Abstract
Background
The effects of rectal surgery on patients’ postoperative quality of life (QOL) and sexual function has been little studied to date. The present study aims to address this issue.
Methods
519 patients who had undergone surgery for rectal cancer from January 1997 to January 2003 were included in the study. The EORTC-QLQ-C-30 questionnaire and an additional, tumor-specific module were administered prospectively multiple times over a 2-year period: before surgery, on discharge from the hospital, and at 3, 6, 12, and 24 months after surgery. Comparisons were made between men and women, different age groups, and different surgical procedures: abdominoperineal resection (APR) versus anterior resection (AR).
Results
There were significant differences between men and women on scales of function and symptoms. Women had worse scores for physical function and overall quality of life and higher values for fatigue. Sexual life was impaired in both men and women, but the impairment was significantly more severe in men, and men felt more distressed by it than women did. Physical function and overall quality of life were better in patients aged 69 and younger, while patients aged 70 and older suffered from fatigue. Younger patients had a more severe impairment of sexuality, which, over the time period of the study, led to severe emotional symptoms. Sexuality was more severely impaired in patients who had undergone APR than in those who had undergone AR.
Conclusion
These findings show that the quality of life is changed by surgery for rectal cancer and is influenced by the patient’s sex and age, as well as by the particular surgical approach used.
Colorectal carcinoma is among the more common malignancies in Germany, with more than 70 000 new cases and approximately 30 000 deaths from this disease each year (1). It is the second most common type of cancer for both men (after lung cancer) and women (after breast cancer). The average age of diagnosis is in the seventh decade of life (1).
The rectum is the most common site of colorectal carcinoma, accounting for 30% of cases (1). The treatment of rectal carcinoma can be associated with incontinence, as well as with impaired sexual function (1– 7). Because of advances in the early detection and treatment of this disease, it is expected that, in the future, an increasing number of patients will be living with its sequelae. Little is currently known about impaired function in everyday life among patients who have been treated for colorectal carcinoma. There is thus a need for assessment in this area. Measuring the quality of life (QOL) has become well established as an appropriate tool for such purposes (2– 5).
Only a few studies have been published to date concerning quality of life and sexuality after rectal resection. These studies have often focused on a comparison between operative techniques (4– 8). Other aspects that have been investigated include the effects of a stoma (7), adjuvant treatment (5), or severe complications (7) on sexuality and quality of life.
Moreover, the patient’s age and sex are also known to be important factors for the postoperative quality of life and sexuality (2– 8).
In most of the studies performed to date, sexual function was difficult to assess, because patients were reluctant to answer questionnaires with detailed information about their degree of sexual impairment (2, 4– 6). Recently, an attempt has been made to improve the accuracy of assessment by studying sexual function pre- and postoperatively with neurophysiological tests (4).
Most studies to date have assessed postoperative sexual function only at one time point, rather than longitudinally. The goal of the present study was to assess the patients’ postoperative sexual function and quality of life over time, with special attention to the important factors that influence sexual function.
Methods
This study included 519 patients who had undergone treatment with curative intent for carcinoma of the rectum or of the rectosigmoid junction from January 1997 to January 2003 in the Department of General and Thoracic Surgery at the Schleswig-Holstein University Clinic, Kiel Campus (Kiel, Germany). All patients gave their informed consent to participation in the study when they were admitted to the hospital for surgery.
The patients were asked to fill out questionnaires before surgery, at discharge from the hospital, and three, six, twelve, and 24 months later. The questionnaires were distributed directly to the patients while they were in the hospital, and sent to them by mail after discharge. Before each mailing, the authors contacted the patients’ family physicians to determine whether the patients were still living or had died of their malignant disease. The questionnaires sent to the surviving patients assessed the influence of social and demographic factors as well as tumor surgery on their quality of life; the questionnaire forms also contained information for patients about the purpose of the study. Data on the patients’ medical treatment, tumor histology, and adjuvant therapy were obtained from their medical records.
The patients’ quality of life was assessed with the EORTC-QLQ-C-30 questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC) (8). This questionnaire consists of 30 items, and the responses are summarized in three scales: a functional scale, a symptom scale, and a global scale. An overview of the number and content of questions (items) is provided in Table 1. In addition, the questionnaire used in this study included a tumor-specific module for patients with colorectal cancer, which was developed according the the EORTC guidelines (10, 11). This supplementary module has been tested for validity (11). It encompasses the areas of fecal incontinence, diarrhea, impaired sexual function, and stoma-related problems. By agreement with the patients’ treating physicians, questions regarding sexuality were asked only after the patients had been discharged from the hospital.
Table 1. The EORTC-QLQ-C-30 contains five functional scales, two symptom scales, eight individual items (questions), and two global items.
| Scales | Number of items | Content | |
| Functional scales | Physical functions | 5 | Carrying suitcases |
| Long walks | |||
| Short walks | |||
| Bed-ridden | |||
| Needs help with personal hygiene | |||
| Role functions | 2 | Occupation | |
| Hobbies | |||
| Emotional functions | 4 | Tension | |
| Worry | |||
| Irritability | |||
| Dejection | |||
| Cognitive functions | 2 | Concentration | |
| Memory | |||
| Social functions | 2 | Family life | |
| Contact with friends | |||
| Symptom scales | Fatigue | 3 | Tiredness |
| Weakness | |||
| Need for rest | |||
| Pain | 2 | Pain | |
| Limitation of activities by pain | |||
| Individual items | Nausea | ||
| Vomiting | |||
| Shortness of breath | |||
| Sleep disturbance | |||
| Loss of appetite | |||
| Constipation | |||
| Diarrhea | |||
| Financial difficulties | |||
| Global scales | Health status | ||
| Quality of life |
The EORTC-QLQ-C-30 and the supplementary module are designed to obtain information about the week just prior to the filling out of the questionnaire. There are four possible answers to each question, ranging from 1 (“not at all”) to 4 (“very much”). High numerical values correspond to better function on the functional scale, but to more severe symptoms (problems) on the symptom scale (9). The appropriate statistical procedures for assessment of responses to the questionnaire have been described in an EORTC manual (12). The mean value of all items contributing to each scale (the raw score) is linearly transformed to yield values that can range from 0 to 100 (12).
The results are stated as mean values with standard deviations. The distributions and frequencies of the clinical parameters (such as tumor stage) were compared with the chi-square test. Non-parametric tests were used for statistical evaluation, in view of the fact that quality-of-life data are not, in general, normally distributed. The global level of statistical significance was set at p <0.05. The authors used the SPSS for Windows program (Version 10.1) for data analysis.
Results
519 patients underwent surgery in the Department of General and Thoracic Surgery of the Kiel University Clinic from January 1997 to January 2003. 105 patients died of their malignancy during the follow-up period; for 50 of these patients, completed questionnaires were available. 33 patients who had undergone an anterior rectal resection because of a gynaecological or benign tumor, and three patients with accompanying mental illness, were excluded from the study. Five patients who were more than 85 years old at the time of surgery were also excluded, and 55 patients were eligible for the study, but declined to participate.
Thus, data were available for at least one evaluation time point for a total of 368 patients. An overview of these patients is provided in Table 2. Their mean age was 64.9 years; 183 were women, and 185 were men. The 5-year survival rate was 67.1%, and the rate of complications was 8.7%. Typical complications included anastomosis failure, wound infection, postoperative hemorrhage, pneumonia, and sepsis (1– 8). The complication rates and the distributions of age, tumor stage, and surgical techniques were comparable among patients who did and did not provide data on their quality of life.
Table 2. The number of patients for whom quality-of-life (QOL) data were available and unavailable*1 at each time point (N = 519).
| Time point | QOL data available | QOL data unavailable |
| Preop | 172 (33.1%) | 347 (72.9%) |
| Postop | 166 (31.9%) | 353 (68.1%) |
| At 3 months | 193 (37.1%) | 326 (62.9%) |
| At 6 months | 170 (32.7%) | 349 (67.3%) |
| At 12 months | 241 (46.4%) | 278 (54.6%) |
| At 24 months | 95 (18.3%) | 424 (81.7%) |
*1Data were available for at least one time point for 368 patients; 105 patients died, for 50 of whom a questionnaire had been filled out. A total of 96 patients were excluded: 33 had a benign or gynecological tumor, 3 were mentally ill, 5 were over 85 years old, and 55 declined to participate in the study
The characteristics of the patient collective are summarized in Table 3. For the purpose of evaluation, groups were defined and analyzed for quality of life and sexuality. The first comparison was between men and women; next, age groups (under 70 versus 70 and above) and surgical techniques were compared.
Table 3. Overview of patients participating in the study.
| QOL data available | QOL data unavailable | Total | |
| Number | 368 | 151 | 519 |
| Average age | 64.9 | 66.7 | 65.5 |
| Complication rate | 8.7% | 10.1% | 9.2% |
| Five-year survival | 67.1% | 66.2% | 66.8% |
| Sex | |||
| Female | 183 (49.7%) | 75 | 258 |
| Male | 185 (50.2%) | 76 | 261 |
| Primary tumor | |||
| Tis | 1 (0.2%) | 0 | 1 (0.1%) |
| T1 | 45 (12.2%) | 13 (8.6%) | 58 (11.1%) |
| T2 | 92 (25%) | 42 (27.8%) | 134 (25.8%) |
| T3 | 185 (50.2%) | 78 (51.6%) | 263 (50.6%) |
| T4 | 34 (9.2%) | 13 (8.6%) | 47 (9%) |
| Tx | 11 (2.9%) | 5 (3.3%) | 16 (3%) |
| Degree of lymph node involvement | |||
| N0 | 190 (51.6%) | 73 (48.3%) | 263 (50.6%) |
| N1 | 94 (25.5%) | 43 (28.4%) | 137 (26.3%) |
| N2 | 78 (21.1%) | 23 (15.2%) | 101 (19.4%) |
| Nx | 6 (1.6%) | 12 (7.9%) | 18 (3.4%) |
| Metastases | |||
| M0 | 270 (73.3%) | 110 (72.8%) | 380 (73.2%) |
| M1 | 88 (23.9%) | 37 (24.5%) | 125 (24%) |
| Mx | 10 (2.7%) | 4 (2.6%) | 14 (2.6%) |
| Operative techniques | |||
| Anterior resection | 223 (60.5%) | 89 (58.9%) | 312 (60.1%) |
| Anterior resection and pouch | 26 (7%) | 11 (7.2%) | 37 (7.1%) |
| Abdominoperineal rectal resection | 38 (10.3%) | 18 (11.9%) | 56 (10.7%) |
| Sigmoid colectomy | 81 (22%) | 33 (21.8%) | 114 (21.9%) |
| Adjuvant therapy*1 | |||
| Chemotherapy only | 52 (13.1%) | 19 (12.5%) | 71 (13.6%) |
| Radiochemotherapy | 177 (48%) | 76 (50.3%) | 253 (48.7%) |
| Unknown | 17 (4.6%) | 56 (37%) | 71 (13.6%) |
*1Adjuvant therapy is not recommended for all tumor stages;Tis, tumor in situ
Functional scales
Table 4 concerns differences between men and women, between patients under 70 years old and patients aged 70 or older, and between patients who had been treated with two different surgical techniques, anterior resection (AR) and abdominoperineal resection (APR). On the functional scales, women rated their pre- and postoperative functional status significantly worse than men did. Accordingly, men rated their quality of life significantly better than women did from three months after surgery until the last time point 24 months after surgery. The comparison between age groups revealed that the younger patients (under 70) rated their functional status and quality of life better than the older patients (70 and above) did, from the first postoperative time point to the last time point 24 months after surgery. No significant differences were found between the two surgical techniques with respect to functional status or quality of life.
Table 4. Significant differences between the comparison groups in the functional and symptom scales of the EORTC QLQ-C-30 (mean values are indicated).
| Preop | At discharge | At 3 months | At 6 months | At 12 months | At 24 months | |
| Functional scales | ||||||
| Men | FS p = 0.04 | FS p = 0.04 | FS p = 0.03 | FS p = 0.03 | FS p = 0.04 | FS p = 0.03 |
| Women | (m = 86.12; | (m = 64.36; | (m = 74.52; | (m = 76.78; | (m = 78.12; | (m = 80.49; |
| w = 78.51) | w = 50.36) | w = 60.38) | w = 66.35) | w = 68.28) | w = 68.86) | |
| Men | n.s. | n.s. | QOL p = 0.03 | QOL p = 0.04 | QOL p = 0.04 | QOL p = 0.04 |
| Women | (m = 61.39; | (m = 63.26; | (m = 65.26; | (m = 66.24; | ||
| w = 54.37) | w = 58.14) | w = 61.03) | w = 62.53) | |||
| ≤ 69 years | FS p = 0.03 | n.s. | FS p = 0.03 | FS p = 0.04 | FS p = 0.02 | FS p = 0.03 |
| ≥ 70 years | (≤ 69 = 85.76; | (≤ 69 = 73.39; | (≤ 69 = 74.80; | (≤ 69 = 77.76; | (≤ 69 = 80.35; | |
| ≥ 70 = 74.94) | ≥ 70 = 56.30) | ≥ 70 = 62.51) | ≥ 70 = 60.60) | ≥ 70 = 64.44) | ||
| ≤ 69 years | QOL p = 0.04 | n.s. | QOL p = 0.04 | QOL p = 0.04 | QOL p = 0.03 | QOL p = 0.04 |
| ≥ 70 years | (≤ 69 = 63.80; | (≤ 69 = 61.79; | (≤ 69 = 62.78; | (≤ 69 = 66.49; | (≤ 69 = 68.12; | |
| ≥ 70 = 54.41) | ≥ 70 = 52.42) | ≥ 70 = 56.03) | ≥ 70 = 55.85) | ≥ 70 = 62.72) | ||
| Symptom scales | ||||||
| Men | n.s. | FT p = 0.04 | FT p = 0.04 | FT p = 0.04 | n.s. | n.s. |
| Women | (m = 50.78; | (m = 39.81; | (m = 43.90; | |||
| w = 64.33) | w = 52.21) | w = 44.58) | ||||
| Men | n.s. | n.s. | SD p = 0.03 | SD p = 0.02 | SD p = 0.04 | n.s. |
| Women | (m = 31.35; | (m = 25.56; | (m = 23.85; | |||
| w = 42.76) | w = 39.58) | w = 35.97) | ||||
| Men | DR p = 0.04 | n.s. | DR p = 0.04 | DR p = 0.04 | DR p = 0.04 | DR p = 0.04 |
| Women | (m = 29.46; | (m = 27.70; | (m = 21.46; | (m = 20.25; | (m = 19.77; | |
| w = 28.39) | w = 34.04) | w = 29.43) | w = 25.08) | w = 22.60) | ||
| ≤ 69 years | n.s. | n.s. | FT p = 0.04 | n.s. | FT p = 0.04 | n.s. |
| ≥ 70 years | (≤ 69 = 42.93; | (≤ 69 = 32.51; | ||||
| ≥ 70 = 52.40) | ≥ 70 = 47.28) | |||||
| APR | n.s. | n.s. | DR p = 0.02 | DR p = 0.01 | DR p = 0.01 | DR p = 0.01 |
| AR | (APR = 8.89; | (APR = 19.23; | (APR = 16.57; | (APR = 5.43; | ||
| AR = 33.33) | AR = 35.88) | AR = 29.23) | AR = 21.12) | |||
FS, functional status; QOL, quality of life; FT, fatigue; SD, sleep disturbance; DR, diarrhea; n.s., not significant; APR, abdominoperineal resection; AR, anterior resection. No Bonferroni correction was performed, because this was an exploratory study
Symptom scales
Women had significantly more severe symptoms of fatigue (tiredness, exhaustion) and more severe sleep disturbances than men did, up to six and twelve months after surgery (respectively). Women also complained more often of diarrhea before surgery and three months afterward. Older patients had more severe symptoms of fatigue up to three months after surgery. Patients who underwent AR had more severe problems with diarrhea up to three months after surgery (table 4).
Sexuality
The authors asked patients two questions: “Has the operation resulted in an impairment of your sexuality?” and “How much does this disturb you?”
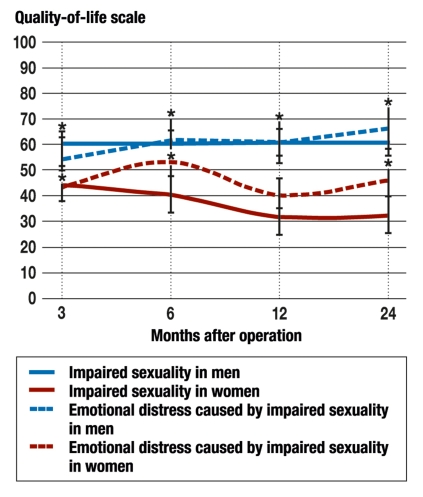
Both sexes experienced an impairment of their sexuality, men more severely than women. The associated emotional distress in men worsened up to the last time point 24 months after surgery; in men, the values for emotional distress were significantly worse at 24 months than at 3 months (figure 1).
Figure 1.
Comparison of the impairment of sexuality caused by surgery (solid lines) and the resulting emotional distress (dotted lines) in men and women (blue and red, respectively). Significant differences (p <0.05) are marked with asterisks (N = 368).
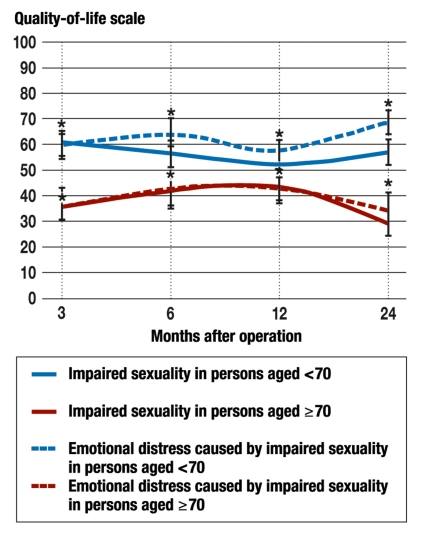
The comparison of age groups revealed that sexuality was markedly impaired in both. Patients under 70 years old experienced a mild improvement of their sexuality over time, yet they nonetheless experienced stronger emotional distress due to impaired sexuality, and this distress worsened up to two years after surgery. The differences between the two age groups over time are shown in Figure 2.
Figure 2.
Comparison of the impairment of sexuality caused by surgery (solid lines) and the resulting emotional distress (dotted lines) in persons less than 70 years old and persons aged 70 and older (blue and red, respectively). Significant differences (p <0.05) are marked with asterisks (N = 368).
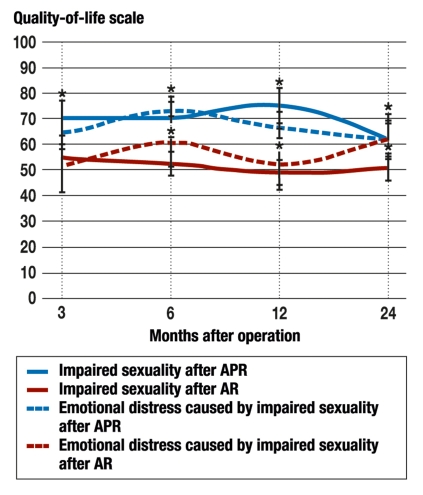
The comparison of the two surgical techniques, AR and APR, revealed that patients who underwent APR experienced a significantly worse impairment of sexuality than patients who underwent AR, over a time range from six to 24 months after surgery. The resulting emotional distress was greater in the APR patients than in the AR patients (figure 3).
Figure 3.
Comparison of the impairment of sexuality caused by surgery (solid lines) and the resulting emotional distress (dotted lines) in persons who underwent abdominoperineal resection (APR) with stoma and in persons who underwent anterior resection (AR) (blue and red, respectively). Significant differences (p <0.05) are marked with asterisks (N = 368).
Discussion
In 1958, the World Health Organization (WHO) defined health as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity” (13). This is important for patients with rectal carcinoma, because multimodal treatment prolongs their survival while increasing their functional and symptomatic burdens. Thus, additional end-points such as quality of life are needed in the evaluation of the results of treatment, especially if the patients’ disease-related impairment in their activities of daily living is to be assessed (2– 9, 11).
In particular, the treatment of colorectal carcinoma can be associated with deleterious effects on sphincter function and on the patients’ sexuality (2– 8). The assessment of sexuality and its impairment is difficult, however, because patients often feel that attempts to evaluate the extent of impairment (e.g., erectile dysfunction) precisely are excessively intrusive (2– 8, 17– 23). Thus, they tend to answer questions of this type incompletely, or not at all. This problem has arisen in many studies and has led to the adoption of various methods of assessment, ranging from interviews to clinical tests, which have yielded variable results (2, 5– 7, 18– 23).
In patients with rectal carcinoma, factors such as age, sex, and the type of operation performed have an influence on postoperative symptoms and on the subjective feeling of well-being, and must therefore be taken into account (2– 8, 18– 23). Moreover, quality of life should be assessed longitudinally at multiple time points, including preoperatively. In summary, the assessment of quality of life and sexuality in patients with rectal carcinoma has been found to have special methodological requirements (2– 8, 18– 23).
The present study meets these special requirements adequately: The QLQ-C-30 und the supplementary, tumor-specific module have been validated (9, 11). The groups of patients for whom quality-of-life data were available and unavailable were comparable in terms of the distribution of tumor stages, adjuvant treatment, and surgical techniques. The same was true of the patient groups under study. The questions regarding sexuality were answered.
Significant differences between men and women were found with respect to physical functioning, quality of life, and fatigue. Furthermore, women suffered more commonly than men from exhaustion and sleep impairment in the first six months after surgery, and were thus subjected to a greater overall burden through their treatment (2, 3, 8, 17, 18). Similar findings were made by Engel et al. (19) in a prospective study of women with rectal carcinoma and breast carcinoma and have been confirmed by other studies as well (2– 4).
As expected, older patients needed a longer time to recover after surgery in various ways, e.g., it took them longer to become accustomed to their stomata (14, 17). Younger patients often continue to bear responsibility in the family and at work and are thus more strongly motivated to get well (17). The authors found that the sole difference between the two operative techniques was with regard to diarrhea; other studies have yielded comparable findings (2– 8).
Women were especially burdened by the medical treatment itself, while, for men, impaired sexuality played a major role and was also associated with greater emotional distress than in women. The reason for impaired sexuality is surgical injury to the hypogastric plexus (1– 8). Surgical removal of the rectum was found 30 years ago to impair male sexual function markedly (1), but women, too, experience sexual dysfunction after surgery in the pelvic area for rectal carcinoma (17, 18). Many recent studies have confirmed this and have thus underscored the importance of sex-specific information to patients before surgery (2– 8). Men of whatever age have been found to experience greater emotional distress than women because of impaired sexual function. This is said to be due to men’s greater level of sexual activity (21, 22).
With regard to impaired sexuality and the related emotional distress, younger patients were found to be more severely affected than older patients by both of these over the entire period of time covered by the study. For many years, persons over age 70 were not perceived to be sexually active (21– 23). This attitude was reflected in science as well, with the result that research on sexuality in old age has begun only recently. Lindau (22) asked 3005 Americans (1550 women, 1455 men) aged 57 to 85 about their sexual behavior: 73% of respondents aged 57 to 64, 53% aged 65 to 74, and 26% aged 75 to 85 stated that they were sexually active (22). Other studies have confirmed that sexuality declines with age, yet remains an important aspect of quality of life for many people (2– 5, 23). The maintenance of quality of life, and of sexuality, in the elderly is now an increasingly important matter, because the population as a whole is becoming older (1). This is true of cancer patients as well. Quality of life and sexuality should be explicitly taken into account in preoperative discussions with the patient (2– 8).
As could have been expected, patients who undergo abdominoperineal resection complained more commonly of impaired sexuality and resulting emotional distress. Other studies have revealed similar findings (14, 17, 18), which are explicable as a consequence of the larger incision. The improved results with regard to sexual function after anterior resection, as currently performed, are attributable to the technique of total mesorectal excision (TME), which spares the nerve plexus (2, 4, 25). TME, introduced by Heald (25), lowers the local recurrence rate and also improves survival (1– 9, 25). The nerve-sparing technique causes less impairment of sexual function, yet it does not limit the radicality of the procedure (2– 7, 25).
Although the findings of this study are clear, a few limitations must be mentioned. The patients’ sexuality and sexual function were not assessed preoperatively, as it was feared that this might cause the patients unnecessary worry before surgery. The authors tried to compensate for this by asking the question, “Has the operation resulted in an impairment of your sexuality?” The study focused on impairments and related emotional distress over the course of the postoperative period. More specific aspects, such as erectile dysfunction or the integrity of the fascia of Denonvilliers, were not addressed. Thus, the patients’ responses are not informative on these points. Moreover, the yield of completed questionnaires declined over the two-year period of postoperative follow-up. Finally, as a consequence of the study design, data were not available for every patient at every time point. Care should therefore be exercised in drawing conclusions from the findings.
Overview
Sexual problems commonly result from surgery for rectal carcinoma (1– 8, 17– 23). This study reveals that, in men, sexual impairment and the related emotional distress tend to increase over time after surgery and are worse than in women. Moreover, younger patients have more difficulty living out their sexuality than older patients did. Finally, different operative techniques affect sexuality differently. The larger the incision, and the more distal the carcinoma, the more likely it becomes that surgery will impair sexuality. Adjuvant treatment (radiation and/or chemotherapy) was not found to affect sexuality in this study.
Awareness of the influence of the surgical technique, as well as of the patients’ age and sex, on the postoperative outcome can help physicians inform their patients completely and effectively before surgery (1– 8, 17– 23).
Key Messages.
Sexual problems are common after surgery for rectal carcinoma.
In men, symptoms of sexual dysfunction increase postoperatively and are more severe than in women.
Younger patients have greater difficulty living out their sexuality than older patients.
The larger the incision, and the more distal the carcinoma, the more likely it becomes that the patient will suffer from impaired sexuality after surgery.
Acknowledgments
Translated from the original German by Ethan Taub, MD
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Schmiegel W, Pox A, Reinacher-Schick G, et al. S3-Leitlinie “Kolorektales Karzinom”: Ergebnisse evidenzbasierter Konsensuskonferenzen am 6./7. 2. 2004 und am 8./9. 6. 2007. Z Gastroenterol. 2008;46:1–73. [Google Scholar]
- 2.Fisher SE, Daniels IR. Quality of life and sexual function following surgery for rectal cancer. Colorectal Dis. 2006;8:40–42. doi: 10.1111/j.1463-1318.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CE, Bestmann B, Küchler T, Kremer B. Factors influencing sexual function in patients with rectal cancer. Int J Impot Res. 2005;17:231–238. doi: 10.1038/sj.ijir.3901276. [DOI] [PubMed] [Google Scholar]
- 4.Pietrangeli A, Pugliese P, Perrone M, Sperduti I, Cosimelli M, Jandolo B. Sexual dysfunction following surgery for rectal cancer—a clinical and neurophysiological study. J Exp Clin Cancer Res. 2009;17 doi: 10.1186/1756-9966-28-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang JT, Lai HS, Lee PH. Laparoscopic pelvic autonomic nerve–preserving surgery for patients with lower rectal cancer after chemoradiation therapy. Ann Surg Oncol. 2007;14:1285–1287. doi: 10.1245/s10434-006-9052-6. [DOI] [PubMed] [Google Scholar]
- 6.Sterk P, Shekarriz B, Günter S, et al. Voiding and sexual dysfunction after deep rectal resection and total mesorectal excision: prospec-tive study on 52 patients. Int J Colorectal Dis. 2005;20:423–427. doi: 10.1007/s00384-004-0711-4. [DOI] [PubMed] [Google Scholar]
- 7.Bloemen JG, Visschers RG, Truin W, Beets GL, Konsten JL. Long-term quality of life in patients with rectal cancer: association with severe postoperative complications and presence of a stoma. Dis Colon Rectum. 2009;52:1251–1258. doi: 10.1007/DCR.0b013e3181a74322. [DOI] [PubMed] [Google Scholar]
- 8.Davies RJ, O’Connor BI, Victor C, MacRae HM, Cohen Z, McLeod RS. A prospective evaluation of sexual function and quality of life after ileal pouch-anal anastomosis. Dis Colon Rectum. 2008;51:1032–1035. doi: 10.1007/s10350-008-9248-x. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Sprangers MA, Cull A, Groenvold M, Bjordal K, Blazeby J, Aaronson NK. The European Organization for Research and Treatment of Cancer approach to developing questionnaire modules: An update and overview. EORTC Quality of Life Study Group. Qual Life Res. 1998;7:291–300. doi: 10.1023/a:1024977728719. [DOI] [PubMed] [Google Scholar]
- 11.Davidson-Homewood J, Norman A, Küchler T, Cunningham D, Watson M. Development of a disease specific questionnaire to supplement a generic tool for QoL in colorectal cancer. Psychooncology. 2003;12:675–685. doi: 10.1002/pon.684. [DOI] [PubMed] [Google Scholar]
- 12.Fayers P, Aaronson N, Bjordal K, Sullivan M. EORTC Quality of Life Study Group. Brüssel: EORTC; 1995. EORTC QLQ-C30 scoring manual. [Google Scholar]
- 13.World Health Organization. Genf: World Health Organization; 1958. The first ten years of the World Health Organization. [Google Scholar]
- 14.Schmidt CE, Bestmann B, Küchler T, Longo WE, Kremer B. Ten-year historic cohort of quality of life and sexuality in patients with rectal cancer. Dis Colon Rectum. 2005;48:483–492. doi: 10.1007/s10350-004-0822-6. [DOI] [PubMed] [Google Scholar]
- 15.Gacci M, Bartoletti R, Figlioli S, et al. Urinary symptoms, quality of life, and sexual function in patients with benign prostatic hypertrophy before and after prostatectomy: a prospective study. BJU. 2003;91:196–200. doi: 10.1046/j.1464-410x.2003.04072.x. [DOI] [PubMed] [Google Scholar]
- 16.Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies: the use of a single question self-assessment in the Massachusetts Male Aging Study. J Impot Res. 2000;12:197–204. doi: 10.1038/sj.ijir.3900542. [DOI] [PubMed] [Google Scholar]
- 17.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Hölzel D. Quality of Life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;238:203–213. doi: 10.1097/01.sla.0000080823.38569.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Hölzel D. Comparison of breast and rectal cancer patients’ quality of life: results of a four year prospective field study. Eur J Cancer Care. 2003;12:215–223. doi: 10.1046/j.1365-2354.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- 19.Fisher SE, Daniels IR. Quality of life and sexual function following surgery for rectal cancer. Colorectal Dis. 2006;8:40–42. doi: 10.1111/j.1463-1318.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 20.Platell CF, Thompson PJ, Makin GB. Sexual health in women following pelvic surgery for rectal cancer. Br J Surg. 2004;91:465–468. doi: 10.1002/bjs.4471. [DOI] [PubMed] [Google Scholar]
- 21.Moreira ED, Jr, Hartmann U, Glasser DB, Gingell C GSSAB Investigators Group. A population survey of sexual activity, sexual dysfunction and associated help-seeking behaviour in middle-aged and older adults in Germany. Eur J Med Res. 2005;10:434–443. [PubMed] [Google Scholar]
- 22.Lindau ST, Schumm LP, Laumann EO, Levinson W, O’Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith LJ, Mulhall JP, Deveci S, Monoghan N, Reid MC. Sex after seventy: a pilot study of sexual function in older persons. J Sex Med. 2007;4:1247–1253. doi: 10.1111/j.1743-6109.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 24.Lippert H, Gastinger U. Medical care of patients with rectal carcinoma in Germany [Versorgung von Patienten mit Rektumkarzinomen in Deutschland) Dtsch Arztebl. 2006;103 [Google Scholar]
- 25.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]