Abstract
FasL (TNFSF6, CD95L) is hypothesized to trigger testicular germ cell apoptosis that normally occurs during a distinct peripubertal period as well as in response to toxicant-induced Sertoli cell injury. To test this hypothesis, we evaluated the testis of FasL gene–deficient mice (FasL−/−) at two distinct developmental ages (postnatal day [PND] 28 and 44) and after toxicant-induced Sertoli cell injury. Testicular cross sections from peripubertal (PND 28) FasL−/− mice showed significant increases in the basal germ cell apoptotic index (AI; 20.58 ± 4.59) as compared to the testis of C57BL/6J wild-type mice (5.16 ± 0.08) and closely correlated with increased expression of TRAIL protein in the testis of FasL−/− mice. A limited, but significant, number of seminiferous tubules in the testis of PND 28 FasL−/− mice showed a severe loss of germ cells with only Sertoli cells present. In contrast, no apparent gross histological changes were observed in the testis of adult (PND 44) FasL−/− mice. However, PND 44 FasL−/− mice did show a 51% reduction in homogenization-resistant elongate spermatids as compared to age-matched C57BL/6J mice. Exposure of PND 28 FasL−/− mice to mono-(2-ethylhexyl) phthalate (MEHP), a well-described Sertoli cell toxicant, unexpectedly caused a rapid decrease in the germ cell AI that paralleled increased levels of the CFLAR (c-FLIP) protein, a known inhibitor of death receptor signaling. In contrast, MEHP treatment did not decrease c-FLIP levels in PND 28 C57BL/6J mice. Taken together, these findings indicate that FasL protein expression is required during the peripubertal period for the proper regulation of germ cell apoptosis that occurs normally during this period. The influence of FasL on the cellular regulation of c-FLIP protein levels appears to be a unique mechanism for modulating germ cell apoptosis after toxicant-induced Sertoli cell injury.
Keywords: apoptosis, FasL, c-FLIP, MEHP, testis
INTRODUCTION
A low level of spontaneous germ cell apoptosis occurs in the testis of adult mammals under physiological conditions. Apoptosis of germ cells serves as a mechanism to delete damaged cells as well as to limit the size of the germ cell population to match the supportive capacity of the Sertoli cells (Lee et al., 1997; Print and Loveland, 2000; Russell et al., 1988). During the peripubertal period, a time when the first wave of spermatogenesis is nearing completion, a significantly higher rate of apoptosis is seen (Russell, 1990; Wang et al., 1998). The mechanisms controlling this “physiological” testicular germ cell apoptosis in both adult and peripubertal animals have not been clearly established.
Apoptotic signaling pathways are generally classified as either extrinsic (death receptor) or intrinsic (mitochondrial) signaling. The extrinsic signaling pathway is characterized by the action of the tumor necrosis factor (TNF) superfamily of protein ligands (TNF-α, FasL, and TRAIL) from one cell acting on their corresponding receptors (TNFR, Fas, and DR5) on another cell (Locksley et al., 2001). The intrinsic pathway is distinguished by the release of factors, such as cytochrome c, from the mitochondria within a cell in response to cellular stress or injury (Garrido et al., 2006; Gogvadze et al., 2006; Kluck et al., 1997). Common to both these signaling pathways is the activation of a family of cysteinyl aspartate proteinases (caspases) that results in characteristic structural, biochemical, and morphological changes associated with cellular apoptosis (Elmore, 2007).
Fas ligand (FasL/CD95L/TNFSF6) and Fas receptor (Fas/CD95/TNFRSF6) are well-characterized proteins of TNF superfamily of ligands and receptors (Ashkenazi and Dixit, 1998). Engagement of homotrimers of Fas by homotrimers of FasL recruits the adapter protein, Fas associated death domain protein (FADD), to bind to Fas (Chinnaiyan et al., 1995). FADD serves, through its C-terminal death domain (DD), to couple Fas receptors to the “initiator” enzyme pro-caspase-8 (also named FADD-like interleukin 1-beta converting enzyme, FLICE), through the mutual association of N-terminal death effector domains (DED) that exist in both FADD and pro-caspase-8. The complex of Fas, FADD, and pro-caspase-8 is referred to as the death-inducing signaling complex (DISC) (Kischkel et al., 1995; Lavrik et al., 2005). The close apposition of two molecules of pro-caspase-8 allows for their further proteolytic processing, resulting in the formation of the active caspase-8 enzyme (Medema et al. 1997). Active caspase-8 can then cleave other distinct “downstream” caspase enzyme family members, resulting in a caspase cascade that cleaves substrates necessary for the instigation of apoptosis. The initiation of this caspase cascade at the DISC is negatively regulated by the binding of cellular FLICE inhibitor (c-FLIP and CFLAR) to the DED of FADD and thereby preventing the recruitment of pro-caspase-8 (Hyer et al., 2006; Krueger et al., 2001; Thome and Tschopp, 2001). Although this simplified description of death receptor–mediated signaling is widely accepted, there are many nuances to its regulation and even evidence for various nonapoptotic functions involved by the activation of Fas or by FasL itself (Peter et al., 2007). Nevertheless, the extent of the recruitment of either procaspase-8 or c-FLIP to the DISC is a recognized determinant for the downstream signaling events keyed off by death receptor activation.
Another member of the TNF family, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL/Apo2L/TNFSF10) (Pitti et al., 1996; Wiley et al., 1995), can induce apoptosis in many type of cancer cell lines but not in many normal cell lines (Ashkenazi et al., 1999; Walczak et al., 1999). TRAIL is a type II transmembrane protein that binds to two receptors in humans, DR4 (TRAIL-R1/TNFRSF10A) and DR5 (TRAIL-R2/TNFRSF10B) (Pan et al. 1997a,b). The interaction between TRAIL and DR4/DR5 triggers a signaling pathway similar to the FasL/Fas system through DISC formation (MacFarlane et al., 1997; Pan et al., 1997a; Schneider et al., 1997; Screaton et al., 1997; Sheridan et al., 1997).
Although the mechanisms responsible for the control of physiological germ cell apoptosis in the testis have not been clearly resolved, the direct involvement of FasL/Fas signaling in triggering testicular germ cell apoptosis has been widely described in rodents after exposure to the Sertoli cell toxicant mono-(2-ethylhexyl) phthalate (MEHP) (Lee et al., 1997; Yao et al., 2007). The functional participation of FasL/Fas signaling in MEHP-induced germ cell apoptosis was shown experimentally by challenging gld (generalized lymphoproliferative disease) mutant mice to MEHP (Richburg et al., 2000). The gld mice have a mutation in a single amino acid in FasL that prevents it from binding to and activating Fas (Takahashi et al., 1994). Although these mice are fertile and show similar basal germ cell apoptotic rates as wild-type mice, a significant reduction in the incidence of germ cell apoptosis was seen in the gld mice in response to MEHP exposure as compared to the C57BL/6J wild-type mice (Richburg et al., 2000). However, since the protection from MEHP-induced germ cell apoptosis was not complete, it is thought that the mutant form of FasL in gld mice may retain some of its activity. In order to adequately test the hypothesis that FasL is required for the regulation of germ cell apoptosis during the distinct peripubertal periods, we report here the evaluation of the testis of FasL gene–deficient (FasL−/−) mice during the first wave of spermatogenesis (postnatal day [PND 28], the peripubertal age) and right after the first wave of spermatogenesis (PND 44, adulthood) as well as the testicular effects in PND 28 mice after MEHP challenge.
MATERIALS AND METHODS
Mice.
All mice used in this study were maintained at the University of Texas Animal Resource Center. Mice were housed at a constant temperature (22 ± 0.5°C) at 35–70% humidity with a 12L:12D photoperiod. Mice were given standard lab chow and water ad libitum. All procedures involving mice were performed in accordance with the guidelines of the University of Texas at Austin's Institutional Animal Care and Use Committee in compliance with guidelines established by the National Institutes of Health.
Breeding pairs of wild-type C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding pairs of FasL heterozygous mice (FasL+/−), which are on a C57BL/6 background, were provided by Dr Saoussen Karray, Institut National de la Santé et de la Recherche Médicale, Paris, France (Karray et al., 2004). FasL gene–deficient mice (FasL−/−) on a C57BL/6J background were generated by mating FasL+/− mice, and their genotypes were confirmed by genomic PCR as described previously (Karray et al., 2004). In order to characterize the basic testicular phenotype, FasL−/− mice were killed at PND 28 and PND 44 by CO2 inhalation. The testes were rapidly removed and either frozen in liquid nitrogen and stored at −80°C for protein analysis or immersion fixed overnight in Bouin's solution (Polysciences, Inc., Warrington, PA), washed in 70% ethyl alcohol-Li2CO3 saturated solution, and embedded in paraffin for histology analysis.
MEHP treatment.
MEHP exposure in peripubertal rodents has been reported to be a good model to test the interaction between Sertoli cells and germ cells by decreasing Sertoli cell support (Bhattacharya et al., 2005; Richburg and Boekelheide, 1996; Richburg et al., 1999; Yao et al., 2009). PND 28 FasL−/− and C57BL/6J mice were given a single dose of MEHP (1 g/kg, TCI America, Portland, OR) by oral gavage at a volume equal to 4 ml/kg, a standard procedure for investigating the consequences of MEHP-induced Sertoli cell injury (Yao et al., 2007). Control animals received a similar volume of vehicle (corn oil). Both control and MEHP-treated mice were killed at 0, 6, 12, and 24 h after MEHP exposure.
Physiologic characterization of the testis.
The body and testis weights of mice at PND 28 and 44 were measured. The numbers of mice used were as follows: PND 28 C57BL/6J mice (n = 26) and FasL−/− mice (n = 40); PND 44 C57BL/6J mice (n = 8) and FasL−/− mice (n = 16).
Testicular histopathology.
Cross sections (5 μm) of paraffin-embedded testes were evaluated for morphological changes by using periodic acid-Schiff-Hematoxylin staining. Testicular cross sections were imaged using a Nikon E800 microscope and captured with a Canon 5D digital camera (Canon U.S.A., Inc., NY). The numbers of mice used for the evaluation of testicular morphology were as follows: PND 28 C57BL/6J mice (n = 7) and FasL−/− mice (n = 8); PND 44 C57BL/6J mice (n = 6) and FasL−/− mice (n = 6).
Testicular spermatid head counts.
Testicular spermatid head counts were performed as previously described with slight modifications (Richburg et al., 2000). Briefly, testes from PND 44 mice were homogenized in dimethyl sulfoxide (DMSO)/saline solution containing 0.9% (wt/vol) NaCl and 10% (vol/vol) DMSO and then homogenization-resistant spermatid heads were counted using a hemocytometer. The average number of spermatid heads was determined from 12 C57BL/6J mouse testes and 13 FasL−/− mouse testes. Each testis sample was counted three times. The daily sperm production per testis was calculated by using 4.84 days as the time divisor (Robb et al., 1978).
Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine triphosphate nick end labeling assay.
Apoptotic fragmentation of DNA in mouse paraffin-embedded testicular cross sections was determined by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine triphosphate nick end labeling (TUNEL) analysis using the ApopTag™ kit (Chemicon, Temecula, CA). The apoptotic index (AI) was calculated as the percentage of essentially round seminiferous tubules in a cross section that contain more than three TUNEL-positive germ cells. At least two testicular cross sections per mouse were analyzed. The AI was determined from six C57BL/6J mice and seven FasL−/− mice at PND 28 and four C57BL/6J mice and five FasL−/− mice at PND 44. The numbers of each group for MEHP-treated C57BL/6J mice were as follows: 0 h (n = 7), 1 h (n = 3), 3 h (n = 3), 6 h (n = 4), 12 h (n = 3), and 24 h (n = 3). The numbers of each group for MEHP-treated FasL−/− mice were as follows: 0 h (n = 8), 1 h (n = 4), 3 h (n = 6), 6 h (n = 4), 12 h (n = 4), and 24 h (n = 3).
Immunohistochemistry.
Expression of cleaved caspase-9 in the germ cells was determined by immunohistochemistry. Cross sections (5 μm) of paraffin-embedded testes from PND 28 C57BL/6J and FasL−/− mice were deparaffinized and rehydrated, and antigens were unmasked by heating in 10mM sodium citrate solution. Sections were incubated with 3% H2O2 to block endogenous peroxidase activity and then incubated in blocking buffer containing 10% horse serum. Primary antibody used in this study was rabbit anti-cleaved caspase-9 (1:100; Cell Signaling, Danvers, MA). Sections were incubated in primary antibody at 4°C overnight. Immunodetection was performed by standard procedures using the VectaStain ABC kit (Vector Laboratories, Burlingame, CA) and 3,3'-diaminobenzidine as the substrate (Vector Labs). At least two testicular cross sections per mouse were analyzed. The numbers of each group for MEHP-treated C57BL/6J mice were as follows: 0 h (n = 3) and 12 h (n = 3). The numbers of each group for MEHP-treated FasL−/− mice were as follows: 0 h (n = 4) and 12 h (n = 3). All sections were observed using a Nikon E800 microscope. The percentage of cleaved caspase-9–positive germ cells was calculated as the number of positive germ cells/total germ cells × 100.
Immunofluorescence.
Expression and localization of c-FLIP in testicular cross sections were determined by immunofluorescence detection of c-FLIP antibody (rabbit-anti-c-FLIP; 1:100; Cell Signaling). Cross sections (5 μm) of paraffin-embedded testes from C57BL/6J and FasL−/− mice were deparaffinized, rehydrated, heated in 10mM sodium citrate, and incubated in blocking buffer containing 10% horse serum, followed by overnight incubation in primary antibodies at 4°C. Sections were then incubated in Alexa Fluor–conjugated anti-rabbit antibody (1:500; Molecular Probes, Gaithersburg, MD) for 1 h and mounted in Vectashield Mounting Medium (Vector Labs). Fluorescent signals were detected using excitation/emission wavelength of 495/521 nm. Images were captured by a Nikon E800 microscope captured with a Nikon Cool-SNAP digital camera and processed using MetaMorph Imaging software (v. 4.1) (Downingtown, PA). Two testicular cross sections per mouse were analyzed. The numbers of each group for MEHP-treated C57BL/6J mice were as follows: 0 h (n = 3) and 12 h (n = 3). The numbers of each group for MEHP-treated FasL−/− mice were as follows: 0 h (n = 4) and 12 h (n = 3).
Total protein preparation and Western blot analysis.
A detailed description of total protein preparation from mice testis and Western blot analysis has been described previously (Yao et al., 2007). Total cellular proteins were detected using primary antibodies against FasL (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), TRAIL (1:250; Invitrogen), and α-tubulin (1:250; Santa Cruz Biotechnology, Inc.) coupled with horseradish peroxidase–conjugated secondary antibody (1:5000; Santa Cruz Biotechnology, Inc.). The ECL chemiluminescent substrate (Amersham Bioscience, Piscataway, NJ) was used as the detection reagent and α-tubulin as the internal control for gel loading. All experiments were performed in triplicate and repeated at least three times.
Statistical analysis.
In this study, the minimum number of animals necessary to achieve statistically significant were used after performing statistical power analysis (α = 0.05 and β = 0.05) to test if the power of sample size was sufficient (Cleophas et al., 2006; Park, 2008). Statistical results are presented as the individual means ± SEM. The data were subjected to a Student’s t-test or parametric one-way ANOVA, and statistical significance using Fisher's protected least significant difference was considered to be achieved when p ≤ 0.05.
RESULTS
Assessment of Testicular Parameters in FasL−/− and C57BL/6J Wild-Type Mice
In order to initially characterize the testis of FasL−/− mice, the body weights, testis weights, and testicular spermatid head counts were measured in peripubertal (PND 28) and adult (PND 44) mice (Table 1). No significant differences in body weights or testis weights are evident between FasL−/− and C57BL/6J mice at PND 28 (Table 1). In adults (PND 44), the body weights of FasL−/− are significantly increased compared to the C57BL/6J mice (22.15 ± 0.27 g and 19.5 ± 0.54 g, respectively), while no differences are observed between their testis weights. The testis/body weight ratio in adult FasL−/− mice is not significantly decreased as compared to C57BL/6J mice (0.0035 and 0.0038, respectively). Measurements of spermatid head counts reveal that adult FasL−/− mice have significantly reduced mature spermatids (51 ± 1.32%) as compared to C57BL/6J mice.
TABLE 1.
Comparison of C57BL/6J wild-type and FasL−/− mice baseline testicular parametersa
| Body weight (g) |
Testis weight (g) |
|||||
| Mouse strain | PND 28 | PND 44 | PND 28 | PND 44 | PND 44 | Spermatid heads/g testis/day (× 106) |
| C57BL/6J | 14.53 ± 0.40 (26) | 19.50 ± 0.54 (8) | 0.0528 ± 0.0026 (26) | 0.0744 ± 0.0024 (8) | 40.24 ± 0.68 (12) | |
| FasL−/− | 15.00 ± 0.58 (40) | 22.15 ± 0.28 (16)b | 0.0489 ± 0.0028 (40) | 0.0794 ± 0.0017 (16) | 21.06 ± 0.11 (13)b | |
Data represent the mean ± SEM, and numbers of examined mice and testis in each strain are indicated in parentheses.
p < 0.05, by ANOVA followed by Fisher's protected least significant differences test.
Abnormal Testicular Histopathology in Young FasL−/− Mice
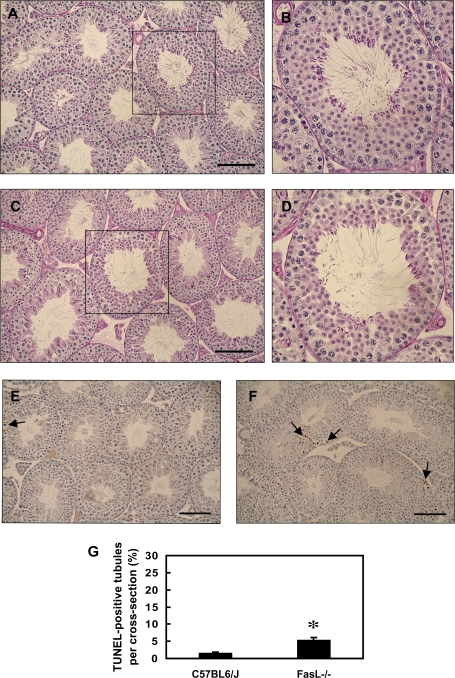
Evaluation of the histology of testicular cross sections from PND 28 FasL−/− mice revealed that 7 of 10 FasL−/− mice had abnormal tubules, and about 3% of the seminiferous tubules show a significant loss of germ cells. However, only 1 of 10 C57BL/6J mice showed similar abnormal tubules. The most severely affected seminiferous tubules were found to be populated by only Sertoli cells and few spermatogonia (Figs. 1A–D). In contrast, the testes of adult PND 44 FasL−/− mice show numerous germ cells and apparently normal histology (Figs. 2A–D), despite the lower numbers of mature spermatids produced. These observations indicate that the seminiferous epithelium of adult FasL−/− mice is able to partially recover from the drastic germ cell loss seen in the peripubertal period.
FIG. 1.
Testicular histopathology of PND 28 C57BL/6J and FasL gene–deficient (FasL−/−) mice. Cross sections (5 μm) of paraffin-embedded testis tissues were stained with periodic acid-Schiff-Hematoxylin staining (A–D) or TUNEL assay (E and F). (A, B, and E) C57BL/6J wild-type mice; (C, D, and F) FasL−/− mice; and (B and D) the magnification of one seminiferous tubule of (A) and (C) (boxed area). Arrowheads indicate Sertoli cell vacuolization. Arrows indicate TUNEL-positive germ cells (brown spots). The bar represents 100 μm. (G) The germ cell apoptotic rate by TUNEL assay. PND 28 FasL−/− mice have a significantly increased incidence of TUNEL-positive germ cells (*p < 0.05, Student’s t-test).
FIG. 2.
Testicular histopathology in PND 44 C57BL/6J and FasL−/− mice. Cross sections (5 μm) of paraffin-embedded testis tissues from PND 44 mice were stained with periodic acid-Schiff-Hematoxylin staining (A–D) and TUNEL assay (E and F). (A, B, and E) C57BL/6J mice; (C, D, and F) FasL−/− mice; and (B and D) the magnification of one seminiferous tubule of (A) and (C) (boxed area). Arrows indicate TUNEL-positive germ cells (brown spots). The bar represents 100 μm. (G) The apoptotic rate in germ cells by TUNEL assay. PND 44 FasL−/− mice have a significantly increased incidence of TUNEL-positive germ cells (*p < 0.05, Student’s t-test).
Increased Basal Apoptotic Rate in Germ Cells in FasL−/− Mice
Contrary to our expectations, FasL−/− mice at both PND 28 (Fig. 1E) and PND 44 (Fig. 2E) show dramatic increases in the basal numbers of TUNEL-positive germ cells as compared to C57BL/6J wild-type mice. The testis of FasL−/− mice shows a basal germ cell AI of 20.58 at PND 28 and 5.26 at PND 44. The testis of C57BL/6J mice had a relatively low germ cell AI at both PND 28 (5.16) and PND 44 (1.50).
Germ Cell Apoptosis was Decreased in FasL−/− Mice after MEHP Exposure
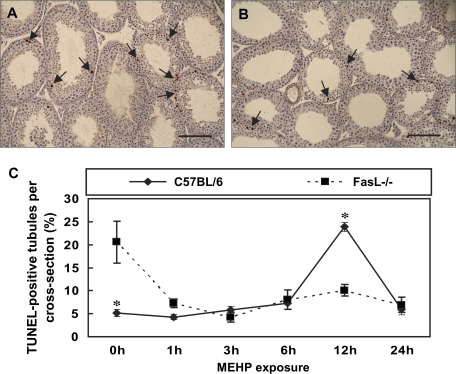
In order to evaluate the effect of MEHP on germ cell apoptosis, PND 28 FasL−/− and C57BL/6J mice were treated with 1 g/kg of MEHP for various time periods, a treatment paradigm that has been employed for the evaluation of death receptor–dependent germ cell apoptosis (Thomas and Thomas, 1984). In C57BL/6J mice, increases in apoptotic germ cells were apparent in a time-dependent manner after MEHP exposure (Fig. 3), findings that are consistent with previous reports (Richburg et al., 2000; Yao et al., 2009). As described above, the basal AI in the testis of FasL−/− mice is 20 ± 4.59, a level very near the maximal AI seen in MEHP-treated C57BL/6J mice (Fig. 1G). In response to MEHP exposure, this high basal AI of FasL−/− mice was found to significantly decrease at 6 h after exposure and remain at this decreased level at both the 12- and the 24-h time points (Fig. 3). The maximal difference in the AI between the FasL−/− and C57BL/6J mice was observed at the 12-h time point with ∼50% difference (10.09 and 23.86, respectively).
FIG. 3.
Germ cell AI in peripubertal FasL gene–deficient mice after MEHP exposure. The AI in mouse paraffin-embedded testicular cross sections was determined by TUNEL assay. PND 28 mice were treated with MEHP for various periods of time. Testicular cross sections show TUNEL-positive germ cells as brown spots (arrows) in MEHP-treated C57BL/6J mice (A) and FasL−/− mice (B) at 12 h of exposure. The bar represents 100 μm. (C) C57BL/6J wild-type mice display an increase in germ cell apoptosis at 12 h of MEHP exposure. The apoptotic rate of germ cell in FasL−/− mice is significantly decreased after MEHP exposure (*p < 0.05, Student’s t-test).
MEHP Exposure Causes no Difference in Cleaved Caspase 9 Expression in FasL−/− Mice
In order to assess the participation of the intrinsic pathway in FasL−/− mice after MEHP exposure, we evaluated the level of cleaved caspase-9 in testicular cross sections by immunohistochemistry. The level of cleaved caspase-9 is decreased in C57BL/6J mice after MEHP treatment, but no significant differences are observed in FasL−/− mice (Fig. 4).
FIG. 4.
Immunohistochemical detection of cleaved caspase-9 in mice testes. Cross sections (5 μm) of paraffin-embedded testis tissues from C57BL/6J and FasL−/− mice (PND 28) were analyzed. (A) Nontreated C57BL/6J mice, (B) C57BL/6J mice treated with MEHP for 12 h, (C) nontreated C57BL/6J mice, and (D) C57BL/6J mice treated with MEHP for 12 h. The arrows indicate the cleaved caspase-9–positive germ cells. The bar represents 100 μm. (E) Testicular cross sections show that the expression level of cleaved caspase-9 is reduced in C57BL/6J mice after MEHP exposure but remains the same expression when FasL signaling is absent (*p < 0.05, Student’s t-test).
Expression of TRAIL Protein in the Testis of Young FasL−/− Mice
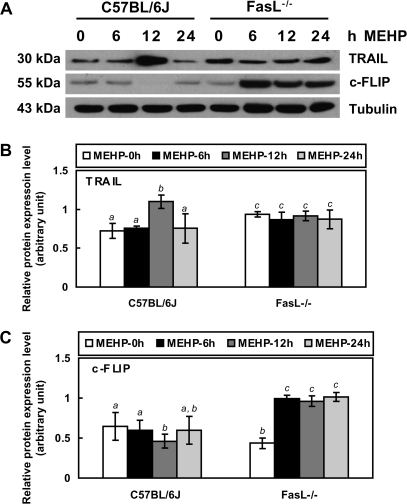
Western blot analysis of testis samples from FasL−/− (PND 28) mice show the expression of TRAIL that is 1.3-fold higher than that seen in C57BL/6J (PND 28) mice (Figs. 5A and 5B). After MEHP exposure, TRAIL levels are increased at 12 h in the testis of C57BL/6J mice, but no significant changes in TRAIL levels are measured in the testis of FasL−/− mice at any time point.
FIG. 5.
Western blot analyses of TRAIL and c-FLIP protein expression in PND 28 FasL gene–deficient mice. Total cellular proteins from whole-testis tissues were detected using primary antibodies against TRAIL (1:250) and c-FLIP (1:1000). FasL−/− mice show a significant increase in TRAIL expression compared to C57BL/6J mice. TRAIL expression in C57BL/6J wild-type mice is enhanced by 12-h MEHP exposure while there is no significant difference between control or MEHP-treated FasL−/− mice. C57BL/6J mice have a very low expression level in c-FLIP. FasL−/− mice show a higher level of c-FLIP protein after MEHP treatment. (A) Western blot, (B) the quantified measurement of TRAIL expression level, and (C) the quantified measurement of c-FLIP expression level. Significant differences between groups are denoted by bars with different letters (p < 0.05, ANOVA).
Increased c-FLIP Expression in FasL−/− Mice After MEHP Exposure
Western blot analysis of testis samples from both C57BL/6J (PND 28) and FasL−/− (PND 28) mice indicates that the basal levels of c-FLIP are very low (Figs. 5A and 5C). However, in response to MEHP exposure, the levels of c-FLIP are found to be significantly increased in the FasL−/− mice (∼2.3-fold) at each of the measured time points but do not increase at any of the time points in the testis from C57BL/6J mice.
Localization of c-FLIP in the Seminiferous Tubules
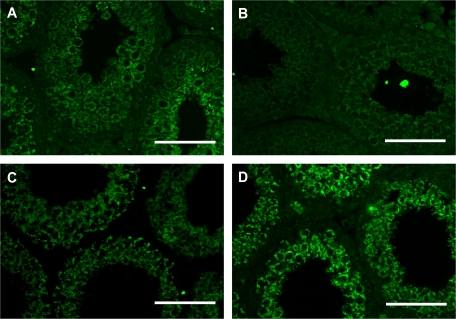
Immunofluorescence detection of c-FLIP in testicular cross sections revealed that it is localized within the cytoplasm of spermatocytes and round spermatids but not in spermatogonia of PND 28 C57BL/6J mouse testes (Fig. 6A). However, PND 28 FasL−/− mice have very low expression level of c-FLIP in the seminiferous tubule as compared to C57BL/6J mice (Fig. 6B). The c-FLIP expression in MEHP-treated C57BL/6J mice remains similar as that observed in nontreated mice (Fig. 6C). However, its expression is increased in treated FasL−/− mouse testis compared to nontreated groups (Fig. 6D).
FIG. 6.
The expression and localization of c-FLIP in the seminiferous epithelium are revealed by immunofluorescence analysis. (A) Nontreated C57BL/6J mice (PND 28), (B) nontreated FasL−/− mice (PND 28), (C) MEHP-treated C57BL/6J mice (PND 28), and (D) MEHP-treated FasL−/− mice (PND 28). Testicular cross sections show that c-FLIP is expressed in the cytoplasm of spermatocytes and spermatids. MEHP-treated FasL mice have higher level of c-FLIP expression in the testis than nontreated mice. The bar represents 50 μm.
DISCUSSION
The FasL/Fas signaling pathway has been described by our laboratory (Richburg, 2000; Richburg et al., 2000; Yao et al. 2007, 2009) and others (Lee et al., 1997, 1999; Pentikainen et al., 1999) to serve as a means to regulate germ cell apoptosis in the testis as part of a physiologic mechanism to match germ cell numbers to the Sertoli cell supportive capacity. In agreement with this concept are our previous observations that gld mice expressing a mutant form of the FasL protein show a decreased sensitivity to germ cell apoptosis triggered by MEHP-induced Sertoli cell injury as compared to wild-type mice (Richburg et al., 2000). Nevertheless, despite this clear indication of the participation of the Fas-signaling system after toxicant-induced Sertoli cell injury, the gld mice are fertile and show normal spermatogenesis indicating that this mutant form of FasL does not drastically disrupt the physiological control of apoptosis and functional spermatogenesis (Richburg et al., 2000). It is possible that the single amino acid mutant form of FasL in gld mice retains a limited ability to bind to and trigger Fas-mediated apoptosis but cannot activate Fas-signaling robustly after toxicant-induced Sertoli cell injury. Therefore, the intent of this study was to evaluate if the complete loss of the FasL expression in the testis would influence the normal physiologic regulation of germ cell apoptosis as well as alter the ability of germ cells to undergo apoptosis after MEHP-induced Sertoli cell injury.
Although adult FasL−/− mice are fertile and display typical testis histology (Figs. 2C and 2D), these mice surprisingly showed a significant reduction in the number of mature sperm they produce. In contrast, we discovered that peripubertal (PND 28) mice display many seminiferous tubules with a drastic disorganization of the seminiferous epithelium (Figs. 1C and 1D). The histological analysis of these peripubertal FasL−/− mouse testis reveals a dramatic loss of germ cells (Figs. 1C and 1D) in some seminiferous tubules as indicated by the absence of differentiating spermatocytes with only the presence of Sertoli cells and spermatogonia in the seminiferous epithelium. However, in adult FasL−/− mice, after the first wave of spermatogenesis has been completed, a partial repopulation of these severely affected tubules apparently occurs (Figs. 2C and 2D) as indicated by near-normal histology, although they produce fewer mature elongate spermatids. These observations suggest a more prominent functional role of FasL in early testis development rather than in the adult testis and the maintenance of spermatogenesis.
The incidence of germ cell apoptosis in FasL−/− (PND 28) mice is about four times higher than that of wild-type mice (Fig. 1G). In adult FasL−/− (PND 44) mice, the basal germ cell AI (5.26) is lower than that seen in the peripubertal FasL−/− mice (20.58) but higher than age-matched adult wild-type C57BL/6J (PND 44) mice (1.5). This increase in the basal incidence of germ cell apoptosis may explain the slight decrease in testis weight measured in these peripubertal mice as well as the decrease in spermatid numbers produced in the adult FasL−/− mice (Table 1). On the other hand, these findings also suggest that compensatory mechanisms may exist in FasL−/− mice to regulate germ cell apoptosis, such as other death ligands/receptors, since it appears that MEHP exposure does not trigger the intrinsic apoptotic signaling (Fig. 4).
In order to understand how germ cell apoptosis is triggered in FasL−/− mice, we further evaluated the participation of TRAIL, another member of the TNF family. The Western blot was shown that TRAIL is strongly expressed in the testis of peripubertal FasL−/− mice but at relatively low levels in wild-type mice (Figs. 5A and 5B). Therefore, it is plausible that the increased level of TRAIL in peripubertal FasL−/− mice may account for, and provide in vivo evidence for, the observed high basal levels of germ cell apoptosis seen in these mice.
In order to assess the sensitivity of FasL−/− mice to toxicant-triggered apoptosis, the MEHP-induced exposure model of prepubertal mice was used. MEHP specifically injures the Sertoli cells of the testis, inducing FasL expression, which leads to initiation of germ cell apoptosis through the extrinsic signaling pathway (Richburg et al., 1999). In wild-type mice, the basal level of germ cell apoptosis is low and is significantly induced after MEHP exposure (Fig. 3), which is consistent with our previous studies (Richburg et al., 2000; Yao et al., 2009). Since no increase in the cleavage of caspase-9 occurred in the testis of FasL−/− mice after MEHP exposure (Fig. 4), it does not appear that the intrinsic pathway participates initiation of germ cell apoptosis in the FasL−/− mice after Sertoli cell injury. We have also previously shown that peripubertal gld mice have a similar basal germ cell AI as the wild-type controls. However, in gld mice, the increase in MEHP-induced germ cell apoptosis is significantly diminished as compared to wild-type mice (Richburg et al., 2000), indicating that the expression of the single amino acid mutation in FasL only affords these mice a partial protection against germ cell apoptosis. However, the germ cells of FasL−/− mice show a much different response to MEHP exposure than either C57BL/6J or gld mice. After exposure of FasL−/− mice to MEHP, a dramatic decrease in the high basal germ cell AI of 20.58 to an AI of 8.04 occurs by 6 h after MEHP exposure and remains at this level through 24 h (Fig. 3). Since no significant changes in the levels of TRAIL protein were observed in FasL−/− mice after MEHP exposure (Fig. 5), it would appear that the negative effect of MEHP on germ cell apoptosis in FasL−/− mice lies downstream of TRAIL.
c-FLIP is a well-described inhibitor of caspase-8 (Peter, 2004), and we have previously suggested that c-FLIP is involved in regulating MEHP-triggered germ cell apoptosis (Chandrasekaran et al., 2006). The testis of the PND 28 C57BL/6J and FasL−/− mice was found to display low levels of c-FLIP, while large increases in the levels of c-FLIP were measured only in the testis of FasL−/− mice following MEHP treatment (Figs. 5A, 5C, and 6). Although increases in c-FLIP levels in FasL−/− mice after MEHP can provide a logical mechanism to account for the observed decreases in germ cell apoptosis, the mechanism underlying this specific increase in c-FLIP protein levels in these mice after MEHP exposure is not readily apparent.
There are several intriguing reports suggesting that the FasL protein itself has an inverse influence on c-FLIP expression. These studies indicate that FasL induces the degradation of c-FLIP via the ubiquitin-proteasomal pathway after the simulation of nitric oxide or reactive oxygen species (Chanvorachote et al., 2005; Wang et al., 2008). Therefore, it would be expected that in FasL−/− mice, the absence of FasL and its negative influence on c-FLIP expression would allow for a build up of c-FLIP in the cell. Moreover, our group previously reported that c-FLIP is induced in p53 gene–deficient (p53−/−) mice after MEHP exposure (Chandrasekaran and Richburg, 2005), and c-FLIP is also induced as a result of the FasL/Fas interaction in vitro when p53 is not activated (Chandrasekaran et al., 2006). It appears that p53 serves as a positive regulator to stimulate FasL/Fas signaling pathway that facilitates the activation of caspase-8 and the degradation of c-FLIP following MEHP exposure, leading to an increase in germ cell apoptosis (Chandrasekaran and Richburg, 2005). These data suggest that linkages may exist in the testis by which FasL/Fas signaling can influence p53-dependent processes that subsequently act to modulate the levels of c-FLIP in germ cells and their sensitivity to undergo death receptor–mediated apoptosis.
Taken together, the present study demonstrates that: (1) loss of FasL protein expression results in decreased production of mature spermatids in the adult testis, likely as a result of alterations in germ cell homeostatsis during the first wave of spermatogenesis, (2) the high baseline incidence of germ cell apoptosis in peripubertal FasL−/− mice is correlated with increase in the levels of TRAIL and/or the decrease in the levels of c-FLIP, and (3) declines in germ cells apoptosis observed after MEHP treatment in FasL−/− mice closely corresponds to the increased levels of c-FLIP that occur. These novel findings provide new insights into the functional roles of FasL in the testis at distinct developmental periods and further indicates that FasL itself is required for the regulation of c-FLIP levels in the testis.
FUNDING
The University of Texas at Austin's Center for Molecular and Cellular Toxicology (Y.-C.L.); National Institute of Environmental Health Sciences (NIEHS)/National Institute of Health (ES016591 to J.H.R.); pilot project from the NIEHS Center Grant (P30ES07784 to J.H.R.).
Acknowledgments
We greatly appreciate Dr Karray for his support in providing the breeding pair of FasL+/− mice. We also thank the assistance of Jessica Cobarrubia and James Harman in their expert editorial assistance in the preparation of this manuscript.
References
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya N, Dufour JM, Vo MN, Okita J, Okita R, Kim KH. Differential effects of phthalates on the testis and the liver. Biol. Reprod. 2005;72:745–754. doi: 10.1095/biolreprod.104.031583. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran Y, McKee CM, Ye Y, Richburg JH. Influence of TRP53 status on FAS membrane localization, CFLAR (c-FLIP) ubiquitinylation, and sensitivity of GC-2spd (ts) cells to undergo FAS-mediated apoptosis. Biol. Reprod. 2006;74:560–568. doi: 10.1095/biolreprod.105.045146. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran Y, Richburg JH. The p53 protein influences the sensitivity of testicular germ cells to mono-(2-ethylhexyl) phthalate-induced apoptosis by increasing the membrane levels of Fas and DR5 and decreasing the intracellular amount of c-FLIP. Biol. Reprod. 2005;72:206–213. doi: 10.1095/biolreprod.104.030858. [DOI] [PubMed] [Google Scholar]
- Chanvorachote P, Nimmannit U, Wang L, Stehlik C, Lu B, Azad N, Rojanasakul Y. Nitric oxide negatively regulates Fas CD95-induced apoptosis through inhibition of ubiquitin-proteasome-mediated degradation of FLICE inhibitory protein. J. Biol. Chem. 2005;280:42044–42050. doi: 10.1074/jbc.M510080200. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Cleophas TJ, Cleophas TJ, Zwinderman AH, Zwinderman AH, Cleophas TF. Statistics Applied to Clinical Trials. Norwell, MA: Kluwer Academic Publishers; 2006. [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Hyer ML, Samuel T, Reed JC. The FLIP-side of Fas signaling. Clin. Cancer Res. 2006;12:5929–5931. doi: 10.1158/1078-0432.CCR-06-2098. [DOI] [PubMed] [Google Scholar]
- Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Levi-Strauss M. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J. Immunol. 2004;172:2118–2125. doi: 10.4049/jimmunol.172.4.2118. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I, Golks A, Krammer PH. Death receptor signaling. J. Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140:852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997a;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997b;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Park HM. Hypothesis Testing and Statistical Power of a Test. Bloomington, IN: The University Information Technology Services (UITS) Center for Statistical and Mathematical Computing, Indiana University; 2008. [Google Scholar]
- Pentikainen V, Erkkila K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am. J. Physiol. 1999;276:E310–E316. doi: 10.1152/ajpendo.1999.276.2.E310. [DOI] [PubMed] [Google Scholar]
- Peter ME. The flip side of FLIP. Biochem. J. 2004;382:e1–e3. doi: 10.1042/BJ20041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Richburg JH. The relevance of spontaneous- and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol. Lett. 2000;112-113:79–86. doi: 10.1016/s0378-4274(99)00253-2. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol. Appl. Pharmacol. 1996;137:42–50. doi: 10.1006/taap.1996.0055. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Nanez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 1999;160:271–278. doi: 10.1006/taap.1999.8786. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Nanez A, Williams LR, Embree ME, Boekelheide K. Sensitivity of testicular germ cells to toxicant-induced apoptosis in gld mice that express a nonfunctional form of Fas ligand. Endocrinology. 2000;141:787–793. doi: 10.1210/endo.141.2.7325. [DOI] [PubMed] [Google Scholar]
- Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Russell LD. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Russell LD, Goh JC, Rashed RM, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol. Reprod. 1988;39:105–118. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr. Biol. 1997;7:693–696. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Thomas MJ. Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit. Rev. Toxicol. 1984;13:283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J. Immunol. 2008;180:3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RA, Nakane PK, Koji T. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol. Reprod. 1998;58:1250–1256. doi: 10.1095/biolreprod58.5.1250. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol. Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. J. Biol. Chem. 2007;282:5420–5431. doi: 10.1074/jbc.M609068200. [DOI] [PubMed] [Google Scholar]