Abstract
Background
Altered renal sodium handling has a major pathogenic role in salt-sensitive hypertension. Renal sodium transporters are present in urinary exosomes. We hypothesized that sodium transporters would be excreted into the urine in different amounts in response to sodium intake in salt-sensitive versus salt-resistant patients.
Methods
Urinary exosomes were isolated by ultracentrifugation, and their content of Na-K-2Cl cotransporter (NKCC2) and Na-Cl cotransporter (NCC) was analyzed by immunoblotting. Animal studies: NKCC2 and NCC excretion was measured in 2 rat models to test whether changes in sodium transporter excretion are indicative of regulated changes in the kidney tissue. Human studies: in hypertensive patients (n = 41), we investigated: (1) a possible correlation between sodium reabsorption and urinary exosomal excretion of sodium transporters, and (2) the profile of sodium transporter excretion related to blood pressure (BP) changes with salt intake. A 24-hour ambulatory BP monitoring and a 24-hour urine collection were performed after 1 week on a low- and 1 week on a high-salt diet.
Results
Animal studies: urinary NKCC2 and NCC excretion rates correlated well with their abundance in the kidney. Human studies:6 patients (15%) were classified as salt sensitive. The NKCC2 and NCC abundance did not decrease after the high-salt period, when the urinary sodium reabsorption decreased from 99.7 to 99.0%. In addition, the changes in BP with salt intake were not associated with a specific profile of exosomal excretion.
Conclusions
Our results do not support the idea that excretion levels of NKCC2 and NCC via urinary exosomes are markers of tubular sodium reabsorption in hypertensive patients.
Key Words: Exosomes, Na-Cl cotransporter, Na-K-2Cl cotransporter, Renal sodium transporters, Salt sensibility, Urine biomarkers
Introduction
Recent progress in proteomic techniques has reopened the investigation of urinary biomarkers. Mass spectrometry studies have shown that normal urine contains hundreds of proteins [1]. The study of these proteins can provide information about the physiological and pathophysiological state of their renal cells of origin, and also provide useful information about diagnostic and therapeutic strategies.
Experimental and clinical studies have emphasized the crucial role of sodium in the regulation of blood pressure, and the implications of abnormal sodium balance in the development of hypertension in animal models as well as in humans. It is known that the response of blood pressure (BP) to changes in sodium intake is heterogeneous, and that altered renal sodium handling has a major pathogenic role in salt-sensitive hypertension [2,3].
Renal tubule sodium absorption is mediated by membrane transport proteins that facilitate sodium movement across the plasma membranes of renal epithelial cells. The major apical sodium transporters are the type 3 Na-H exchanger NHE3 (proximal tubule), the type 2 Na-K-2Cl cotransporter NKCC2 (thick ascending limb of Henle), the Na-Cl cotransporter NCC (distal convoluted tubule) and the epithelial sodium channel ENaC (collecting duct).
Several studies in experimental animals point to an association between alterations in the expression of some renal sodium transporters and the salt sensitivity of BP [4,5,6,7]. A few studies have been performed on humans to investigate the renal sodium handling in this type of hypertension [8]. In humans, no studies have been done to investigate in the kidney the pathogenic role of sodium transporters in salt-sensitive hypertension due to the ethical conflict/difficulty of obtaining kidney tissue to study this pathology. However, the presence of apical sodium transporters in the urine has recently been demonstrated [9,10]. One of the major pathways by which apical membrane proteins are excreted in the urine seems to be by exosome release [11,12,13,14,15]. Exosomes are tiny membrane-bound vesicles that originate as the internal vesicles of multivesicular bodies in several cell types. Membrane proteins are endocyted into the cell, and through several signals are delivered into multivesicular bodies. When those multivesicular bodies are fused with the apical membrane, exosomes are released to the urinary space.
Taking into account this background, we designed the present work. First, in animals, to study if the excretion of sodium transporters in urinary exosomes correlates with their abundance in renal tissue. Second, in hypertensive patients, with the following objectives: (1) investigate a possible correlation between tubular sodium reabsorption and urinary excretion of NKCC2 and NCC sodium transporters contained in exosomes, and (2) study the profile of excreted renal sodium transporters related to changes in BP levels with low and high salt intake.
Methods
Animal Studies
Animal Models
Escape from Aldosterone-Induced Sodium Retention. The experiment was conducted as reported previously [16]. Briefly, all rats initially received an aldosterone infusion of 200 μg/day by osmotic minipump, and were ration-fed a low-sodium diet (0.02 mEq/day) for 3 days. The sodium intake was then increased to 2.0 mEq/day for the escape rats (n = 6), while control rats (n = 6) continued to receive 0.02 mEq/day of sodium. Rats were euthanized on the fourth day after the increase in sodium intake.
Sodium Restriction Model. Rats were ration fed a high-salt (2.0 mEq/day; n = 6) or a low-salt (0.02 mEq/day; n = 6) gel food diet for 3 days [17,18].
Ration Feeding Protocol. For rat studies, the intakes of sodium, calories, and water were carefully controlled by ration feeding of a fixed daily amount of a gelled diet that contains all of the nutrients, NaCl, and water that the rat receives in a day [19]. The baseline low-sodium intake was achieved by feeding a gelled mixture of a synthetic low-sodium diet (Formula 53140000; Ziegler Brothers, Gardner, Pa., USA), deionized water (25 ml/15 g of food), and agar (0.125 g/25 ml of water). The sodium-replete diet was the same, except for addition of 2 mEq sodium per 15 g food prior to gelation. All animals received the equivalent of 15 g/200 g body weight per day of rat chow, determined by weighing the gelled mixture. Analysis of the diet demonstrated that this protocol provides approximately 0.02 mEq/200 g body weight per day of sodium for the low-sodium mixture and 2.0 mEq/200 g body weight per day for the sodium-replete diet.
Processing of Urine and Kidneys from Rats
The rats were placed in metabolism cages, allowing daily ration feeding and collection of urine. The urine samples were centrifuged to isolated urinary exosomes (see ‘Urinary Exosome Isolation’). Loading of gels was normalized to the urinary output of each animal according to equal time of collection as described previously [9]. This technique allows the comparison of protein excretion for the same period of time in all rats.
Rats were euthanized by decapitation. The kidneys were rapidly removed and homogenized. Total protein was measured by using the BCA Protein Assay reagent kit (Pierce Chemical Company, Rockford, Ill., USA).
Urinary exosomes and kidney homogenates were solubilized for immunoblotting as previously described [10].
Dot Blots
Dot blots were carried out by applying the samples directly to nitrocellulose membranes and probing with the primary antibody [17]. The secondary antibody was Alexa 488-conjugated goat anti-rabbit antibody (A-11008; Molecular Probes, Eugene, Oreg., USA). Signals were detected and quantified by Molecular Imager FX (Bio-Rad, Hercules, Calif., USA).
Human Studies
Experimental Procedure
The study was conducted on 52 patients with mild-moderate hypertension (31 women, 21 men). The exclusion criteria were: (1) pharmacologic treatment of hypertension, and (2) presence of another sodium and water balance disorder, such as congestive heart failure or liver cirrhosis.
Patients were on a low-salt diet for 1 week, followed by another week with a high sodium intake. Salt restriction was obtained by providing careful dietary instructions (menu lists) to reach a daily sodium excretion <100 mmol Na/24 h during the low-salt-intake period. At the end of each dietary period, 24-hour urine was collected, a blood sample was drawn, and 24-hour ambulatory BP monitoring (ABPM) was recorded. BP measurement was performed at 20-min intervals during the day, and at 30-min intervals during the night (Diasys Integra II; Novacor, Paris, France). A urine aliquot (100 ml) from the 24-hour urine collection was frozen at −80°C until assayed.
The study was approved by the Ethics Committee of the institution.
BP Analyses
Systolic BP, diastolic BP and mean BP (MBP; from the day, night and over 24 h) and the cardiac rate were measured by ABPM. ABPM data was edited, and, for patients with sleeping disturbances due to BP monitoring, night and 24-hour BP were not analyzed. Pulse pressure was calculated by systolic BP minus diastolic BP. In addition, the 24-hour circadian patterns were also assessed. We classified the patients into 4 categories according to the following definitions: (1) dipper: patient with a fall in night MBP compared to day MBP ≥10–20%, (2) non-dipper: fall in night MBP compared to day MBP <10%, (3) riser: increase in night MBP compared to day MBP, and (4) extreme dipper: fall in night MBP compared to day MBP >20% [20].
Assessment of Salt Sensitivity
We calculated the percentage of change in the 24 h MBP between the end of the low-salt diet and the end of the high-salt diet. As in previous studies, salt-sensitive patients were those who presented an increase ≥10% in the 24-hour MBP with the changes in salt intake, while patients with an increase ≤5% were considered salt-resistant. Patients with an increase in their 24 h MBP between >5 and <10% were considered salt-indeterminate [21]. When there was interference to the ABPM from the sleep of the patients, we used the day MBP from both diet periods to assess the salt sensitivity.
Blood and Urine Analyses
Plasma and urine osmolality were determined from the osmometric depression of the freezing point (Osmometer 3300; Advanced Instruments, Needham Heights, Mass., USA). Sodium, potassium, albumin, and creatinine were determined by standard analytical methods. Plasma concentrations of aldosterone, plasma renin activity, dopamine, adrenaline, and noradrenaline were determined by radioimmunoassay (Clinical Assays, Cambridge, Mass. USA; Diagnostic and Products, Los Angeles, Calif, USA; Bühlman, Basel, Switzerland; CAIBL, Hamburg, Germany, respectively).
Urinary Exosome Isolation
Urine samples were thawed and centrifuged at 17,000 g for 15 min at 4°C to remove cells, cellular debris, and large membrane fractions. These samples were then ultracentrifuged (Ultra Beckman Coulter OPTIMA™ L-90K Ultracentrifuge, Beckman TFT 55.38 rotor; Beckman Coulter, Fullerton, Calif., USA) at 200,000 g for 2 h at 4°C to purify the exosomes as previously described [10]. The resulting pellets were resuspended in isolation solution containing 250 mmol/l sucrose and 10 mmol/l triethanolamine (Calbiochem, La Jolla, Calif., USA) with 1 ug/ml leupeptin (Bachem, Torrance, Calif., USA) and 0.1 mg/ml phenylmethylsulfonyl fluoride (United States Biochemical, Cleveland, Ohio, USA).
Electrophoresis and Immunoblotting
Gel loading of urinary exosome sodium transporters was normalized by urine creatinine as described previously [22]. Urine samples were extensively vortexed immediately after thawing. Electrophoresis was run for the entire set of samples on 7.5% polyacrylamide/SDS gels.
Polyclonal Antibodies. Rabbit polyclonal antibodies prepared against the thick ascending limb isoform of the NKCC2 cotransporter and the NCC of the distal convoluted tubule have been described previously [23]. They were raised against carrier-conjugated synthetic peptides corresponding to hydrophilic portions of the proteins. These antibodies were affinity purified against the immunizing peptides. Specificity of the antibodies has been demonstrated by showing unique peptide-abatable bands on immunoblots (data not shown). These rabbit polyclonal antibodies recognized both rat and human NCC and NKCC2 transporters (data not shown).
Measurement of Urinary NKCC2 and NCC in Exosomes
Two urine samples from each patient, 1 from each diet period, were run in the same gel. NKCC2 and NCC levels were quantified by densitometric analysis of the immunoblot bands using Gel Doc 2000 (Quantity One software, Bio-Rad). The values were normalized (divided by the mean level of transporters from all patients) in order to homogenize the data and perform a graphic representation. We compared the levels of NKCC2 and NCC urinary exosomal excretion in high salt intake versus low salt intake for each subject. Moreover, we calculated the ratio of the normalized values of NKCC2 and NCC of each patient in the high-salt diet with respect to the low-salt diet and compared that with the salt-sensitivity profile.
Presentation of Data and Statistical Analyses
Animal Studies. Kidney protein measurements were presented as normalized densitometry values from immunoblots (means ± SE) and urinary measurements were presented as normalized excretion rates (means ± SE).
Human Studies. Quantitative data are presented as means ± SD. The statistical significance of differences between the 2 periods was evaluated by paired Student's t test.
Statistical comparisons were performed using ANOVA, the unpaired t test (assuming equal variance), and the Mann-Whitney rank-sum test (assuming unequal variance). Values of p < 0.05 were considered statistically significant.
Results
Animal Studies
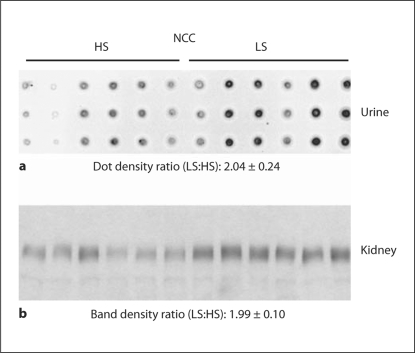
In the animal studies, we investigated whether rats with perturbed sodium balance manifest changes in urinary sodium transporter excretion that correlate with abundance changes in renal tissue. The first rat model we examined was escape from aldosterone-induced sodium retention [16]. Normalized densitometry values for the kidney and normalized excretion rates for the urinary exosomes measurements were summarized in table 1. As previously demonstrated, the experimental rats escaped from aldosterone-induced sodium retention through a reduction in NCC expression of the distal convoluted tubules (normalized densitometry values: escape, 0.27 ± 0.04 vs. control, 1.00 ± 0.35). This response was paralleled by a marked reduction in NCC urinary excretion (normalized densitometry values: escape, 0.16 ± 0.05 vs. control, 1.00 ± 0.05). NKCC2 did not exhibit substantial changes in renal abundance nor urinary exosome excretion. In the second rat model, we examined NCC excretion in urinary exosomes (fig. 1) in dietary sodium restriction rats by dot blotting. Urinary measurement of NCC excretion rates from these animals revealed a statistically significant increase in NCC excretion (normalized excretion rates: low salt, 2.04 ± 0.24 vs. high salt, 1.00 ± 0.23) associated with an increase in NCC abundance in the kidney (normalized band densities: low salt, 1.99 ± 0.10 vs. high salt, 1.00 ± 0.12) in response to salt restriction. Thus, the overall results indicate that urinary exosome excretion from NKCC2 and NCC correlated well with their abundance in the kidney.
Table 1.
Sodium transporters in aldosterone-escape rats (n = 6) and control rats (n = 6)
| Urine |
Kidney |
|||
|---|---|---|---|---|
| control | escape | control | escape | |
| NKCC2 | 1.00 ± 0.26 | 0.52 ± 0.19 | 1.00 ± 0.09 | 0.73 ± 0.08 |
| NCC | 1.00 ± 0.17 | 0.16 ± 0.05∗ | 1.00 ± 0.35 | 0.27 ± 0.04∗ |
Data are expressed as means ± SE. All values normalized by control.
p < 0.05.
Fig. 1.
Sodium restriction study in rats; high salt (HS; n = 6) vs. low salt (LS; n = 6). NCC excretion in urine by dot blots (a; triplicates) paralleled with NCC abundance in the kidney by Western blots (b).
Human Studies
Demographic, Biochemical and ABPM Data
Eleven hypertensive patients were not included in the analysis due to poor compliance to the low-salt diet or due to the presence of urinary infections. Therefore, 41 hypertensive patients completed the protocol: 20 females (48.8%) and 21 males (51.2%); with a mean age of 51 ± 16 years. Mean urinary sodium excretion was 44 ± 17 mmol/24 h at the end of the low-salt-intake period, and 162 ± 60 mmol/24 h at the end of the high-salt-intake period. As expected, levels of plasma renin activity and aldosterone were increased as a consequence of the activation of the renin-angiotensin-aldosterone system under the salt-restricted diet (table 2). There was no difference in urinary albumin excretion at the end of the two salt-intake periods (table 2).
Table 2.
Twenty-four-hour ABPM, hormones and biochemical data from plasma and urine (n = 41) at the end of each diet period in patients
| Reference values | Low-salt diet | High-salt diet | p value | |
|---|---|---|---|---|
| ABPM | ||||
| 24-hour MBP, mm Hg | 99.1 ± 8.3 | 102.0 ± 8.7 | 0.041∗ | |
| Day MBP, mm Hg | 103.8 ± 9.2 | 107.1 ± 10.9 | 0.048∗ | |
| Night MBP, mm Hg | 82.6 ± 9.1 | 86.8 ± 9.8 | 0.003∗ | |
| Pulse pressure, mm Hg | 49.4 ± 14.1 | 49.4 ± 7.9 | 0.997 | |
| 24-hour cardiac rate, beat/min | 78.7 ± 8.7 | 77.8 ± 9.1 | 0.371 | |
| Hormones | ||||
| PRA, μg/l/h | 0.4–4.0 | 1.0 ± 0.7 | 0.4 ± 0.5 | <0.0001∗∗ |
| Aldosterone, nmol/l | 0.22–0.57 | 0.48 ± 0.20 | 0.24 ± 0.16 | <0.0001∗∗ |
| Aldosterone: PRA ratio | 30 ± 41 | 38 ± 39 | 0.042∗ | |
| Noradrenaline, pg/ml | 135–300 | 374 ± 209 | 276 ± 199 | 0.001∗ |
| Adrenaline, pg/ml | 20–60 | 23 ± 12 | 20 ± 8 | 0.236 |
| Dopamine, pg/ml | 10–150 | 27 ± 16 | 23 ± 14 | 0.297 |
| Plasma | ||||
| Sodium, mmol/l | 137–143 | 138 ± 2 | 138 ± 2 | 0.57 |
| Potassium, mmol/l | 3.5–4.8 | 4.3 ± 0.4 | 4.2 ± 0.4 | 0.054 |
| Creatinine, μmol/l | 60–130 | 75 ± 16 | 73 ± 17 | 0.085 |
| Osmolality, mosm/kg | 280–300 | 290 ± 3 | 291 ± 5 | 0.138 |
| Urine | ||||
| Sodium, mmol/24 h | 44 ± 17 | 162 ± 60 | <0.0001∗∗ | |
| Potassium, mmol/24 h | 76 ± 25 | 73 ± 25 | 0.419 | |
| Osmolality, mosm/kg | 403 ± 180 | 567 ± 229 | <0.0001∗∗ | |
| Creatinine, mmol/24 h | 8–18 | 11 ± 4 | 11 ± 4 | 0.160 |
| Albuminuria, mg/l | <30 | 10 ± 10 | 12 ± 13 | 0.462 |
Data are expressed as means ± SD. PRA = Plasmatic renin activity.
p < 0.05;
p < 0.0001.
ABPM interfered with sleep in 9 patients, from whom day MBP was used to assess the salt sensitivity. 24-hour ABPM data at the end of both diet periods showed elevations in: 24-hour MBP (3.0 mm Hg), day MBP (3.2 mm Hg) and night MBP (4.2 mm Hg) when patients changed from the low- to the high-salt diet (table 2). We did not detect significant variations in 24-hour pulse pressure or in cardiac rate. In this study, we detected 6 salt-sensitive patients (15%) and 29 salt-resistant patients (70%), while the remaining 6 patients (15%) were salt-indeterminate according to the definition of BP salt sensitivity by Weinberger [21]. According to the circadian rhythm at the end of the low-salt diet, we detected 8 patients with a dipper profile, 4 patients with a non-dipper profile, and 20 patients with an extreme-dipper profile. At the end of the high-salt diet, we detected 15 patients with a dipper profile, 3 patients with a non-dipper profile and 14 patients with an extreme-dipper profile. There were no patients with a riser profile.
Tubular Sodium Reabsorption and NKCC2 and NCC Excretion in Urinary Exosomes
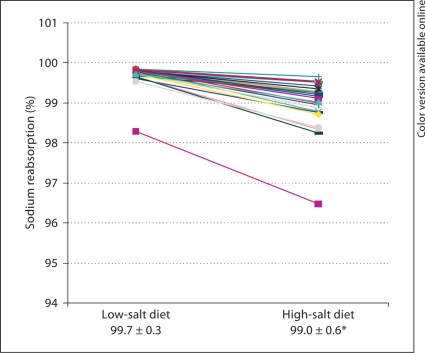
The sodium filtration rate was calculated from the values of creatinine clearance, plasma sodium concentration, and 24-hour urine sodium excretion in the two diet periods. At the end of the low-salt-intake period, the mean 24-hour sodium filtration rate was 20.286 mmol/ 24 h; however, the sodium filtration increased to 20.838 mmol/24 h at the end of the high-salt diet (table 3). From these values, we calculated the fractional sodium reabsoption and the fractional sodium excretion at the end of the two diet periods. As expected, we observed a reduction in the fractional sodium reabsorption when the patients changed from the low-salt to the high-salt diet (99.7 vs. 99.0%, respectively; fig. 2).
Table 3.
Creatinine clearance, sodium filtration rate and fractional sodium excretion and reabsorption at the end of each diet period in patients
| Low-salt diet | High-salt diet | |
|---|---|---|
| Creatinine clearance, ml/min | 101.9 ± 43.4 | 104.8 ± 45.6 |
| Sodium filtration rate, mmol/24 h | 20.286 ± 8.582 | 20.838 ± 9.243 |
| Fractional sodium excretion, % | 0.3 ± 0.28 | 1.0 ± 0.59∗∗ |
| Fractional sodium reabsorption, % | 99.7 ± 0.28 | 99.0 ± 0.59∗∗ |
Data are expressed as means ± SD.
p < 0.05;
p < 0.0001.
Fig. 2.
Fractional sodium reabsorption at the end of the low- and high-salt diets in patients. Data presented as means ± SD. * p < 0.05.
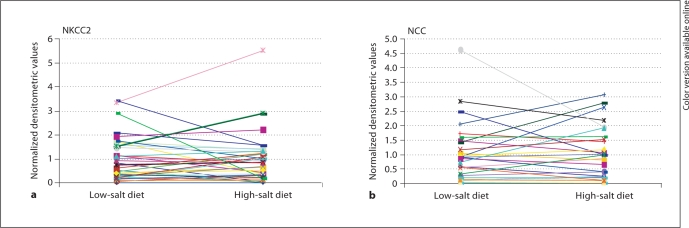
Figure 3 shows the urinary exosome excretion of NKCC2 and NCC at the end of the low- and the high-salt diets. Although at the end of the low-salt diet we found an increase in the fractional sodium reabsorption and at the end of the high-salt diet a decrease in the fractional sodium reabsorption, we observed a variable excretion of NKCC2 and NCC in urinary exosomes. Some patients showed no differences regarding the excretion of the transporters at the end of the two periods, while others showed an increase or a decrease in either or both transporters when changed from the low-salt to the high-salt diet.
Fig. 3.
NKCC2 (a; n = 41) and NCC (b; n = 31) urinary exosomal excretion at the end of the low- and high-salt diets in patients.
NCC is well known to be upregulated by aldosterone [19]. Thus, in the present work, there was an expected increase in the aldosterone plasma levels at the end of the low-salt diet. However, we did not observe any correlation between the aldosterone levels and the excretion of NCC in urinary exosomes. Due to technical problems, we only have the results of the exosomal excretion of NCC from 31 patients.
BP Changes with Salt Intake and NKCC2 and NCC Excretion in Urinary Exosomes
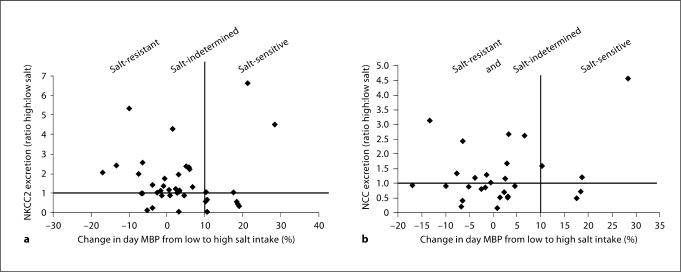
We analyzed BP changes related to salt intake with the profile of NKCC2 and NCC urinary exosome excretion. As we previously mentioned, not all the patients had a reliable 24-hour MBP; therefore, we used the day MBP for these patients and in the graphic representations. We hypothesize that sodium transporters in urinary exosomes would be excreted into the urine in a different pattern in response to sodium intake in salt-sensitive patients in comparison to salt-resistant patients. Our results showed that there is not a clear relationship between the difference in NKCC2 and NCC excretion in exosomes and the variation in the BP related to salt intake (fig. 4).
Fig. 4.
Human study. Changes in BP with salt intake and NKCC2 and NCC urinary exosomal excretion. The x-axes represent the percentage change in day MBP from the low- to the high-salt diet: ≥10% = salt-sensitive patients, <10 or >5% = salt-indeterminate patients, and ≤5% = salt-resistant patients. The y-axes represent the ratio of sodium transporters, NKCC2 (a) and NCC (b), in urinary exosomes between the high- and low-salt diets. The cutoff at 1 is the point at which there are no changes in the excretion of the sodium transporter between the two diet periods. A value >1 means an increase in the urinary excretion of the transporter at the end of the high-salt diet, and a value <1 a decrease in the urinary excretion of the sodium transporter at the end of the high-salt diet.
When we classified the patients according to their circadian rhythm, we could not detect any relationship between the circadian pattern (dipper, non-dipper, and extreme dipper) and the excretion of NKCC2 or NCC in urinary exosomes in the two diet periods (data not shown).
Discussion
Basic research has provided important information regarding the molecular mechanisms of renal disease; however, translation of these findings to the clinical practice remains challenging. Development of urinary biomarkers requires three major steps, which are discovery, validation, and implementation. Pisitkun et al. [10] discovered the presence of small vesicles named exosomes containing apical plasma membrane proteins, such as tubular sodium transporters, in urine. In the present work, we showed in two models of sodium imbalance the correlation of apical sodium transporters from the loop of Henle (NKCC2) and the distal tubule (NCC) in urinary exosomes with the abundance of these transporters in the kidney. Thereafter, we studied the excretion of NKCC2 and NCC in urinary exosomes in humans as potential markers of segment-specific tubular sodium reabsorption. In addition, we investigated the renal excretion profile of NKCC2 and NCC in urinary exosomes in salt-sensitive, salt-resistant, and salt-indeterminate hypertensive patients because altered renal sodium handling has a major pathogenic role in salt-sensitive hypertension.
Tubular Sodium Reabsorption and Urinary Excretion of Exosomes Containing NKCC2 and NCC
The kidney plays a central role in sodium balance. Sodium reabsorption along the nephron is mediated by ion transporters located in the apical membrane of tubular cells, and by the sodium pump located in the basolateral membrane. Under conditions of low salt intake, the kidney increases the tubular sodium reabsorption in order to maintain blood volume and BP. When the sodium intake increases, the tubular sodium reabsorption decreases to avoid significant elevation in BP. This is the concept of pressure natriuresis postulated by Guyton [2], and the kidney-fluid system is the predominant method of establishing long-term pressure control. In the present study, we also observed that during the period of low salt intake the fractional sodium reabsorption is increased, and that this reabsorption decreases during the period of high salt intake. During the period of high sodium reabsorption, we did not however observe an increase in the urinary exosomal excretion of sodium transporters from the loop of Henle (NKCC2) or the distal tubule (NCC) as an indirect marker of raised renal tubular sodium transport in these segments. In addition, during the period of high salt intake, when tubular sodium reabsorption diminishes, we did not observe a decrease in urinary NKCC2 or NCC exosomal excretion. Therefore, in the present work we found a lack of correlation between renal sodium reabsorption and excretion of NKCC2 and NCC in urinary exosomes in humans.
Salt Intake, BP Changes, and Urinary Excretion of Exosomes Containing NKCC2 and NCC
The regulation of BP is closely linked to the capacity of the kidney to excrete sodium. Thus, our study population was classified as salt-sensitive, salt-resistant, or salt-indeterminate depending on their level of BP changes in response to a high-salt diet.
Recent scientific evidence suggests that salt sensitivity in human populations may be linked directly or indirectly to sodium transport in the kidney [5,6,24]. Genetic studies have demonstrated that an increase in the activity of the apical sodium transporters NKCC2, NCC, and the epithelial sodium channel ENaC are associated with a salt-sensitive form of hypertension. Together, these observations indicate that salt sensitivity reflects a physiological defect in the ability of the kidneys to excrete sodium.
Hypothetically, salt-sensitive patients could not sufficiently increase the sodium excretion during the high-salt diet, leading to an elevation of their BP, so they would not be able to regulate any or some of the renal sodium transporters in response to the increase in salt intake. On the other hand, salt-resistant patients would be able to modify sodium transporter levels according to the salt intake, and therefore they could enhance sodium excretion as long as salt ingestion increased, so their BP would be independent of the salt level in the diet. In the present study, we did not observe a specific profile of NKCC2 and NCC urinary exosomal excretion in salt-sensitive, salt-resistant, or salt-indeterminate hypertensive patients. When comparing the level of excretion of these sodium transporters in exosomes at the end of the low- and high-salt diets for each patient, we found a heterogeneous response. These results suggest that there is not a clear correlation between NKCC2 and NCC urinary exosomal excretion and the salt sensitivity of BP. However, we can not rule out a relationship between the level of renal sodium transporters in the kidney and changes in BP levels with low and high salt intake in hypertensive patients. There are several possible explanations for the lack of correlation that we found in this study. First, we do not have direct data from the kidney tissue. It is clear that there is no indication for a kidney biopsy in the hypertensive patients studied. Thus, we used urine as an alternative source that can provide indirect information about kidney tissue. Second, we have hypothesized that the main mechanism by which renal sodium transporters reach the urine is by exosomal release. However, renal sodium transporters can also be released in the urine by other pathways, such as cell shedding, direct secretion by the epithelial cell, and membrane fragmentation. Since we have purified only the exosomal fraction by ultracentrifugation of the urine, we have not been able to measure these other possible sources of tubular protein excretion. To date, there are no studies that have investigated specifically how apical sodium transporters of renal tubules are excreted into the urine in humans. There are, however, several works that have shown a correlation between the amount of aquaporin-2 in the kidney and the amount present in the urine of experimental animals. Furthermore, the majority of aquaporin-2 excreted into the urine is via the exosomal pathway; thus, making exosomal analysis a useful tool for investigating aquaporin-2 [14]. Third, another possible explanation for the lack of correlation is that the urinary excretion of sodium transporters might not reflect the activity of the transporters in the kidney. Fourth, in the present study we did not analyze other renal sodium transporters present in the apical plasma membrane such a NHE-3 from the proximal tubule and ENaC from the collecting ducts. Indeed, experimental studies have observed a correlation between NHE-3 and ENaC in rats with salt-sensitive hypertension. Loffing et al. [5] also observed that there is a redistribution of ENaC from the intracellular space to the apical plasma membrane under dietary sodium restriction. Chiolero et al. [8] have investigated proximal sodium reabsorption indirectly measured by lithium clearance in patients with salt-sensitive and salt-resistant hypertension. Lithium is mainly reabsorbed in the proximal tubule and, to a lesser extent, in other tubular segments. In this study, the patients with a salt-sensitive form of hypertension had an increase in lithium clearance that suggests a rise in proximal sodium reabsorption. These results suggest that we cannot rule out the possibility that proximal sodium reabsorption or the interaction between different sodium transporters may be implicated in this type of hypertension. Finally, the etiology of hypertension is highly heterogeneous and different factors, both genetic and non-genetic, are implicated in the development of high BP. These factors might also be interacting with the sodium renal transporters analyzed, and make it difficult to interpret the results of the present study.
BP follows a circadian rhythm with a physiologic decrease during the night. Some works have postulated that the ability to eliminate sodium during the day is indeed a key determinant of the circadian rhythm of BP[25]. In the present study, we did not observe a specific profile of renal sodium transporters (NKCC2 and NCC) excretion in urinary exosomes in the two salt periods in patients with a non-dipper rhythm. However, the number of patients included in this analysis was relatively small to yield a final conclusion.
In conclusion, our results indicate that in rats urinary NKCC2 and NCC excretion correlated with the abundance in the kidney. In humans, however, the study of renal sodium transporters NKCC2 and NCC excreted in urinary exosomes does not predict which part of the renal tubule might be implicated in the pathogenesis of salt-sensitive hypertension. Contemporary treatment of hypertension remains largely empirical, so improving our understanding of how different sodium transporters may influence the salt sensitivity of BP can be extremely useful in making therapeutic decisions with the different types of diuretics. However, there are still many unanswered questions. Given the non-invasive nature of the urine sample collection and the large amount of kidney information potentially available in that source, more studies need to be performed in the field of urinary exosomes in biomarker discovery.
Acknowledgements
The authors thank Patricia Ruiz for expert technical assistance; Jordi Ordónez-Llanos, Jose Luis Sánchez-Quesada, Sònia Benítez and Cristina Bancells from Servei de Bioquímica, Hospital de la Santa Creu i Sant Pau for supplying the Beckman ultracentrifuge. Funding for this study was received from Fondo de Investigación Sanitaria (FIS-02/1547 to P.F.L.) and the Spanish Society of Nephrology (to P.F.L.).
References
- 1.Pieper R, Gatlin CL, McGrath AM, Makusky AJ, Mondal M, Seonarain M, Field E, Schatz CR, Estock MA, Ahmed N, Anderson NG, Steiner S. Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1,400 distinct protein spots. Proteomics. 2004;4:1159–1174. doi: 10.1002/pmic.200300661. [DOI] [PubMed] [Google Scholar]
- 2.Guyton AC. Blood pressure control – special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 3.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 4.Capasso G, Rizzo M, Garavaglia ML, Trepiccione F, Zacchia M, Mugione A, Ferrari P, Paulmichl M, Lang F, Loffing J, Carrel M, Damiano S, Wagner CA, Bianchi G, Meyer G. Upregulation of apical sodium-chloride cotransporter and basolateral chloride channels is responsible for the maintenance of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2008;295:F556–F567. doi: 10.1152/ajprenal.00340.2007. [DOI] [PubMed] [Google Scholar]
- 5.Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol. 2000;279:F252–F258. doi: 10.1152/ajprenal.2000.279.2.F252. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Guerra M, Garay RP. Renal Na-K-Cl cotransporter NKCC2 in Dahl salt-sensitive rats. J Hypertens. 2002;20:721–727. doi: 10.1097/00004872-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Hoagland KM, Flasch AK, Dahly-Vernon AJ, dos Santos EA, Knepper MA, Roman RJ. Elevated BSC-1 and ROMK expression in Dahl salt-sensitive rat kidneys. Hypertension. 2004;43:860–865. doi: 10.1161/01.HYP.0000120123.44945.47. [DOI] [PubMed] [Google Scholar]
- 8.Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Proximal sodium reabsorption: an independent determinant of blood pressure response to salt. Hypertension. 2000;36:631–637. doi: 10.1161/01.hyp.36.4.631. [DOI] [PubMed] [Google Scholar]
- 9.McKee JA, Kumar S, Ecelbarger CA, Fernández-Llama P, Terris J, Knepper MA. Detection of Na(+) transporter proteins in urine. J Am Soc Nephrol. 2000;11:2128–2132. doi: 10.1681/ASN.V11112128. [DOI] [PubMed] [Google Scholar]
- 10.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanno K, Sasaki S, Hirata Y, Ishikawa S, Fushimi K, Nakanishi S, Bichet DG, Marumo F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N Engl J Med. 1995;332:1540–1545. doi: 10.1056/NEJM199506083322303. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen RS, Bentzen H, Bech JN, Nyvad O, Pedersen EB. Urinary aquaporin-2 in healthy humans and patients with liver cirrhosis and chronic heart failure during baseline conditions and after acute water load. Kidney Int. 2003;63:1417–1425. doi: 10.1046/j.1523-1755.2003.00858.x. [DOI] [PubMed] [Google Scholar]
- 13.Esteva-Font C, Baccaro ME, Fernández-Llama P, Sans L, Guevara M, Ars E, Jiménez W, Arroyo V, Ballarín JA, Ginès P. Aquaporin-1 and aquaporin-2 urinary excretion in cirrhosis: relationship with ascites and hepatorenal syndrome. Hepatology. 2006;44:1555–1563. doi: 10.1002/hep.21414. [DOI] [PubMed] [Google Scholar]
- 14.Wen H, Frokiaer J, Kwon TH, Nielsen S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol. 1999;10:1416–1429. doi: 10.1681/ASN.V1071416. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-Scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest. 2001;108:215–222. doi: 10.1172/JCI10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and τ subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masilamani S, Wang XY, Kim GH, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F1403–F1421. doi: 10.1152/ajprenal.00016.2002. [DOI] [PubMed] [Google Scholar]
- 19.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. doi: 10.1161/01.cir.81.2.700. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Yuen PS, Pisitkun T, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–1476. doi: 10.1038/sj.ki.5000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Llama P, Ageloff S, Fernández-Varo G, Ros J, Wang X, Garra N, Esteva-Font C, Ballarin J, Barcelo P, Arroyo V, Stokes JB, Knepper MA, Jiménez W. Sodium retention in cirrhotic rats is associated with increased renal abundance of sodium transporter proteins. Kidney Int. 2005;67:622–630. doi: 10.1111/j.1523-1755.2005.67118.x. [DOI] [PubMed] [Google Scholar]
- 24.Rossier BC. The epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997;8:980–992. doi: 10.1681/ASN.V86980. [DOI] [PubMed] [Google Scholar]
- 25.Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. doi: 10.1161/01.HYP.0000240268.37379.7c. [DOI] [PubMed] [Google Scholar]