proteins and/or partial dissociation of PRAS40. So, the AMPK pathway exerts two inhibitory effects on mTOR via phosphorylation of TSC2 and Raptor similary to the Akt pathway, which exerts two stimulatory effects via phosphorylation of TSC2 and PRAS40.
Metformin activity in breast cancer
Metformin is a biguanide, and a widely prescribed oral medication used as front-line therapy for type 2 diabetes. Population studies suggest that metformin decreases the incidence of cancer and cancer-related mortality in diabetic patients (28,29). Clinical and epidemiologic evidence linking hyperinsulinemia, insulin resistance, and diabetes to poor breast cancer outcomes (30). Further, insulin can promote tumorigenesis via a direct effect on epithelial tissues, or indirectly by affecting the levels of other modulators, such as insulin-like growth factors, sex hormones, and adipokines (31–35). Exciting preclinical studies have demonstrated that metformin can inhibit the growth of cancer cells, including breast cancer, in vitro and of tumors in vivo (36–40). More recently, a retrospective study of patients who received neoadjuvant chemotherapy for breast cancer showed that diabetic cancer patients receiving metformin during their neoadjuvant chemotherapy had a higher pathological complete response rate than diabetic patients not receiving metformin (24% vs 8%, p=0.007) (41).
The antineoplastic effects of metformin in breast cancer are supported by a biological rationale involving important factors associated with breast cancer prognosis. In the liver, metformin inhibits transcription of key gluconeogenesis genes and increases glucose uptake in skeletal muscle. It reduces levels of circulating glucose, increases insulin sensitivity, and reduces insulin resistance associated hyperinsulinemia (42). At the level of cell signaling, several mechanisms of metformin action have been proposed; the most important one relates with the activation of AMPK (43). AMPK, the central cellular key energy sensor with an unique ability of directly sense cellular energy, places it in an ideal position to ensure that cell division, which is a highly energy-consuming process, only proceeds if cells have sufficient metabolic resources (44,45). Once activated, it leads to suppression of many of the metabolic processes that highly depend on sufficient cellular adenosine triphosphate (ATP) supply (gluconeogenesis, protein and fatty acid synthesis, cholesterol biosynthesis) and that promote catabolic processes (glycolysis, fatty acid beta oxidation) (46). Further, the AMPK pathway exerts two inhibitory effects on mTOR, via phosphorylation of TSC2 and raptor. AMPK is activated when ATP levels are lower, switching off the mTOR pathway over the positive effects of amino acids (47) or growth factors via phosphorylation of TSC2 by AMPK which stimulates its Rheb-GAP activity. Metformin and its analogs also activate AMPK in the absence of TSC2 through raptor phosphorylation (2,48). This effect appears to be a direct effect on mTOR kinase activity, possibly involving increased binding of 14-3-3 proteins and/or partial dissociation of PRAS40 (49).
Clinical-Translational Advances
Pre-clinical studies
Initial experiments, showed that metformin was capable of reducing proliferation in prostate, colon and breast cancer cell lines through cell cycle inhibition demonstrated by an important decrease of cyclin D1 protein level. Subsequently in vivo experiments using intraperitoneal or oral metformin in nude mice resulted in tumor growth inhibition up to 55% (39). To evaluate the effect of metformin on cell proliferation, investigators looked at the effect of this drug in vitro on a group of breast, ovarian and prostate cancer cells lines. In MCF-7 human breast cancer cells, metformin acted as a growth inhibitor rather than an insulin sensitizer. Further, they found that exposure to a growth inhibitory concentration of drug by means of the AMPK pathway activation and mTOR inhibition can lead to decreased protein synthesis, blocking both growth and proliferation (50,51). Subsequent experiments looking specifically at breast cancer cell lines by hormone receptor status confirmed that AMPK stimulation by metformin results in complete cell growth inhibition in estrogen receptor (ER)-positive cell lines, but partial inhibition in the ER-negative. Interestingly, there was a significant increase in vascular endotheliagrowth factor in ER-negative cell lines. Furthermore, in ER-negative, orthotopic MDA-MB-435 xenograft models, metformin treatment lead to increased tumor growth, increased cancel cell viabilityand angiogenesis (52). In contrast, in a more recent report, investigators found that nude mice bearing tumor xenografts of the triple receptor negative cell line MDA-MB-231, show significant reductions in tumor growth and cell proliferation when treated with metformin, as compared to controls (53).
Lastly, there are now data showing that metformin selectively kills breast cancer stem cells. Investigators took four genetically different types of breast cancer cells and added metformin to doxorubicin. The combination was able to kill both non-stem and cancer stem cells in culture, and reduced tumor mass, prolonged remission more than with either drug alone in a xenograft mouse model (54).
These extensive pre-clinical data showing the anticancer effects of metformin in all breast cancer subtypes as well as in cytotoxic therapy resistant models provided a rational to study the drug in the clinic.
Clinical studies
The provocative results of metformin in retrospective clinical as well as in preclinical studies in breast cancer have lead to research in the clinic, both for treatment and prevention. Metformin is being investigated using different approaches.
1. Window of opportunity studies
In this setting, patients with early operable breast cancer of high risk breast epithelial neoplasia get a baseline biopsy, and then go on to receive either placebo or different doses of metformin for a short period of time (2–6 weeks); tissues are collected at the time of surgery. The main goal of this design is to evaluate whether the use of different doses of metformin can modulate tissue and serum biomarkers involved in cancer growth and to try to establish the lowest dose at which the drug modulation occurs. There are at least five on-going clinical trials world-wide looking at the molecular effects of metformin in breast cancer using this design (55).
2. Phase II, neoadjuvant randomized trials
In this setting, patients are randomized to receive a full course of neoadjuvant systemic therapy with or without metformin. Tissues (tumor and blood) are collected from all patients at baseline, at a set time point during treatment and at the time of surgery. The purpose of these studies is to evaluate the benefit of combining metformin with the standard neoadjuvant systemic therapy. They look at clinical and pathological outcomes, but also look at the differences of tumor and blood biomarkers of pathway modulation and their correlation with the outcome endpoints. Although randomized, these studies may not be powered to definitively answer the clinical question, but may help set the basis for patient selection for subsequentconfirmatory phase III studies. Several clinical trials are ongoing or planned targeting patients with hormone receptor-positive breast cancer and patients with HER2-positive breast cancer (56,57).
3. Phase I–II trials in metastatic disease
These studies are usually done to find the dose limiting toxicities, safety and initial efficacy data of the single agent or the combination with existing breast cancer therapies. Currently two of these trials are on-going. One focuses on obese patients with metastatic hormone receptor-positive breast cancer, and uses the combination of exemestane and Avandamet (metformin and rosiglitazone), and the second one looks at the combination of metformin and temsirolimus, an mTOR inhibitor in solid tumors and lymphoma (55).
4. Phase III, randomized, placebo-controlled trials
These trials can be done in the therapeutic or in the prevention settings. In the therapeutic setting, the National Cancer Institute of Canada will conduct the MA-32 trial: a phase III randomized study of the effect of metformin versus placebo in early stage breast cancer. Three thousand five hundred and eighty two patients will be randomized to metformin 850mg a day for 5 years vs. placebo. Patients will be stratified by hormone receptor status, HER2 status, and chemotherapy use. The primary endpoint is invasive disease-free survival. A parallel large neoadjuvant phase III randomized trial in early breast cancer is being designed. Patients will be randomized to six cycles of docetaxel, doxorubicin and cyclophosphamide (TAC) plus or minus metformin. Primary endpoint will be pathological complete response. Tissue and blood collections for correlative studies are planned. At this time, there is insufficient data on minimal dose needed to have antiproliferative effect, as well as long-term safety of metformin in patients without diabetes. Thus further work is needed before initiation of phase III prevention studies.
Biomarkers
In general, an adequate predictive biomarker should be able to identify the tumors that are sensitive to a specific therapy. In order to develop such markers it is necessary to obtain serial biopsies to test a large range of pharmacodynamic endpoints and their correlation with clinical outcomes. The markers should be tested in independent sets, preferably in the setting of a randomized clinical trial. Currently there are several potential markers studied at the preclinical level that relate to metformin mechanisms of action. They constitute the ideal initial set to explore, and include components of the IGF1R axis, the AMPK and PI3K/Akt/mTOR signaling pathway and metabolism serum markers such as insulin, c-peptide and leptin. The above mentioned clinical trials should serve as the training and validation sets to develop biomarkers of response that can be used to personalize metformin-based cancer therapy.
Adverse effects to be considered
Metformin has been extensively used in patients with type 2 diabetes and less frequently, in patients with polycystic ovarian syndrome. The drug has shown a very good safety profile. The most common toxicity is gastrointestinal distress, including transient mild nausea and moderate diarrhea, with a few patients having to discontinue treatment due to the latter (57–59). The only potential major adverse event from metformin therapy is lactic acidosis. This condition, although rare, is limited to patients with renal and/or liver disorders, and it should be taken into consideration in cancer care, since these patients undergo diagnostic imaging tests requiring contrast media that can increase their risk for it. Rare side effects include hirsutism and vitamin B12 malabsorption after long-term exposure. No teratogenic effects have been reported (58).
Conclusion
Metformin is a widely prescribed oral medication used as front-line therapy for type 2 diabetes. It has been shown to inhibit the growth of cancer cell lines, including breast cancer, in vitro and of in vivo tumors models. Population and retrospective studies showed that metformin decreases the incidence of cancer and cancer-related mortality, and increases the response to neoadjuvant chemotherapy in diabetic patients. Metformin induces AMPK activation which decreases insulin levels and leads to inhibition of protein synthesis pathways, decreasing cancer cell proliferation and growth. As a result, metfromin is being investigated as a therapeutic agent in different clinical settings for all breast cancer subtypes.
Acknowledgments
This work was supported by ASCO Career Development Award, NCI 1K23CA121994 (to A.M.G.), 1R01CA112199 (to F.M.B.), and the Cancer Center Core Grant No. CA16672 from The University of Texas, M. D. Anderson Cancer Center
References
- 1.Towel MC, Hardie DG. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circulation Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 2.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 3.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 4.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–27. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 5.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 6.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–76. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 8.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 9.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–56. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-Aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 12.Wijkander J, Landstrom TR, Manganiello V, Belfrage P, Degerman E. Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology. 1998;139:219–227. doi: 10.1210/endo.139.1.5693. [DOI] [PubMed] [Google Scholar]
- 13.Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–54. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- 14.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–7. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 15.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 17.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor R. Regulation of IGF-I receptor signaling in tumor cells. Horm Metab Res. 2003;35:771–7. doi: 10.1055/s-2004-814166. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 20.Sell C, Dumeril G, Deveaud C, et al. Effect of a null mutation of the insulin like factor I receptor gene on growth and transformation of mouse embryo filbroblasts. Mol Cancer Biol. 1994;14:3604–12. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez T, Hanahan D. Elevated levels IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;26:339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones RA, Campbell CI, Gunther EJ, et al. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–44. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- 23.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 24.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 26.Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–9. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 20006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin PJ. Insulin in the adjuvant breast cancer setting: a novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26:833–4. doi: 10.1200/JCO.2007.14.7132. [DOI] [PubMed] [Google Scholar]
- 31.Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–11. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 32.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 33.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–36. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 35.Yee D. Targeting insulin-like growth factor pathways. Br J Cancer. 2006;94:465–8. doi: 10.1038/sj.bjc.6602963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:910–5. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 37.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 38.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 39.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–86. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 40.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 41.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cusi K, DeFronzo RA. Metformin: A review of its metabolic effects. Diabetes Reviews. 1998;6:89–131. [Google Scholar]
- 43.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 45.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circulation Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 46.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 47.Kraus U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem. 2002;269:3751–59. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- 48.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardie DG. AMPK and Raptor: Matching Cell Growth with Energy Supply. Mol Cell. 2008;30:263–65. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 51.Dowling RJO, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin Inhibits Mammalian Target of Rapamycin-Dependent Translation Initiation in Breast Cancer Cells. Cancer Res. 2007;67:10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 52.Phoenix KN, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERa negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat. 2009;113:101–11. doi: 10.1007/s10549-008-9916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:13, 2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 54.Hirsch HA, liopoulos D, Tsichlis PN, Struhl K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009;69:6507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. http://www.clinicaltrials.gov/ct2/results?term=breast+cancer+AND+metformin&recr=&rslt=&type=&cond=&intr=&outc=&lead=&spons=&id=&state1=&cntry1=&state2=&c ntry2=&state3=&cntry3=&locn=&gndr=&rcv_s=&rcv_e=&lup_s=&lup_e= (accessed on 11/23/09)
- 56.Martin-Castillo B, Dorca J, Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Garcia M, Del Barco S, Menendez JA. Incorporating the antidiabetic drug metformin in HER2-positive breast cancer treated with neo-adjuvant chemotherapy and trastuzumab: an ongoing clinical-translational research experience at the Catalan Institute of Oncology. Ann Oncol. 2009 Nov 2; doi: 10.1093/annonc/mdp494. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it Time to Test Metformin in Breast Cancer Clinical Trials? Cancer Epidemiol Biomarkers Prev. 2009;18:702–5. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 58.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 59.Ehrmann DA, Cavaghan MK, Imperial J, et al. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:524–30. doi: 10.1210/jcem.82.2.3722. [DOI] [PubMed] [Google Scholar]