Abstract
Daunorubicin (daunomycin; NSC 82151) is a fermentation-derived anthracycline antibiotic that is clinically useful in the treatment of human leukemias. Daunorubicin itself is found rarely in microbial fermentations, but is present normally in the form of glycoside derivatives that yield the free drug on simple acid hydrolysis. A major by-product of daunorubicin fermentations is usually the structurally related anthracyclinone epsilon-rhodomycinone. We have used mutants of a daunorubicin-producing Streptomyces species to study the biosynthetic relationship between epsilon-rhodomycinone and daunorubicin. We found that exogenously added epsilon-rhodomycinone can be converted to daunorubicin glycosides by a nonproducing mutant and by a mutant that produces daunorubicin glycosides but not epsilon-rhoeomycinone. Molar conversion efficiences were in the 15 to 30% range. The latter mutant was also shown to convert exogenous 14C-labeled epsilon-rhodomycinone to 14C-labeled daunorubicin glycosides, again at conversion efficiencies of about 25%. The same biotransformation was observed with daunorubicin production strain C5, which normally accumulates both epsilon-rhodomycinone and daunorubicin glycosides. A significant percentage (16 to 37%) of exogenously added epsilon-[14C]rhodomycinone was metabolized by strain C5, and 22 to 32% of the metabolized radioactivity could be recovered as daunorubicin glycosides. A mathematical model of epsilon-rhodomycinone metabolism was constructed based on plausible assumptions concerning the kinetics of epsilon-rhodomycinone accumulation and catabolsim. When analyzed according to this model, our data indicate that most (63 to 73%), but not all, of the daunorubicin glycosides accumulated in the experiments with production strain C5 derived from epsilon-rhodomycinone. A pathway network for the biosynthesis of daunorubicin glycosides is proposed that is in agreement with these data. In this proposed pathway network, epsilon-rhodomycinone is an intermediate in one of at least two pathways which yield daunorubicin glycosides.
Full text
PDF

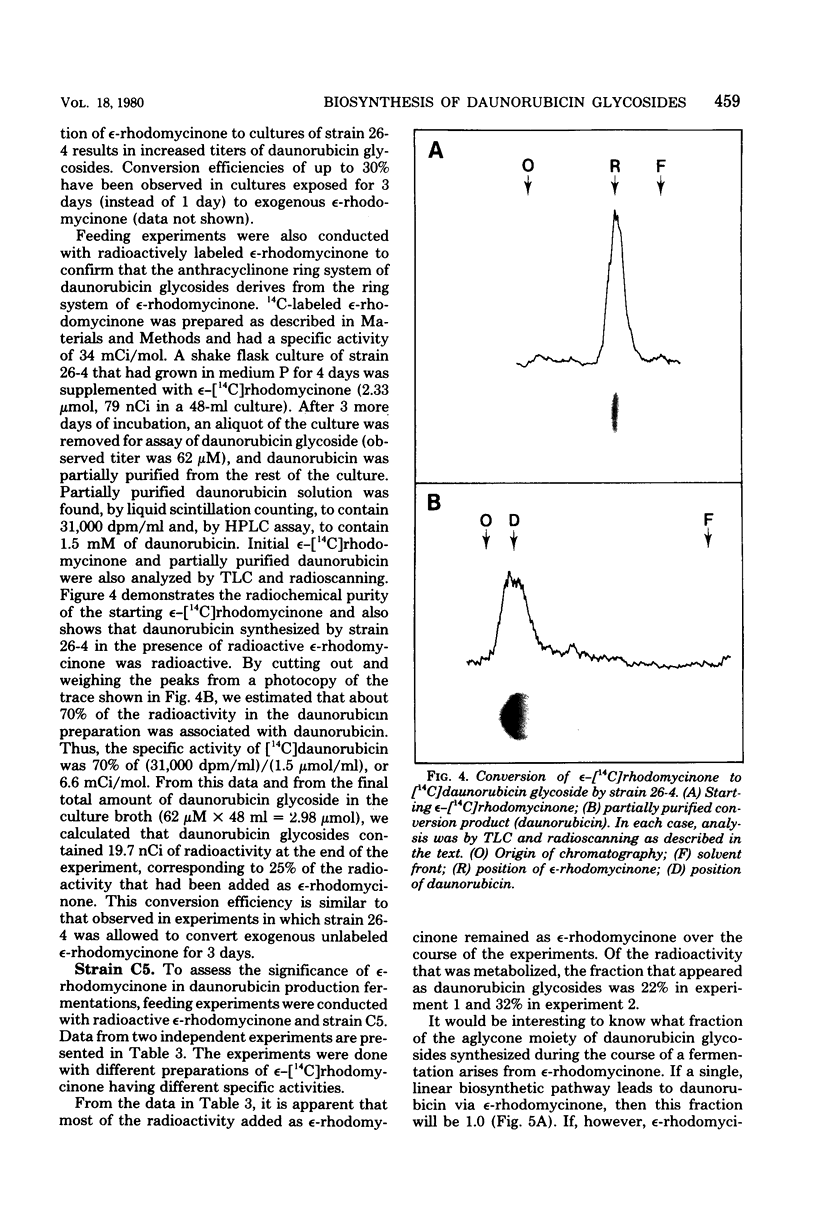
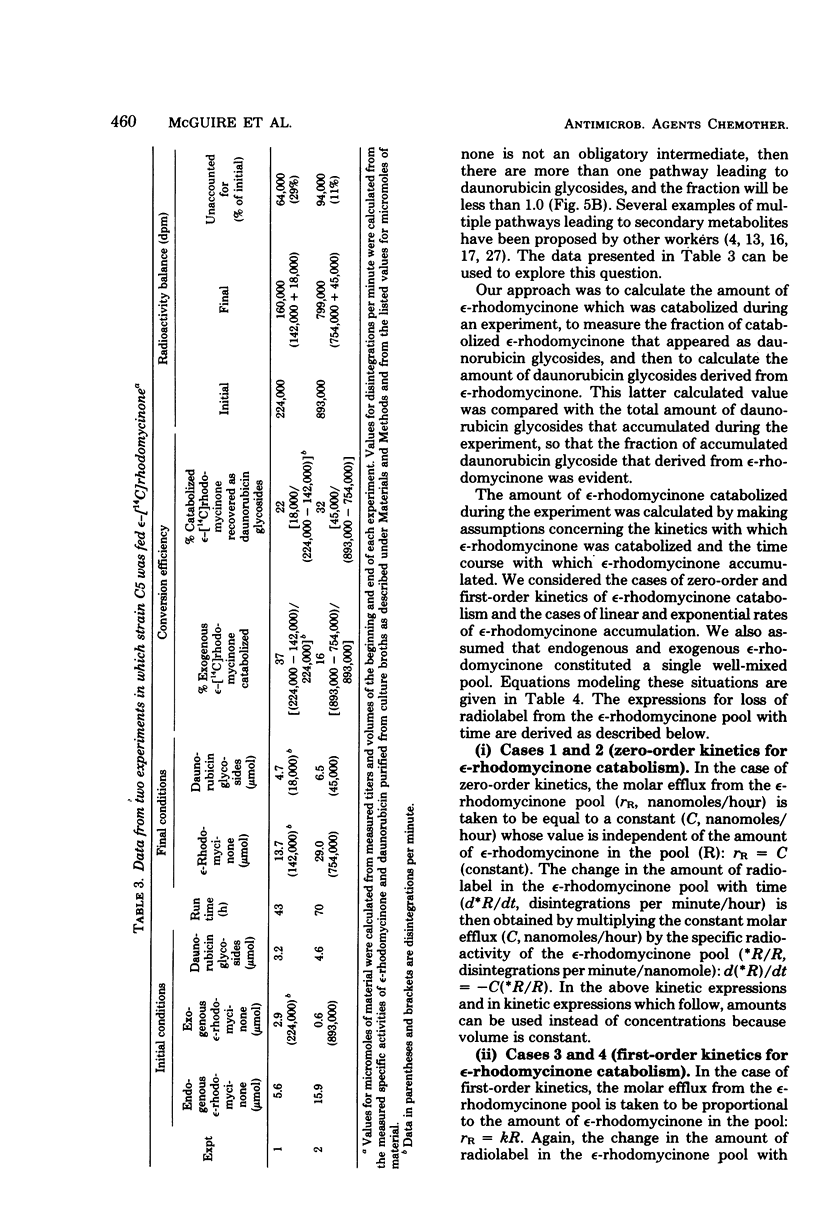
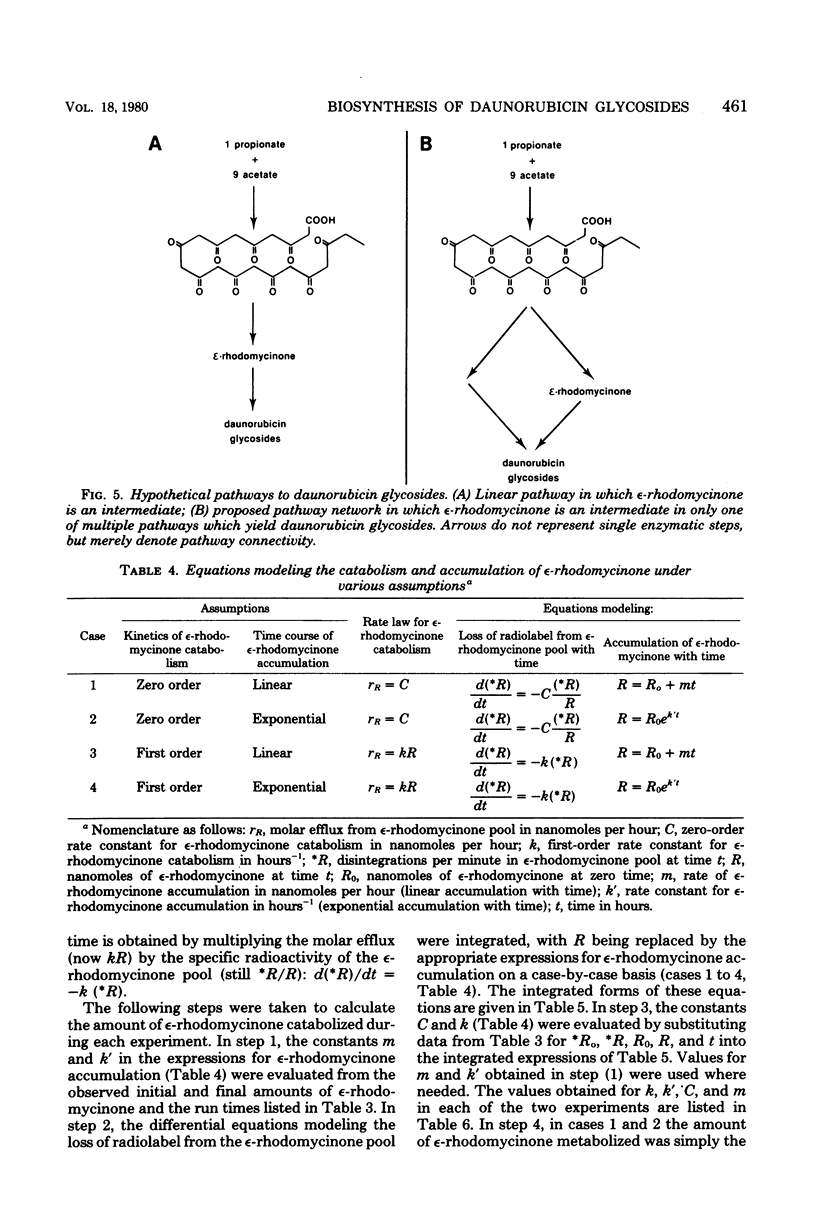
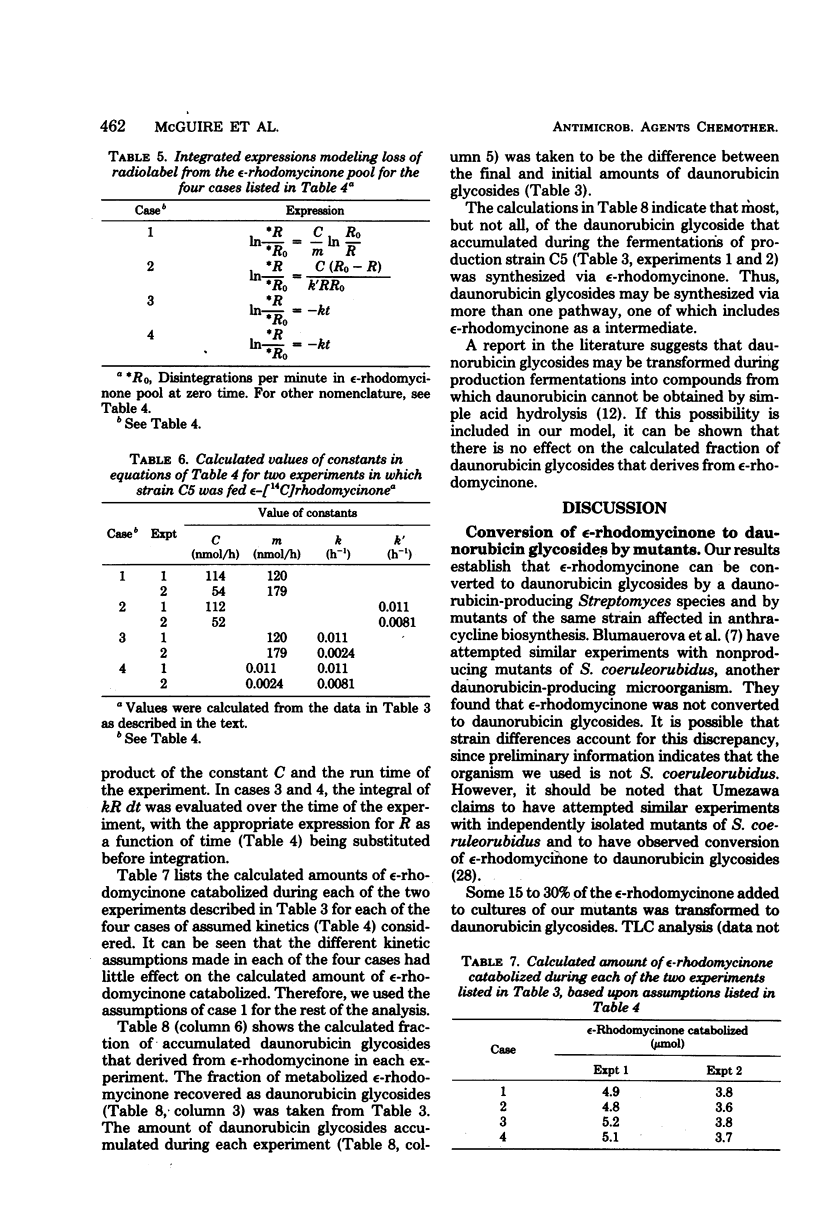

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F. New antitumor anthracyclines. Lloydia. 1977 Jan-Feb;40(1):45–66. [PubMed] [Google Scholar]
- Blumauerová M., Královcová E., Matejů J., Jizba J., Vanek Z. Biotransformations of anthracyclinones in Streptomyces coeruleorubidus and Streptomyces galilaeus. Folia Microbiol (Praha) 1979;24(2):117–127. doi: 10.1007/BF02927295. [DOI] [PubMed] [Google Scholar]
- Blumauerová M., Královocová E., Hostálek Z., Vanek Z. Intra- and interspecific cosynthetic activity of mutants of Streptomyces coeruleorubidus and Streptomyces galilaeus impaired in the biosynthesis of anthracyclines. Folia Microbiol (Praha) 1979;24(2):128–135. doi: 10.1007/BF02927296. [DOI] [PubMed] [Google Scholar]
- Blumauerová M., Matejů J., Stajner K., Vanek Z. Studies on the production of daunomycinone-derived glycosides and related metabolites in Streptomyces coeruleorubidus and Streptomyces peucetius. Folia Microbiol (Praha) 1977;22(4):275–285. doi: 10.1007/BF02877657. [DOI] [PubMed] [Google Scholar]
- Davis H. L., Davis T. E. Daunorubicin and adriamycin in cancer treatment: an analysis of their roles and limitations. Cancer Treat Rep. 1979 May;63(5):809–815. [PubMed] [Google Scholar]
- Essery J. M., Doyle T. W. The synthesis of epsilon-rhodomycinone- and carminomycin-11-methyl ethers. J Antibiot (Tokyo) 1979 Mar;32(3):247–249. doi: 10.7164/antibiotics.32.247. [DOI] [PubMed] [Google Scholar]
- Kern D. L., Bunge R. H., French J. C., Dion H. W. The identification of epsilon-rhodomycinone and 7-deoxy-daunorubicinol aglycone in daunorubicin beers. J Antibiot (Tokyo) 1977 May;30(5):432–434. doi: 10.7164/antibiotics.30.432. [DOI] [PubMed] [Google Scholar]
- Komiyana T., Matsuzawa Y., Oki T., Inui T., Takahashi Y., Naganawa H., Takeuchi T., Umezawa H. Baumycins, new antitumor antibiotics related to daunomycin. J Antibiot (Tokyo) 1977 Jul;30(7):619–621. doi: 10.7164/antibiotics.30.619. [DOI] [PubMed] [Google Scholar]
- Paranosenkova V. I., Karpov V. L. Izuchenie biosinteza uglevodnogo fragmenta rubomitsina. Antibiotiki. 1976 Apr;21(4):299–301. [PubMed] [Google Scholar]
- Paulick R. C., Casey M. L., Whitlock H. W. Letter: A 13C nuclear magnetic resonance study of the biosynthesis of daunomycin from 13CH313CO2Na. J Am Chem Soc. 1976 May 26;98(11):3370–3371. doi: 10.1021/ja00427a052. [DOI] [PubMed] [Google Scholar]
- Rozencweig M., De Sloover C., Von Hoff D. D., Tagnon H. J., Muggia F. M. Anthracycline derivatives in new drug development programs. Cancer Treat Rep. 1979 May;63(5):807–808. [PubMed] [Google Scholar]
- Vanek Z., Tax J., Komersová I., Sedmera P., Vokoun J. Anthracyclines. Folia Microbiol (Praha) 1977;22(2):139–159. doi: 10.1007/BF02881640. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Rozencweig M., Slavik M. Daunomycin: an anthracycline antibiotic effective in acute leukemia. Adv Pharmacol Chemother. 1978;15:1–50. doi: 10.1016/s1054-3589(08)60480-9. [DOI] [PubMed] [Google Scholar]