Abstract
During organ formation and regeneration a proper balance between promoting and restricting growth is critical to achieve stereotypical size. Limb bud outgrowth is driven by signals in a positive feedback loop involving fibroblast growth factor (Fgf) genes, sonic hedgehog (Shh) and Gremlin1 (Grem1)1. Precise termination of these signals is essential to restrict limb bud size2–4. The current model predicts a sequence of signal termination consistent with that in chick limb buds4. Our finding that the sequence in mouse limb buds is different led us to explore alternative mechanisms. By analyzing compound mouse mutants defective in genes comprising the positive loop, we uncovered genetic evidence that FGF signaling can repress Grem1 expression, revealing a novel Fgf/Grem1 inhibitory loop. This repression occurs in both mouse and chick limb buds, and is dependent on high FGF activity. These data support a mechanism where the positive Fgf/Shh loop drives outgrowth and an increase in FGF signaling, which triggers the Fgf/Grem1 inhibitory loop. The inhibitory loop then operates to terminate outgrowth signals in the order observed in either mouse or chick limb buds. Our study unveils the concept of a self-promoting and self-terminating circuit that may be used to attain proper tissue size in a broad spectrum of developmental and regenerative settings.
List of key genes: Fibroblast growth factor (Fgf), Fgf4, Fgf8, Fgf receptor (Fgfr), Fgfr1, Fgfr2, sonic hedgehog (Shh), gremlin 1 (Grem1), bone morphogenetic protein 4 (Bmp4), Spry4, Msx2, Brachyury (T), Prx1
Several models have recently been formulated to explain the control of appendage size5–8. The models focus on how a signal in constant supply is translated into a threshold of growth capability. Evidence from vertebrate limb development suggests that precise termination of growth signals is a key mechanism that restricts limb bud size2–4. These signals include Fgfs (Fgf4, Fgf8, Fgf9 and Fgf17) expressed in the Apical Ectodermal Ridge (AER and AER-Fgfs), and Shh and Grem1 expressed in the underlying mesenchyme. They function in a transcriptional feedback loop (Fgf/Shh loop) to induce and sustain each other’s expression1,9–11.
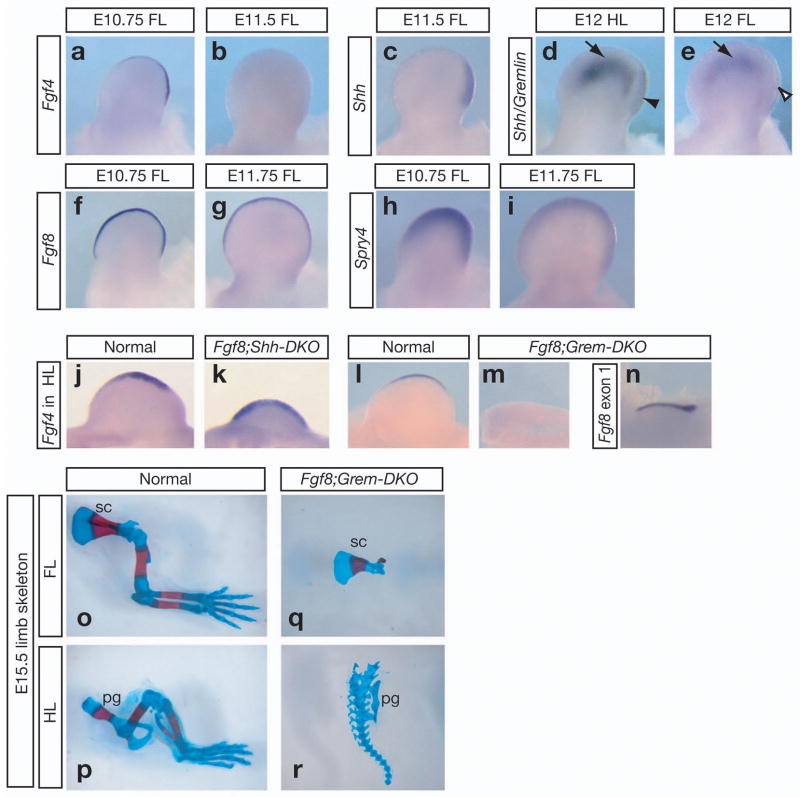
The current model for breakdown of the Fgf/Shh loop is based on the observation that current and former Shh-expressing cells (Shh-lineage cells) are unable to express Grem1 in response to SHH induction4. Expansion of the Shh-lineage would lead to cessation of Grem1 expression followed by that of Fgf4 and then Shh4. This sequence of signal termination is consistent with that observed in chick. However, we found that in mouse limb buds, Fgf4 expression ceases first, followed by Shh and then Grem1 (Fig. 1a–e). To identify alternative mechanisms of signal termination, we investigated the regulation of Fgf4, the first gene of the loop that ceases to be expressed in mouse limb buds. Although Fgf4 itself is not essential for limb development9, it is regulated by essential genes, including Shh and Grem110,12,13. Furthermore, termination of Fgf4 expression coincides with a drop in collective AER-FGF activity14 (Fig. 1f–i). Therefore the extinction of Fgf4 expression serves as readout for the trigger that breaks down the Fgf/Shh loop.
Figure 1. Fgf8 repression of Fgf4 expression is dependent on Grem1 but not Shh.
(a–n) Gene expression in mouse forelimb (FL) or hindlimb (HL) buds. (a–i) In wild-type mouse limb buds, Fgf4 expression terminates first, followed by Shh and then Grem1. In d and e, a combination of RNA probes is used to detect non-overlapping patterns of Shh (arrowhead) and Grem1 (arrow) expression. Both genes are expressed in the E12 hindlimb bud, which is at an earlier developmental stage than the E12 forelimb bud from the same embryo, where only Grem1 is expressed(n=4). Downregulation of Spry4 expression at E11.75 compared to E10.75 reflects decreased AER-FGF activity14, consistent with loss of Fgf4 and reduced Fgf8 expression. (j–m) In E10.5 hindlimb buds, Fgf4 expression is detected in the posterior two-thirds of the AER in normal, expanded through the entire AER in the Fgf8;Shh-DKO mutant and absent in Fgf8;Grem1-DKO mutant. (n) Detection of the remaining exon 1 of the truncated Fgf8 mRNA indicates that the AER is present. (o–r) No forelimb or hindlimb elements are observed in Fgf8;Grem-DKO skeletons. Fgf8;Shh-DKO embryos were generated by mating Msx2cre;Fgf8del/flox;Shh+/− males to Fgf8flox/flox;Shh+/− females15,17. Fgf8;Grem-DKO embryos were generated by crossing Msx2cre;Fgf8del/+;Grem1+/− males to Fgf8flox/flox;Grem1+/− females13,15. sc, scapula; pg, pelvic girdle.
Fgf4 expression is severely reduced in Shh mutant and absent in Grem1 mutant limb buds, but expanded and prolonged in Fgf8 AER-knockout (Fgf8-KO) forelimb buds10–13,15,16. We investigated whether these regulators act genetically upstream or downstream of each other to control Fgf4 expression. To address if Fgf8 represses Fgf4 expression by inhibiting Shh maintenance of Fgf4, we inactivated both Fgf8 and Shh in the limb buds by introducing a null allele of Shh17 into the Fgf8-KO background15 (Msx2cre;Fgf8fl/fl;Shh−/− mutant, or Fgf8;Shh-DKO for double knockout). In Fgf8;Shh-DKO forelimb and hindlimb buds, Fgf4 is detected in an expanded pattern in the entire AER (Fig. 1j, k and data not shown), demonstrating that Fgf8 repression of Fgf4 expression is genetically downstream of Shh.
Grem1 functions downstream of Shh to induce Fgf4 expression10,11. To address if Fgf8 represses Fgf4 expression by inhibiting Grem1, we inactivated both Fgf8 and Grem1 in the limb buds by introducing a null allele of Grem113 into the Fgf8-KO background (Msx2cre;Fgf8fl/fl;Grem1−/− mutant, or Fgf8;Grem-DKO). In Fgf8;Grem-DKO limb buds, Fgf4 is no longer maintained, even though the AER is present (Fig. 1l–n). With AER-Fgf expression severely compromised, all limb skeletal elements are absent (Fig. 1o–r), similar to the phenotype in Fgf4 and Fgf8 double mutant limbs18. This loss of Fgf4 expression in Fgf8;Grem-DKO limb buds demonstrates that Fgf8 repression of Fgf4 is dependent on Grem1.
To understand the mechanism of this dependence, we investigated whether Fgf8 represses Grem1 expression. Consistent with this possibility, the Grem1 domain is closer to the AER than normal in Fgf8-KO limb buds (Fig. 2a,b). As all AER-FGFs perform similar roles in limb bud outgrowth19, we tested a more general hypothesis that collective AER-FGF signaling could repress Grem1 expression. In support of this, the Grem1 expression domain is closer to the AER in various other Fgf and Fgf receptor (Fgfr) mutants (Supplementary Fig. 1). One caveat is that these mutant limb buds are smaller than normal, raising the possibility that the Grem1 domain is closer to the AER due to reduction of the distal mesenchyme. To test FGF repression of Grem1 more rigorously, we inactivated Fgfr1 and Fgfr2 in a small portion of the limb bud mesenchyme (Shhcre;Fgfr1co/co;Fgfr2c/c mutant, or Fgfr1;r2-DKO)20–22. We found that though FGF signaling is severely disrupted in Fgfr-inactivated cells, Fgfr1;r2-DKO limb buds exhibit normal size, shape, and cell survival at E11.5 (Fig. 2c,d and data not shown). In this setting, Grem1 is ectopically expressed within Fgfr-inactivated domain (Fig. 2e–g, Supplementary Fig. 2). Our loss-of-function data complement a previous observation that FGF-soaked beads can inhibit Grem1 expression in chick limb buds23. These data demonstrate that AER-FGF signaling is sufficient and necessary to repress Grem1 expression in the distal mesenchyme.
Figure 2. FGF signaling represses Grem1 expression.
(a–k) Gene expression in mouse forelimb buds at (a,b) E10.75, (c–i) E11 and (j,k) E11.5. (a) The yellow bracket indicates distance between AER and high Grem1 expression. (c,d) Reduced Spry4 expression in the posterior mesenchyme delineates Fgfr-inactivated domain. Arrows in d, f, i, k indicate anterior boundary of Shhcre-mediated receptor inactivation domain. (e–g) Grem1 is ectopically expressed in the distal portion of the Fgfr-inactivated domain. Limb buds shown in d and f are contralateral limb buds from the same embryo. Boxed region in f is magnified in g. (h,i) Bmp4 is reduced in Fgfr-inactivated domain, but is present in the overlying AER. (j,k) No ectopic Grem1 expression is detected in Shhcre;Bmpr1afl/fl (Bmpr1a-KO) limb buds. (l) A diagram depicting gene expression regulation within the Fgfr-inactivated domain in Fgfr1;r2- DKO limb buds as shown in g. Bmps from the AER may be required to promote ectopic Grem123–27, leading to higher Grem1 in the distal portion of the Fgfr inactivated domain as shown in Fig. 2g, Fig. 3l,n. Fgfr1;r2-DKO embryos were generated by mating Shhcre;Fgfr1co/co;Fgfr2c/+ males to Fgfr1co/co;Fgfr2c/+ females20–22. Bmpr1a-DKO embryos were generated by mating Shhcre;Bmpr1afl/+ males to Bmpr1afl/fl females21,30.
High levels of exogenous Bone Morphogenetic Protein (BMP) have been shown to inhibit Grem1 expression23–25. We found that Bmp4 and Bmp7 expression is reduced in the Fgfr-inactivated cells in Fgfr1;r2-DKO limb buds, raising the possibility that AER-FGFs repress Grem1 by maintaining high BMP signaling (Fig. 2h,i and data not shown). However, inactivation of Bmpr1a with Shhcre does not lead to ectopic Grem1 expression (Fig. 2j,k), suggesting that AER-FGF repression of Grem1 is not mediated through BMPs. It remains possible that BMP signaling may be required to promote Grem1 expression in parallel to FGF repression of Grem123–27 (Fig. 2l).
To investigate the threshold requirement for FGF repression of Grem1, we compared Grem1 expression to changes in FGF signaling. In mouse limb buds, downregulation of Grem1 in the distal mesenchyme correlates with progressively higher levels of FGF signaling as development proceeds (Fig. 3a–e). This result is consistent with that observed in chick limb buds23,25. These gene expression data led us to hypothesize that AER-FGF signaling represses Grem1 in a dose-sensitive manner.
Figure 3. AER-FGF repression of Grem1 expression is dose-sensitive.
(a–e) Correlation between Grem1 repression in the distal mesenchyme and increased AER-FGF signaling (yellow brackets in c–e)14. (f–i) Beads (circle) soaked in 1mg/ml FGF2 suppress Grem1 expression distal to the bead, possibly working in combination with FGFs expressed from the AER (n=4/6). No Grem1 suppression is observed with 0.1mg/ml FGF2 (n=7). Beads were implanted in stage 21 limb buds and gene expression was assayed after 12 hours of incubation. (j–n) While ectopic Grem1 expression is more intense in E11.5 Fgfr1;r2-DKO limb buds than in Fgfr1-DKO limb buds, Grem1 expression outside of the Fgfr-inactivated domain remains comparable. Boxed regions in k,m are magnified in l,n, respectively. (o,p) Though absent in E11.75 normal limb bud, Fgf4 expression persists in the posterior AER overlying the Fgfr-inactivated domain in Fgfr1;r2-DKO limb buds (delineated by yellow dashed line).
We tested this hypothesis in both mouse and chick limb buds. In chick, implantation of beads soaked in 1mg/ml of FGF2 leads to a clear repression of Grem1 (n=4/6, Fig. 3i), consistent with previous observation23. This repression is not observed using beads soaked in 0.1mg/ml of FGF2 (n=0/7), even though Spry2 upregulation is detected adjacent to the beads, confirming FGF activity (data not shown). In mouse, ectopic Grem1 expression is more intense in Fgfr1;r2-DKO limb buds compared to Shhcre;Fgfr1co/co (Fgfr1-KO) limb buds (Fig. 3j–n). As there is less residual FGF signaling in Fgfr1;r2-DKO limb buds than that in Fgfr1-KO limb buds (based on expression of FGF readouts, data not shown), lower FGF signaling correlates with less Grem1 repression. Thus data from both chick and mouse limb buds support the scenario that, during limb bud outgrowth, a progressive increase in AER-FGF level leads to increasing repression of Grem1 in the distal mesenchyme.
To return to our question regarding the mechanism that abolishes Fgf4 expression and triggers Fgf/Shh loop termination, we found that Fgf4 expression is prolonged in Fgfr1;r2-DKO forelimb buds at E11.75 (Fig. 3o,p), likely as a result of ectopic Grem1 expression11–13,23. These data demonstrate that FGF repression of Grem1 plays a critical role in triggering the termination of limb bud outgrowth signals.
The finding that AER-FGF signaling can repress Grem1 expression reveals an inhibitory feedback loop (Fgf/Grem1 loop) that is interconnected with the existing Fgf/Shh positive feedback loop (Fig. 4a). The dosage dependency of this repression led us to propose a model (Fig. 4b) whereby positive and inhibitory feedback loops are coordinated first to promote (in phase I, with Fgf/Shh positive loop only) and later to terminate limb bud outgrowth (in phase II, with the induction of Fgf/Grem1 inhibitory loop). In a wild-type limb bud in phase I (e.g. ~E9.5–E10.5 in mouse forelimb bud, ~stage18–23 in chick wing bud), we hypothesize that in phase I, AER-FGF concentration is too low to efficiently repress Grem1 (Fig. 3c,d,g,i). Instead, AER-FGFs act through Shh and BMPs to upregulate Grem110,24,25. As a result, Grem1 is expressed in the distal mesenchyme abutting the AER (Fig. 3a) and efficiently promotes AER-Fgf expression 3,26,28,29. In phase I, the positive Fgf/Shh loop induces and sustains limb outgrowth signals leading to a progressive increase in collective AER-FGFs entering phase II (Fig. 3c–e).
Figure 4. A model describing a self-promoting and self-terminating mechanism to control limb bud outgrowth signals.
(a) A schematic of the inhibitory loop (outlined in red) in relation to the existing positive loop. Arrows indicate activation, while “T” lines indicate inhibition. BMP regulation of AER architecture indirectly affects Fgf8 expression12,13. Grem1 is also positively regulated by BMP signaling11,24,25,27. (b) A model explaining how the two loops are utilized to first promote (phase I) and then terminate (phase II) signals. Dashed lines represent diminishing regulation while dashed line with “X” emphasizes absence of regulation. In phase I, the positive regulatory loop operates to increase all signals. Transition to phase II occurs when AER-FGFs reach a level that confers efficient Grem1 repression (represented by “T” in both distal and posterior mesenchyme). Together with mesenchymal growth, the Grem1-negative domain expands. Increasing distance between Grem1-expressing cells and Fgf or Shh-expressing cells leads to inability of signals to maintain one another at the end of phase II.
We hypothesize that the transition to phase II occurs when AER-FGF signaling surpasses the threshold needed for Grem1 repression in the distal mesenchyme, triggering the Fgf/Grem1 inhibitory loop (Fig. 4b, e.g. ~E10.5–E12 in mouse forelimb bud, ~stage 23–27 in chick wing bud). This repression establishes a Grem1-negative domain separating Grem1-expressing cells and the AER (Fig. 2a). As development proceeds, the Grem1-negative domain expands both distally and posteriorly due to mesenchymal growth. We postulate that this expansion would trigger different rate-limiting steps in mouse versus chick limb buds, leading to distinct sequences of signal termination. In a mouse limb bud, the size of the Grem1-negative domain would first exceed the distal range of GREM1 protein diffusion, leading to downregulation of collective AER-FGFs followed by loss of Shh and then Grem1 expression (Fig. 4b, end of phase II). Loss of Grem1 expression would mark the beginning of AER degeneration and gradual extinction of Fgf8 expression12,13. Conversely in a chick limb bud, the size of the Grem1-negative domain would first exceed the anterior range of SHH diffusion, leading to loss of Grem1 expression followed by extinction/reduction of different AER-Fgfs and then termination of Shh. Thus, this model can explain the sequence of signal abrogation in both mouse and chick. We further postulate that in a wider spectrum of divergent species, parameters such as signal diffusion range, threshold requirement of signaling activity and extent of mesenchyme expansion dictate the timing of outgrowth signal termination.
There are two key differences between our model and the existing model of signal termination4 (Shh-lineage model). First, the Shh-lineage model only accounts for Grem1 repression in posterior mesenchyme. Our model explains Grem1 repression in both the posterior and distal mesenchyme, which accommodates the sequence of signal termination in both mouse and chick limb buds. Second, the molecular mechanisms at the core of the two models are distinct. In the Shh-lineage model, the factor responsible for cell-autonomous repression of Grem1 in Shh-lineage cells has not been identified. Our finding that Fgfr inactivation allows Grem1 expression in Shh-lineage cells (Fig. 2f,g, Fig. 3k–n) suggests that maintenance of FGF signaling is essential for Grem1 repression in this lineage. In our model, signal termination relies on FGF repression of Grem1 expression. The finding that an FGF bead placed in the anterior chick limb bud downregulates Grem1 expression23 (Fig. 3i) indicates that FGF repression of Grem1 can occur independent of the Shh-lineage influence.
In this study, we identified an inhibitory Fgf/Grem1 feedback loop that operates in both mouse and chick limb buds. We propose a model whereby the known positive Fgf/Shh feedback loop acts to increase AER-FGF concentration, triggering the inhibitory loop, which in turn leads to extinction of outgrowth signals. These interconnected positive and inhibitory loops direct a limb outgrowth program that once initiated, can propagate and self-terminate.
Supplementary Material
Acknowledgments
We are grateful to Drs. John Fallon, Gail Martin, Rebecca Bacon, Grace Boekhoff-Falk, Brian Harfe, Mark Lewandoski, Deneen Wellik as well as members of the Sun laboratory, in particular Lisa Abler for discussions and critical reading of the manuscript. We thank Drs Richard Behringer, Chin Chiang, Chuxia Deng, Brian Harfe, Richard Harland, Peter Lonai, Yuji Mishina, and Cliff Tabin for mouse strains. We are grateful to Amber Lashua, Minghui Zhao, and Jennifer Heinritz for technical assistance. J.M.V. was supported by the NIH funded predoctoral training program in Genetics (5T32GM07133). This work was supported by a March of Dimes Basil O’Connor award 5-FY03-13 (to X.S.) and NIH grant RO1 HD045522 (to X.S.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Figure 4 of the main text summarizes the findings of this paper.
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
- 1.Niswander L. Interplay between the molecular signals that control vertebrate limb development. Int J Dev Biol. 2002;46:877–81. [PubMed] [Google Scholar]
- 2.Sanz-Ezquerro JJ, Tickle C. Fgf signaling controls the number of phalanges and tip formation in developing digits. Curr Biol. 2003;13:1830–6. doi: 10.1016/j.cub.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999;126:883–94. doi: 10.1242/dev.126.5.883. [DOI] [PubMed] [Google Scholar]
- 4.Scherz PJ, Harfe BD, McMahon AP, Tabin CJ. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science. 2004;305:396–9. doi: 10.1126/science.1096966. [DOI] [PubMed] [Google Scholar]
- 5.Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–26. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Day SJ, Lawrence PA. Measuring dimensions: the regulation of size and shape. Development. 2000;127:2977–87. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Bellido AC, Garcia-Bellido A. Cell proliferation in the attainment of constant sizes and shapes: the Entelechia model. Int J Dev Biol. 1998;42:353–62. [PubMed] [Google Scholar]
- 8.Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci U S A. 2007;104:3835–40. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, et al. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- 10.Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- 11.Panman L, et al. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133:3419–28. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- 12.Michos O, et al. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–10. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 13.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34:303–7. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 14.Minowada G, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–75. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 15.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 16.Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26:455–9. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang C, et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 19.Lu P, Minowada G, Martin GR. Increasing Fgf4 expression in the mouse limb bud causes polysyndactyly and rescues the skeletal defects that result from loss of Fgf8 function. Development. 2006;133:33–42. doi: 10.1242/dev.02172. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Qiao W, Li C, Deng CX. Generation of Fgfr1 conditional knockout mice. Genesis. 2002;32:85–86. doi: 10.1002/gene.10028.abs. [DOI] [PubMed] [Google Scholar]
- 21.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–28. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Eswarakumar VP, et al. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129:3783–3793. doi: 10.1242/dev.129.16.3783. [DOI] [PubMed] [Google Scholar]
- 23.Merino R, et al. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development. 1999;126:5515–22. doi: 10.1242/dev.126.23.5515. [DOI] [PubMed] [Google Scholar]
- 24.Nissim S, Hasso SM, Fallon JF, Tabin CJ. Regulation of Gremlin expression in the posterior limb bud. Dev Biol. 2006;299:12–21. doi: 10.1016/j.ydbio.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Capdevila J, Tsukui T, Rodriquez Esteban C, Zappavigna V, Izpisua Belmonte JC. Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol Cell. 1999;4:839–49. doi: 10.1016/s1097-2765(00)80393-7. [DOI] [PubMed] [Google Scholar]
- 26.Selever J, Liu W, Lu MF, Behringer RR, Martin JF. Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev Biol. 2004;276:268–79. doi: 10.1016/j.ydbio.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Ovchinnikov DA, et al. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol. 2006;295:103–15. doi: 10.1016/j.ydbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay A, et al. Genetic Analysis of the Roles of BMP2, BMP4, and BMP7 in Limb Patterning and Skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–68. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- 30.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.