Abstract
The pathogenicity of Clostridium difficile (C. difficile) is mediated by the release of two toxins, A and B. Both toxins contain large clusters of repeats known as cell wall binding (CWB) domains responsible for binding epithelial cell surfaces. Several murine monoclonal antibodies were generated against the CWB domain of toxin A and screened for their ability to neutralize the toxin individually and in combination. Three antibodies capable of neutralizing toxin A all recognized multiple sites on toxin A, suggesting that the extent of surface coverage may contribute to neutralization. Combination of two noncompeting antibodies, denoted 3358 and 3359, enhanced toxin A neutralization over saturating levels of single antibodies. Antibody 3358 increased the level of detectable CWB domain on the surface of cells, while 3359 inhibited CWB domain cell surface association. These results suggest that antibody combinations that cover a broader epitope space on the CWB repeat domains of toxin A (and potentially toxin B) and utilize multiple mechanisms to reduce toxin internalization may provide enhanced protection against C. difficile-associated diarrhea.
Key words: Clostridium difficile, toxin neutralization, therapeutic antibody, cell wall binding domains, repeat proteins, CROPs, mAb combination
The most common cause of nosocomial antibiotic-associated diarrhea is the gram-positive, spore-forming anaerobic bacillus Clostridium difficile (C. difficile). Infection can be asymptomatic or lead to acute diarrhea, colitis, and in severe instances, pseudomembranous colitis and toxic megacolon.1,2
The pathological effects of C. difficile have long been linked to two secreted toxins, A and B.3,4 Some strains, particularly the virulent and antibiotic-resistant strain 027 with toxinotype III, also produce a binary toxin whose significance in the pathogenicity and severity of disease is still unclear.5 Early studies including in vitro cell-killing assays and ex vivo models indicated that toxin A is more toxigenic than toxin B; however, recent gene manipulation studies and the emergence of virulent C. difficile strains that do not express significant levels of toxin A (termed “A− B+”) suggest a critical role for toxin B in pathogenicity.6,7
Toxins A and B are large multidomain proteins with high homology to one another. The N-terminal region of both toxins enzymatically glucosylates small GTP binding proteins including Rho, Rac and CDC42,8,9 leading to altered actin expression and the disruption of cytoskeletal integrity.9,10 The C-terminal region of both toxins is composed of 20 to 30 residue repeats known as the clostridial repetitive oligopeptides (CROPs) or cell wall binding (CWB) domains due to their homology to the repeats of Streptococcus pneumoniae LytA,11–14 and is responsible for cell surface recognition and endocytosis.12,15–17
C. difficile-associated diarrhea is often, but not always, induced by antibiotic clearance of the normal intestinal flora followed by mucosal C. difficile colonization resulting from preexisting antibiotic resistant C. difficile or concomitant exposure to C. difficile spores, particularly in hospitals. Treatments for C. difficile include administration of metronidazole or vancomycin.2,18 These agents are effective; however, approximately 20% of patients relapse. Resistance of C. difficile to these antibiotics is also an emerging issue19,20 and various non-antibiotic treatments are under investigation.20–25
Because hospital patients who contract C. difficile and remain asymptomatic have generally mounted strong antibody responses to the toxins,26,27 active or passive immunization approaches are considered hopeful avenues of treatment for the disease. Toxins A and B have been the primary targets for immunization approaches.20,28–33 Polyclonal antibodies against toxins A and B, particularly those that recognize the CWB domains, have been shown to effectively neutralize the toxins and inhibit morbidity in rodent infection models.31 Monoclonal antibodies (mAbs) against the CWB domains of the toxins have also demonstrated neutralizing capabilities; however, their activity in cell-based assays is significantly weaker than that observed for polyclonal antibody mixtures.33–36
We investigated the possibility of creating a cocktail of two or more neutralizing mAbs that target the CWB domain of toxin A with the goal of synthetically re-creating the superior neutralization properties of polyclonal antibody mixtures. Using the entire CWB domain of toxin A, antibodies were raised in rodents and screened for their ability to neutralize toxin A in a cell-based assay. Two mAbs, 3358 and 3359, that (1) both independently demonstrated marginal neutralization behavior and (2) did not cross-block one another from binding toxin A were identified. We report here that 3358 and 3359 use differing mechanisms to modify CWB-domain association with CHO cell surfaces and combine favorably to reduce toxin A-mediated cell lysis.
Results
Production and characterization of neutralizing antibodies against toxin A.
Anti-C. difficile toxin A mAbs were obtained by immunizing mice against a fragment of toxin A encompassing the entire CWB domain (ToxA:40R; contains 40 repeat units). Ten hybridoma clones were generated that exhibited high specific antibody titers against ToxA:40R as measured by ELISA. Supernatants from the ten clones were tested alone or in combination with one another in a toxin A neutralization assay (data not shown). The neutralization assay measures the amount of live cells after incubation for two days with toxin A/mAb mixtures relative to untreated cells. Of the ten mAbs, one, denoted 3358 (IgG2a/kappa), had the highest neutralization activity. A second mAb, denoted 3359 (IgG1/kappa), was moderately neutralizing, but paired well with several other monoclonals, including 3358, for increased toxin A neutralization. In the same immunization, we also generated and subsequently characterized as a control an antibody, 3356 (IgG1/kappa), which bound toxin A with high affinity, but had little neutralizing capability.
Purified anti-toxin A mAbs were further characterized by titration against two toxin A concentrations in the neutralization assay (Table 1). A recombinant chimeric form (mouse variable and human IgG1/kappa constant domains) of the anti-toxin A monoclonal PCG-4,32,37 and the publicly available mouse PBA-3 (IgG1/kappa) were generated and included in the neutralization assay. A completely neutralizing polyclonal antibody mixture against toxins A and B was included as a positive control. The 3358, 3359 and rPCG-4 antibodies exhibited weak neutralization at a high toxin A concentration (4 µg/mL) and moderate neutralization at a lower toxin A concentration (0.8 µg/mL, Table 1). Binary combinations of 3358 or 3359 with rPCG-4 afforded greater protection than the mAbs in isolation (Table 1).
Table 1.
Toxin A neutralization by anti-toxin A antibodies
| [Toxin A] 4.0 µg/mL (15 nM) | [Toxin A] 0.8 µg/mL (3 nM) | |||
| Average cell survival (%) | S.D. (+/−) | Average cell survival (%) | S.D. (+/−) | |
| Media only | 98.0 | 2.0 | 97 | 3.61 |
| Toxin A | 2.7 | 2.1 | 6.7 | 1.53 |
| Control polyclonal (2.5 µg) | 76.0 | 3.6 | 92.3 | 2.08 |
| Control mouse IgG (2.5 µg) | 4.7 | 0.6 | 6.0 | 1.00 |
| 3358a (2 µg; 130 nM) | 37.3 | 2.5 | 63.3 | 7.23 |
| 3359a (2 µg) | 26.3 | 1.5 | 38.7 | 3.21 |
| rPCG-4a (2 µg) | 22.3 | 2.5 | 37.0 | 3.00 |
| PBA-3a (2 µg) | 2.0 | 1.0 | 15.3 | 2.52 |
| 3358 & 3359 (2 µg + 2 µg) | 49.0 | 5.6 | 73.0 | 6.24 |
| 3358 & 3359 (1 µg + 1 µg) | 31.7 | 1.5 | 52.7 | 4.04 |
| 3358 & rPCG-4 (1 µg + 1 µg) | 41.7 | 2.1 | 73.3 | 5.69 |
| 3359 & rPCG-4 (1 µg + 1 µg) | 61.0 | 6.6 | 64.3 | 8.14 |
a3358, 3359 and PBA-3 were mouse antibodies (IgG2a, IgG1 and IgG1, respectively) produced from hybridoma culture. All three have mouse kappa light chains. rPCG-4 is a recombinant chimeric mAb with mouse PCG-4 variable domains and human (IgG1/kappa) constant domains.
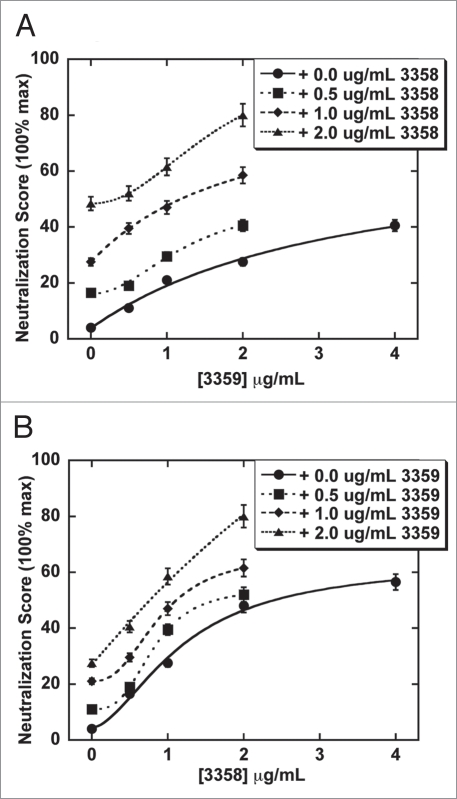
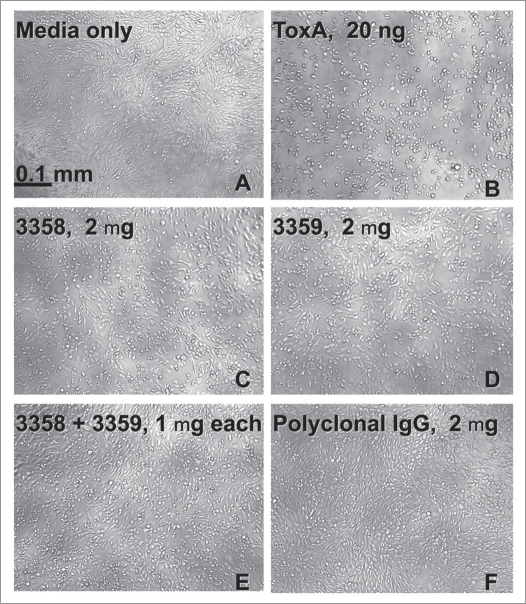
Expansion of the 3358 and 3359 concentrations used in the neutralization assay revealed a significant increase in neutralization activity for the 3358/3359 combination over that of the isolated antibodies (Fig. 1). Increasing the concentration of 3359 or 3358 to 4 µg (and higher) led to a saturating neutralization level or “plateau” well below the 100% cell survival of the control. The “plateau” was significantly increased towards a higher percentage of living cells when the 3358 and 3359 mAbs were combined. These results were also confirmed by observation of CHO cell morphologies. Various combinations of the 3358, 3359 and rPCG-4 antibodies came much closer than the isolated antibodies to mimicking the fully differentiated (nonrounded) cell morphology afforded by the polyclonal control (Fig. 2).
Figure 1.
Analysis of the neutralization capability of 3359 and 3358 combinations following a co-incubation with 80 ng (0.8 µg/mL) toxin A on CHO cell surfaces. (A) Titration of 3359 in the presence of 0, 0.5, 1 and 2 µg 3358. (B) Titration of 3358 in the presence of 0, 0.5, 1 and 2 µg 3359. The combination of the two antibodies (2 µg 3358 + 2 µg 3359) was significantly more neutralizing than 4 mg of 3358 or 4 mg of 3359 (p = 0.03 and p = 0.01, respectively).
Figure 2.
Images from culture plates 48 h after seeding with CHO-K1 cells in the absence (A) and presence of toxin A (B). As a positive neutralization control, an anti-C. difficile toxin polyclonal antibody mixture (TE CHLAB®) was added to the medium with toxin A (F). mAbs 3358 (C) and 3359 (D) were added separately and together (E) with toxin A to the culture media. Addition of 20 ng (0.2 mg/mL) toxin A to the CHO cell media led to >90% cell rounding following 2 days of incubation.
Epitope mapping.
We evaluated the ability of three partially neutralizing antibodies, rPCG-4, 3358 and 3359, and two inactive antibodies, rPBA-3 and 3356 from this study, to interact with 4 recombinant toxin A CWB domain constructs11,32,34,37 using surface plasmon resonance (SPR). Binding of the rPCG-4 Fab was also evaluated. While the kinetic data for each antibody/ toxin binding experiment fit well to a 1:1 binding model, the antibodies are functionally bivalent (excluding the rPCG-4 Fab), and the repeat domains potentially have many analogous antibody binding sites. Therefore, the apparent KD values were used as relative affinity measures only. The two most neutralizing single antibodies, 3358 and rPCG-4, recognized all toxin A CWB domain constructs with high apparent affinity (KD < 30 nM, Table 2). The two non-neutralizing antibodies, rPBA-3 and 3356, only bound with high apparent affinity to the full-length ToxA:40R and select CWB fragments (Table 2).
Table 2.
Apparent antibody affinities for the full-length and truncated toxin A cell wall binding repeat regions (KD, nM; columns 2–5) determined using the kinetic binding curves
| mAb | ToxA:6R (1800–1945) kinetic | ToxA:7R (2078–2234) kinetic | ToxA:11R (2459–2710) kinetic | ToxA:40R (1800–2710) kinetic | ToxA:40R (1800–2710) equilibrium | #ToxA:40R binding sites |
| rPCG-4 Fab | 1.3 | 1.2 | 19 | 0.8 | <0.1 | 5.2 ± 0.2 |
| rPCG-4 | 1.2 | 0.3 | 29 | 0.5 | 0.1 ± 0.05 | 5.6 ± 0.2 |
| rPBA-3 | — | 55 | 60 | 16 | N.D. | N.D. |
| 3358 | 0.6 | 3.4 | 0.7 | 0.3 | <0.1 | 14 ± 1 |
| 3359 | 100 | 13 | 12 | 1.2 | 0.7 ± 0.3 | 8.9 ± 1 |
| 3356 | 3.2 | 106 | 20 | 0.3 | N.D. | N.D. |
Column 6 represents the solution equilibrium KDs calculated by fitting the competition curves. Column 7 represents the number of ToxA:40R binding sites determined by the amount of Fab/mAb necessary to eliminate the presence of free ToxA:40R from solution.
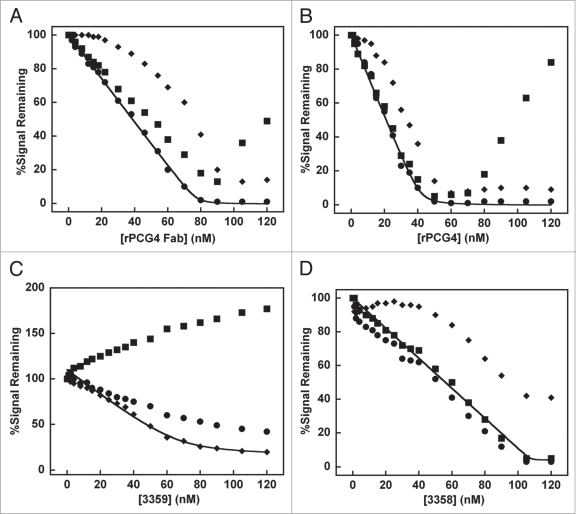
We modified the SPR experiment to measure the apparent equilibrium affinity, stoichiometry of binding, and crossblocking properties of the 3358, 3359 and rPCG-4 antibodies. To fit the data, a simplistic model where all the binding sites were of equivalent affinity was used because a more complicated model that allowed for multiple affinities would be very difficult to interpret. First, solutions containing soluble ToxA:40R (15 nM) were titrated with rPCG-4 Fab, rPCG-4 antibody, 3358, or 3359 (Fig. 3) and injected over sensorchip surfaces containing immobilized rPCG-4 antibody, 3358, or 3359. Overall, the equilibrium data agreed with the rank ordering determined by the kinetic experiments (Table 2). The apparent affinities of both rPCG-4 and 3358 were too strong (KD < 0.1 nM) to be measured using the equilibrium methodology. The apparent affinity of the 3359 mAb for ToxA:40R measured using both techniques were comparable.
Figure 3.
Antibody competition for ToxA:40R binding studied by surface plasmon resonance. Solutions containing 15 nM ToxA:40R were injected over surfaces containing immobilized rPCG-4 (circles), 3359 (triangles) or 3358 (squares). ToxA:40R was titrated with (A) rPCG-4 Fab, (B) rPCG-4 antibody, (C) 3359 or (D) 3358.
Fits of the equilibrium data enabled the determination of the number of high affinity binding sites each mAb has on the ToxA:40R surface. Soluble rPCG-4 Fab completely inhibited soluble ToxA:40R binding to the rPCG-4 antibody surface at a 1:5.2 toxin:Fab ratio, while the bivalent rPCG-4 mAb inhibited at a 1:2.8 ratio (Fig. 3A and B), suggesting that rPCG-4 is capable of simultaneously binding 5 to six separate sites on ToxA:40R with high affinity. The data does not preclude the existence of low affinity rPCG-4 binding sites (KD > 10 nM) on ToxA:40R as observed for the C-terminal CWB domain construct, ToxA:11R. The 3358 mAb saturated its own ToxA:40R binding sites at a 1:7 toxin:mAb ratio (Fig. 3D), suggesting up to 14 separate antibody binding sites on ToxA:40R. In contrast, addition of 120 nM 3359 (∼20 µg/mL, 8-fold molar excess over ToxA:40R) did not entirely abrogate ToxA:40R binding to immobilized 3359 (Fig. 3C). This is likely due to the existence of moderate to low affinity 3359 binding sites on the surface of ToxA:40R. The fit to the data suggest approximately nine separate antibody binding sites for 3359.
The equilibrium studies also demonstrated that rPCG-4 and 3358 compete directly with one another for binding to ToxA:40R (Fig. 3). The rPCG-4 antibody and 3359 also competed with one another for toxin binding; however, 3358 and 3359 did not compete directly with one another, and titration of 3359 into ToxA:40R actually led to the apparent binding of 3359/ToxA:40R complexes by the 3358 immobilized surface (Fig. 3C). Addition of excess rPCG-4 Fab or mAb beyond the level necessary to saturate 3358 binding sites led to an increase in signal from the surface containing immobilized 3358. One explanation is that excess rPCG-4 may lead to an allosteric change within the toxin molecule allowing 3358 access to additional binding sites. Another explanation may be that rPCG-4 occupation of low affinity binding sites occludes its own access to high affinity sites while leaving overlapping 3358 binding surface(s) open.
Mechanistic variability of toxin A neutralizing antibodies.
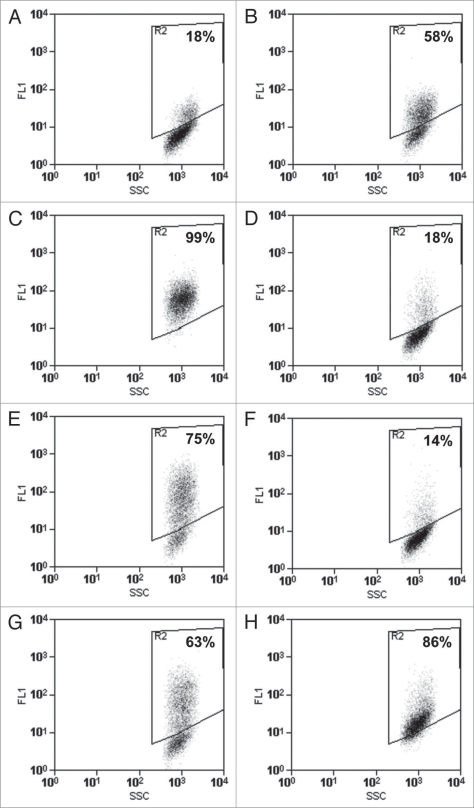
We developed a novel flow cytometry assay to study CWB domain association with CHO cells.11 ToxA:11R was chosen for the flow cytometry assay based on its native folding properties and cell-binding characteristics. By tuning ToxA:11R and Ca2+ concentrations within the experiment, we could reproducibly induce an ∼50% fluorescence shift in CHO cell populations, allowing us to observe antibody-induced increases or decreases in ToxA:11R detected on the CHO surface (Fig. 4).11 Addition of 3358 or rPCG-4 to ToxA:11R during the binding step significantly increased the detectable amount of ToxA:11R on CHO cell surfaces (Fig. 4C and E). Alternately, addition of 3359 during the ToxA:11R binding step decreased the fluorescent CHO population to baseline levels (Fig. 4D). Combination of 3359 and 3358 inhibited ToxA:11R binding to the CHO cell surface similar to the behavior of 3359 alone (Fig. 4F). rPCG-4 combined with an equimolar amount of 3359 led to an increase in ToxA:11R binding to cell surfaces, indicating that rPCG-4 dictated the behavior of the two antibodies when introduced at identical concentrations in the assay (Fig. 4G). This result correlates with the data demonstrating that 3359 and rPCG-4 cross-block one another while 3358 and 3359 do not.
Figure 4.
CHO cell surface binding of ToxA:11R in the presence and absence of anti-toxin A antibodies as determined by flow cytometry. Fluorescence of the CHO cell population (A) in the absence of ToxA:11R; (B) in the presence of 50 µg/mL ToxA:11R alone; and in the presence of (C) 250 µg/mL 3358, (D) 250 µg/mL 3359, (E) 250 µg/mL rPCG-4, (F) 250 µg/mL 3358 and 250 µg/mL 3359, (G) 250 µg/mL 3359 and 250 µg/mL rPCG-4 or (H) 250 µg/mL 3358 and 250 µg/mL rPCG-4.
Discussion
We demonstrated that while mAbs 3358 and 3359, and a previously characterized anti-toxin A mAb, PCG-4, partially neutralized toxin A as single agents in an in vitro cell-based neutralization assay, specific combinations involving the three mAbs were significantly more neutralizing than any single mAb tested in the study. Unlike many mammalian repeat proteins, the repeat sequences found in the CWB domains of both toxin A and toxin B are highly homologous to one another. Antibodies raised against either CWB domain, therefore, may potentially recognize multiple sites on the toxin. rPCG-4 and 3358 both recognized multiple regions of the toxin A CWB domain with high apparent affinity. The toxin A binding results with PCG-4 described here are consistent with the previous work of Frey and Wilkins,37 and highlight the existence of at least one additional high-affinity epitope for the PCG-4 antibody, as well as the possible existence of one or more lower-affinity binding sites. While 3359 did not recognize all the toxin A CWB domain fragments with high affinity and was not as strongly neutralizing as 3358 or rPCG-4 in isolation, it paired well with both for toxin A neutralization, perhaps because its epitope was non-overlapping with 3358 and only partially overlapping with rPCG-4. This may have enabled broader coverage of the CWB domain when used in combination. Recently, a crystal structure was reported of a truncated toxin A CWB domain (similar to ToxA:11R used in the flow cytometry studies) complexed with two Galα1-3Galβ1-4GlcNAc moieties.17 The results of this study strongly suggest there are numerous carbohydrate binding sites across the entire 40-repeat CWB domain of toxin A. Additivity between weak binding sites is a likely mechanism for generating strong interactions between the CWB domain and mammalian cell surfaces. Thus, the ability to target multiple receptor/carbohydrate binding sites on the CWB domains, particularly using antibody combinations, may contribute to stronger toxin neutralization.
Because the CWB domains of toxins A and B are considered the main receptor binding domains of the toxins,4,12,25,38 the most straightforward mechanism of antibody neutralization would be to block CWB domain binding to cell surfaces. The flow cytometry experiments described here, however, suggest that multiple neutralization mechanisms may exist. rPCG-4 and 3358, which contain overlapping epitopes, led to an increase in the detection of ToxA:11R on the surface of CHO cells, while the 3359 antibody, which did not compete well with 3358 or rPCG-4 for toxin binding, inhibited the ability to detect ToxA:11R on the surface of CHO cells. The apparent increase in ToxA:11R upon binding of 3358 and rPCG-4 suggests these antibodies may lead to accumulation of toxin A on the cell surface, perhaps by reducing internalization or by promoting the binding activity of the CWB-domains (C-terminal receptor binding domains). The first of these explanations may be more realistic, as these antibodies are neutralizing and not agonistic. Similar results were observed by Babcock and coworkers,39 who showed that a toxin A neutralizing antibody, denoted CDA1, that recognized the CWB domains did not stop the hemagglutination ability of Toxin A. If CDA1 blocked toxin A binding to cells, one would expect the hemagglutination behavior to be reduced. These data and our flow cytometry data with 3358 and rPCG-4 suggest that neutralization of toxin A by antibodies to the CWB-domains may occur through different mechanisms. Some may indeed neutralize toxin A by blocking receptor binding (3359), while others may inhibit cytotoxicity in a different fashion, perhaps by inhibiting toxin internalization. Additional experiments are warranted to define these mechanisms further.
Many reports, especially in the realm of anti-infectives, have demonstrated that the combination of two or more mAbs can have synergistic neutralizing effects often bridging multiple mechanisms of action.40–42 Babcock and coworkers39 show that combination of anti-toxin A and anti-toxin B mAbs can be more efficacious than the administration of a single mAb in the hamster mortality model. Here, we provide evidence that mAb mixtures that neutralize by binding multiple inhibitory epitopes on a single toxin may lead to enhanced efficacy against the single toxins themselves beyond what is achievable with single mAbs. Similarly, we have generated preliminary data suggesting that the multiple mAb approach may be more efficacious in the neutralization of the highly homologous C. difficile toxin B (unpublished data), suggesting the approach may be applicable to this entire class of clostridial toxins. With the emergence of robust multispecific antibody platforms,43–45 as well as new approaches to simultaneously express and co-develop multiple mAbs,46 combinatorial targeting of bacterial antigens as described here may become feasible in the near future.
Materials and Methods
Generation and screening of antitoxin A monoclonal antibodies.
Recombinant constructs consisting of the entire toxin A CWB domain (ToxA:40R, residues 1800–2710) and truncated CWB domain regions were cloned, expressed in E. coli, and purified as described.11 Fifteen mice (5 BALB/c and 10 Swiss-Webster) were immunized with 25 µg ToxA:40R every 21 days for 4 cycles. After 12 weeks, all mice developed high antitoxin A antibody titers. Hybridoma fusions were initiated with spleen cells from these mice, and the hybridoma clones expressing anti-toxin A mAbs were grown in DMEM (Dulbecco’s Minimal Essential Medium, Gibco/Invitrogen), 10% FBS (Fetal Bovine Serum, Sigma), and 1Xglutamine, penicillin and streptomycin (Gibco/Invitrogen). A total of 1,920 cell lines were plated from the fusions (20 96-well plates).
Antibodies were produced by culturing selected hybridoma cell lines at 37°C in a CO2 incubator for 3 or 4 days past the plateau in their growth phase. Cell suspensions were spun down, and the supernatants were collected, filtered, given protease inhibitors, and stored at 4°C until purified.
ELISA testing for anti-toxin A antibodies.
ToxA:40R was biotinylated using the EZ-Link Biotin-LCASA kit at a 4:1 biotin:protein ratio according to manufacturer protocols (Pierce). Microtiter Streptavidin plates (Sigma) were coated with 200 ng per well of biotinylated ToxA:40R, diluted into PBS buffer and incubated overnight at 4°C. The plates were washed with TBST (Sigma). Culture supernatants (diluted 10-, 100-, 1,000- and 10,000-fold with TBST to a final 100 µL volume) were added to the plates and incubated for 1 h at room temperature. The plates were washed, and AP-conjugated rabbit anti-mouse IgG(H + L) (Zymed, cat#61-6522) was added to each well at a 1:1,000 dilution for 1 h at room temperature. The plates were washed, and 100 µL of P-nitrophenylphosphate (Sigma) was added. Absorption was measured at 405 nm using a Molecular Devices νmax kinetic microplate reader.
Production of recombinant anti-toxin A rPBA-3 and rPCG-4 antibodies.
Anti-C. difficile toxin A hybridoma cell line PBA-3 was purchased (ATCC#HB-8713). The cell line was grown as described for the anti-toxin A hybridomas. RNA was isolated from 107 hybridoma cells using the RNeasy Mini kit (Qiagen) procedure. Primers for amplification of the PBA-3 heavy and light chain variable domains were designed as described.47,48 The PCR products were subcloned into pCEP4 mammalian expression vectors modified in-house to encode for a signal peptide and human IgG1 heavy chain constant region or kappa CL domain (Invitrogen).
Using sequences from Genbank (accession # X82691 and X82692), DNA inserts for the variable domains of the PCG-4 mAb were created by overlap extension PCR49 and cloned into a custom pBK-CMV vector containing the human IgG1 heavy chain CH1 and human kappa light chain CL domains, respectively, for periplasmic production of Fab in E. coli. The variable domains were also subcloned into the same pCEP4 vector system described for the rPBA-3 chimera.
The heavy and light chain plasmids of both rPBA-3 and rPCG-4 were transformed into XL1-blue bacteria. Plasmid DNA was prepared as described in the endotoxin-free MaxiPrep kit (Qiagen). Heavy and light chain plasmids were co-transfected at 0.5 µg/mL into adenovirus-transformed HEK293F cells at 106 cells/mL using 293fectin (Invitrogen). Cells were cultured in 293F-FreeStyle media at 37°C in a CO2 incubator. After 7 days, supernatants containing antibody were collected by centrifugation at 1,200 rpm.
Antibody purification/quantification.
The 3358, 3359 and 3356 mAbs and rPBA-3 and rPCG-4 chimeric antibodies were purified from supernatants by capture onto 5 mL HiTrap protein G columns (Amersham) using PBST (Sigma, cat# P-3563) as the running buffer and 0.1 M glycine, pH 2.7 as the elution buffer. Eluants were neutralized with 1 M TrisHCl, pH 8.0 and dialyzed against PBS. rPCG-4 Fab was obtained from bacterial pellets using the B-PER lysis reagent (Pierce) and purified via the C-terminal 6HisTag on the light chain using Ni2+-NTA agarose (Qiagen). Antibody stock concentrations were determined by Bradford analysis using a commercial myeloma IgG1 as a standard. The rPCG-4 Fab produced in E. coli and the rPCG-4 mAb produced in mammalian cells both had the expected physiochemical properties as assessed by SDS-PAGE and size-exclusion chromatography and both exhibited the expected anti-toxin A reactivity.
Toxin A neutralization assay.
CHO-K1 cells (ATCC#CCL-61) were maintained in DMEM supplemented with 10% FBS at 37°C in a CO2 incubator. Prior to the experiment, cells were split into T75 flasks at 1.5 × 106 cells/flask. Cells reached confluency within 2 days. CHO cells were seeded from these stocks into 96-well culture plates and incubated for 4–6 hours. Toxin A and toxin A/antibody mixtures were incubated at 37°C in cell culture media for 1 h, added to the 96-well plates, and incubated for 48 h. At 48 h, 10 µL WST-1 (Roche) was added to each well, and the plates were incubated for 1 h at 37°C. Absorbance at 450 nm was determined using a Molecular Devices Spectramax Plus 96-well plate reader. The data in Table 1 were normalized to the signal generated by control cells incubated in the absence of toxin A. Each data point was performed in triplicate. Toxin A was purchased from List Biological Laboratories. A polyclonal anti-toxin control was from TECHLAB®.
Determination of the relative affinities and epitopes of the anti-toxin A antibodies.
Surface plasmon resonance (SPR) experiments were performed at 31°C on a Biacore3000 instrument. Toxin A CWB-domain constructs11 were immobilized to CM5 chip surfaces using a 100 mM acetate buffer, pH 4 and standard EDC/NHS coupling chemistry. Kinetic analyses were performed by injecting a series of anti-toxin A Fab or antibody concentrations (0.5, 2, 6, 20 and 100 nM) at 30 µL/min over chip surfaces containing single toxin A CWB domain constructs. Chip surfaces were regenerated by injecting 10 µL 0.1 M glycine, pH 1.5 followed by a 10 µL injection of 50 mM NaOH.
Equilibrium affinity, stoichiometry and cross-blocking studies with the 3358, 3359 and rPCG-4 antibodies were performed by immobilizing each antibody (>6000 RU above baseline) to separate CM5 surfaces using the same protocol described above. Fifteen nM ToxA:40R was injected over antibody-coupled sensorchip surfaces in the presence of 0, 1, 3, 5, 8, 12, 15, 20, 25, 30, 35, 40, 50, 60, 70, 80, 90, 105 and 120 nM concentrations of soluble 3358, 3359, rPCG4 and rPCG4 Fab for competitive inhibition. The injections (200 µL) were performed at 10 µL/min. The linear portion of the kinetic binding phase was used to measure the percentage of toxin binding to each sensorchip surface. After each injection, the flow rate was increased to 30 µL/min, and regeneration was achieved using 2 × 10 µL injections of 0.1 M glycine, pH 2.0. Equilibrium solution affinity and stoichiometry measurements for each antibody were measured by determining the concentration of free ToxA:40R based on its initial velocity of binding to the CM5 surface containing the same immobilized antibody.50,51 The initial velocities were directly fit to obtain affinity and stoichiometry data using the following relationship:
 |
where Vi = initial rate of binding, m = slope of the ToxA:40R concentration-dependent standard curve, [ToxA:40R]f = unbound ToxA:40R concentration = Vi/m, [ToxA:40R]t = total ToxA:40R concentration, and [mAb]t = the total mAb concentration. To simplify the calculations, the assumption was made that the multiple binding sites for each antibody on ToxA:40R are equivalent and we use the equilibrium data to both compare to the apparent affinity rank ordering obtained using the kinetic data and to obtain an approximate number of high affinity binding sites.
Flow cytometry.
Cell culture, cell labeling, flow cytometry, and fluorescence detection were performed as described previously.11 Incubation using CaCl2 and ToxA:11R-6HisTag at 1 mM and 50 µg/mL, respectively, during the labeling step led to a reproducible shift in the fluorescent cell population between 35–60% after addition of the PENTA-HIS-Alexafluor 488 conjugated antibody (Qiagen).11 To study antibody neutralization mechanisms, anti-toxin A antibodies were combined with ToxA:11R during the labeling step at concentrations of 250 or 500 µg/mL yielding molar ToxA:11R:antibody ratios of 1:1 and 1:2, respectively.
Acknowledgements
The authors would like to thank Lisa Bibbs and Dr. Xuqiu Tan and the sequencing and cloning groups at Diversa for their support, in particular Sylvia Park and Lisa Ramos. Dr. Gerhard Frey and Dr. Stefan Andrae created the synthetic version of the PCG-4 Fab. Excellent technical assistance for the flow cytometry experiments was provided by Trevin Holland. The authors also thank Dr. Antony Blanc and other members of the BioPharma team from Syngenta for their support and Dr. Kristin Demarest for critical reading of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/11220
References
- 1.Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am. 2001;30:753–777. doi: 10.1016/s0889-8553(05)70209-0. [DOI] [PubMed] [Google Scholar]
- 2.Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile-associated diarrhea. Arch Int Med. 2001;161:525–533. doi: 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
- 3.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: Its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pothoulakis C, LaMont JT. Microbes and microbial toxins: paradigms for microbial mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280:178–183. doi: 10.1152/ajpgi.2001.280.2.G178. [DOI] [PubMed] [Google Scholar]
- 5.Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North Americ and Europe. Clin Microbiol Infect. 2008;12:2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 6.Lyras D, O’Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1181. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rupnik M. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol Rev. 2008;32:541–555. doi: 10.1111/j.1574-6976.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 8.Aktories K, Just I. Monoglucosylation of low-molecular-mass GTP-binding Rho proteins by clostridial cytotoxin. Trends Cell Biol. 1995;5:441–443. doi: 10.1016/s0962-8924(00)89107-2. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 10.Donelli G, Fiorentini C. Bacterial protein toxins acting on the cell cytoskeleton. New Microbiol. 1994;17:345–362. [PubMed] [Google Scholar]
- 11.Demarest SJ, Salbato J, Elia M, Zhong J, Morrow T, Holland T, et al. Structural characterization of the cell wall binding domains of Clostridium difficile toxins A and B; Evidence that Ca2+ plays a role in toxin A cell surface association. J Mol Biol. 2005;346:1197–1206. doi: 10.1016/j.jmb.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 12.Von Eichel-Streiber C, Sauerborn M. Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene. 1990;96:107–113. doi: 10.1016/0378-1119(90)90348-u. [DOI] [PubMed] [Google Scholar]
- 13.Ho JG, Greco A, Rupnik M, Ng KK-S. Crystal structure of receptor-binding C-terminal repeats from Clostridium difficile toxin A. Proc Natl Acad Sci USA. 2005;102:18373–18378. doi: 10.1073/pnas.0506391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García JL, Sánchez-Beato AR, Medrano FJ, López R TA, editors. Streptococcus pneumoniae. New Rochelle: Mary Ann Liebert, Inc.,; 2000. Versatility of Choline-Binding Domain; pp. 155–177. [Google Scholar]
- 15.Tucker KD, Wilkins TD. Toxin A of Clostridium difficile binds to the human carbohydrate antigens, I, X and Y. Infect Immun. 1990;59:73–78. doi: 10.1128/iai.59.1.73-78.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krivan HC, Clark GF, Smith DF, Wilkins TD. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco A, Ho JGS, Lin S-J, Palcic MM, Rupnik M, Ng KK. Carbohydrate recognition by Clostridium difficile toxin A. Nat Struct Mol Biol. 2006;13:460–461. doi: 10.1038/nsmb1084. [DOI] [PubMed] [Google Scholar]
- 18.Brazier JS, Fawley W, Freeman J, Wilcox MH. Reduced susceptibility of Clostridium difficile to metronidazole. J Antimicrob Chemother. 2001;48:741–742. doi: 10.1093/jac/48.5.741. [DOI] [PubMed] [Google Scholar]
- 19.Pelaez T, Alcala L, Alonso R, Rodriguez-Creixems M, Garcia-Lechuz JM, Bouza E. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother. 2005;46:1647–1650. doi: 10.1128/AAC.46.6.1647-1650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland LV. Alternative treatments for Clostridium difficile disease: what really works? J Med Microbiol. 2005;54:101–111. doi: 10.1099/jmm.0.45753-0. [DOI] [PubMed] [Google Scholar]
- 21.Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519. doi: 10.1016/s0140-6736(87)92646-8. [DOI] [PubMed] [Google Scholar]
- 22.Chia JK, Chan SM, Goldstein H. Baker’s yeast as adjunctive therapy for relapses of Clostridium difficile diarrhea. Clin Infect Dis. 1995;20:1581. doi: 10.1093/clinids/20.6.1581. [DOI] [PubMed] [Google Scholar]
- 23.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterol. 1989;96:981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 24.Palace GP, Lazari P, Norton K. Analysis of the physicochemical interactions between Clostridium difficile toxins and cholestyramine using liquid chromatography with post-column derivatization. Biochem Biophys Acta. 2001;1546:171–184. doi: 10.1016/s0167-4838(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 25.Castagliuolo I, LaMont JT, Qiu B, Nikulasson ST, Pothoulakis C. A receptor decoy inhibits the enterotoxic effects of Clostridium difficile toxin A in rat ileum. Gastroenterol. 1996;111:433–438. doi: 10.1053/gast.1996.v111.pm8690209. [DOI] [PubMed] [Google Scholar]
- 26.Kyne L, Warny M, Oamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 27.Kyne L, Warny M, Oamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 28.Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, et al. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003;71:1608–1610. doi: 10.1128/IAI.71.3.1608-1610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, et al. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999;67:527–538. doi: 10.1128/iai.67.2.527-538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kink JA, Williams JA. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyerly DM, Bostwick EF, Binioin SB, Wilkins TD. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyerly DM, Phelps CJ, Toth J, Wilkins TD. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986;54:70–76. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyerly DM, Johnson JL, Frey SM, Wilkins TD. Vaccination against lethal enterocolitis with a nontoxic recombinant peptide of toxin A. Curr Microbiol. 1990;21:29–32. [Google Scholar]
- 34.Corthier G, Muller MC, Wilkins TD, Lyerly DM, L’Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59:1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernie DS, Thomson RO, Batty I, Walker PD. Active and passive immunization to protect against antibiotic-associated caecitis in hamsters. Dev Biol Stand. 1982;53:325–332. [PubMed] [Google Scholar]
- 36.Lyerly DM, Phelps CJ, Wilkins TD. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985;21:12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey SM, Wilkins TD. Localization of two epitopes recognized by monoclonal antibody PCG-4 on Clostridium difficile toxin A. Infect Immun. 1992;60:2488–2492. doi: 10.1128/iai.60.6.2488-2492.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauerborn M, Leukel P, von Eichel-Streiber C. The C-terminal ligand-binding domain of Clostridium difficile toxin A (TcdA) abrogates TcdA-specific binding to cells and prevents mouse lethality. FEMS Microbiol Lett. 1997;155:45–54. doi: 10.1111/j.1574-6968.1997.tb12684.x. [DOI] [PubMed] [Google Scholar]
- 39.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, et al. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks JD. Deciphering antibody properties that lead to poten botulinum neurotoxin neutralization. Movement Disorders. 2004;19:101–108. doi: 10.1002/mds.20023. [DOI] [PubMed] [Google Scholar]
- 41.Volk WA, Bizzini B, Snyder RM, Bernhard E, Wagner RR. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect Immun. 1984;45:604–609. doi: 10.1128/iai.45.3.604-609.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwick MB, Wang M, Poignard P, Stiegler G, Katinger H, Burton DR, et al. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol. 2001;75:12198–12208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 44.Michaelson JS, Demarest SJ, Miller B, Amatucci A, Synder WB, Wu X, et al. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTβR. mAbs. 2009;1:128–141. doi: 10.4161/mabs.1.2.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimasi N, Gao C, Fleming R, Woods RM, Yao XT, Shirinian L, et al. The design and characterization of oligospecific antibodies for simultaneously targeting multiple disease mediators. J Mol Biol. 2009;393:672–692. doi: 10.1016/j.jmb.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Wiberg FC, Rasmussen SK, Frandsen TP, Rasmussen LK, Tengbjerg K, Coljee VW, et al. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng. 2006;94:396–405. doi: 10.1002/bit.20865. [DOI] [PubMed] [Google Scholar]
- 47.Coloma MJ, Hastings A, Wims LA, Morrison SL. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 48.Dattamajumdar AK, Jacobson DP, Hood LE, Osman GE. Rapid cloning of any rearranged mouse immunoglobulin variable genes. Immunogenetics. 1996;43:141–151. doi: 10.1007/BF00176675. [DOI] [PubMed] [Google Scholar]
- 49.Casimiro DR, Toy-Palmer A, Blake RC, Dyson HJ. Gene synthesis, high-level expression, and mutagenesis of Thiobacillus ferrooxidans rusticyanin: His 85 is a ligand to the blue copper center. Biochemistry. 1995;34:6640–6648. doi: 10.1021/bi00020a009. [DOI] [PubMed] [Google Scholar]
- 50.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–1931. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 51.Doern A, Cao X, Sereno A, Reyes CL, Altshuler A, Huang F, et al. Characterization of inhibitory antiinsulin-like growth factor receptor antibodies with different epitope specificity and ligand-blocking properties: implications for mechanism of action in vivo. J Biol Chem. 2009;284:10254–10267. doi: 10.1074/jbc.M809709200. [DOI] [PMC free article] [PubMed] [Google Scholar]