Abstract
Purpose
To report the results from a prospective study of a series of locoregionally advanced head-and-neck cancer patients treated with platinum-based chemotherapy and intensity-modulated radiotherapy and to discuss the findings of their pre-/mid-treatment [18F]-misonidazole (18F-FMISO) positron emission tomography (PET) scans.
Methods and Materials
A total of 28 patients agreed to participate in this study. Of these 28 patients, 20 (90% with an oropharyngeal primary cancer) were able to undergo the requirements of the protocol. Each patient underwent four PET scans: one pretreatment fluorodeoxyglucose PET/computed tomography scan, two pretreatment 18F-FMISO PET/computed tomography scans, and a third 18F-FMISO PET (mid-treatment) scan performed 4 weeks after the start of chemoradiotherapy. The 18F-FMISO PET scans were acquired 2–3 h after tracer administration. Patients were treated with 2–3 cycles of platinum-based chemotherapy concurrent with definitive intensity-modulated radiotherapy.
Results
A heterogeneous distribution of 18F-FMISO was noted in the primary and/or nodal disease in 90% of the patients. Two patients had persistent detectable hypoxia on their third mid-treatment 18F-FMISO PET scan. One patient experienced regional/distant failure but had no detectable residual hypoxia on the mid-treatment 18F-FMISO PET scan.
Conclusion
Excellent locoregional control was observed in this series of head-and-neck cancer patients treated with concurrent platinum-based chemotherapy and intensity-modulated radiotherapy despite evidence of detectable hypoxia on the pretreatment 18F-FMISO PET/computed tomography scans of 18 of 20 patients. In this prospective study, neither the presence nor the absence of hypoxia, as defined by positive 18F-FMISO findings on the mid-treatment PET scan, correlated with patient outcome. The results of this study have confirmed similar results reported previously.
Keywords: Intensity-modulated radiotherapy, IMRT, Hypoxia, 18F-FMISO, PET, chemoradiotherapy
INTRODUCTION
A plethora of both laboratory and clinical data exist linking hypoxia to poor prognosis in patients with tumors (1–4). Immunohistochemical studies have demonstrated that hypoxia can be seen at the microscopic level as nests of cells ranging up to several hundred micrometers in diameter (5). Compared with aerobic cells, hypoxia is more resistant to radiation and has been associated with a more aggressive phenotype (6). Clinical data, including data reported for head-and-neck cancer, has shown hypoxia to be an important determinant of locoregional control and overall survival (7–9).
Strategies aimed at overcoming hypoxia for head-and-neck cancer have been disappointing (10). Recently, interest has been renewed in targeting hypoxia using a hypoxia cytotoxin (11). Tirapazamine not only targets hypoxia, but also potentiates radiation/cisplatin cytotoxicity (12, 13). Several reports from the Peter MacCallum Cancer Center have shown great promise with tirapazamine in treating advanced head-and-neck cancer (14, 15). However, recent results from a Phase III trial did not show a significant benefit in terms of overall or failure-free survival or interval to locoregional failure with the addition of tirapazamine to the current standard of cisplatin plus radiotherapy (RT) for head-and-neck cancer (16). A second, but smaller, Phase III trial examining the added value of tirapazamine to treatment of head-and-neck cancer was also recently closed because of unexpected early deaths (17).
Direct measurements using an Eppendorf electrode is one approach to measuring hypoxia. However, this invasive technique is highly operator dependent and is subject to sampling error (3, 18, 19). An alternative approach can be achieved through positron emission tomography (PET) (20, 21). Rasey et al. (22) have demonstrated the efficacy of PET with [18F]-misonidazole (18F-FMISO) in quantifying hypoxia in head-and-neck cancer patients and observed a marked variability/heterogeneity between tumors at the same site or of the same histologic type (22). Other investigators have published similar findings (23–26). Rischin et al. (15) recently reported that patients with detectable hypoxia on pretreatment 18F-FMISO PET scan and who received non–tirapazamine-containing chemoradiotherapy had a greater risk of locoregional failure compared with those who were treated with tirapazamine-containing chemoradiotherapy.
Owing to our own interest in tumor hypoxia, we initiated a prospective trial to determine the feasibility of incorporating pre-/mid-treatment 18F-FMISO PET/computed tomography (CT) scans in head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Patients in this trial were treated with standard chemoradiotherapy. We report the preliminary clinical outcome and PET findings for these patients.
METHODS AND MATERIALS
Design
A trial titled “Feasibility study of using 18F-FMISO PET/CT to detect hypoxia in head-and-neck cancer” was opened from August 2004 to October 2005 [IRB (#04–070)]. All patients provided written informed consent. Seven subsequently withdrew, with the remaining 21 patients undergoing all four PET/CT scans (one 18F-fluorodeoxyglucose [FDG] and three 18F-FMISO scans), except for 1 patient who did not undergo his second and third 18F-FMISO scan and 2 who did not complete their third 18F-FMISO scan. Of these 2 patients, 1 was further excluded because he underwent surgery plus RT instead of chemoradiotherapy. Scoring of the 18F-FMISO scans was as follows: 0, uptake of 18F-FMISO less than background; 1, uptake equal to background; 2, uptake mildly greater than background; and 3, uptake moderately greater than background. Patients with scores of 2 (mild uptake) or 3 (moderate uptake) were considered to have positive 18F-FMISO scans.
All patients were seen by our multidisciplinary team of physicians and underwent standard pretreatment tests.
FDG and 18F-FMISO PET/CT
All patients were required to fast for 6 h before intravenous injection with 15 mCi of FDG. The patients underwent scanning on a PET/CT scanner 45–60 min after injection. One day later, the patients returned for the first 18F-FMISO PET/CT scan. Patients were injected with 10 mCi of 18F-FMISO. Fasting was not required. The patients were placed on the PET/CT scanner and immobilized in a head-and-neck cast at ~2–2.5 h after injection. A second pretreatment 18F-FMISO PET scan was performed 3 days after the first scan to verify consistency of the hypoxia PET results. The details of the PET/CT scan procedure and the radiopharmaceutical synthesis of 18F-FMISO have been previously published (27, 28). A third 18F-FMISO PET/CT scan was performed 4 weeks after the start of chemoradiotherapy. All baseline pretreatment 18F-FDG/18F-FMISO scans were conducted using the same GE Discovery LS PET/CT scanner and the same patient positioning for the purposes of accurate image registration and image analysis. For logistical reasons, the third 18F-FMISO study was performed on a GE Advance PET scanner using 68Ge rods to acquire the transmission scan used for attenuation correction and for co-registration with the pretreatment PET studies. The GE Advance and Discovery LS scanners have exactly the same PET cameras, with the exception of the LS containing a CT scanner. Phantom studies comparing the quantification between 18F tracer studies using transmission rod and CT attenuation correction have resulted in insignificant differences between the two scanners (29).
The defined gross tumor volume (GTV) was contoured on the CT component of the 18F-FDG PET/CT scan. This CT scan was used as the reference for co-registration of the CT and transmission image sets accompanying the 18F-FMISO PET scans, which were co-registered by the mutual information registration technique. The defined GTV also contained the segmented GTV drawn using the iterative segmentation technique by Nehmeh et al. (30). 18F-FMISO PET/CT lesions were scored qualitatively by our nuclear medicine physician (H.S.). The 18F-FMISO PET/CT scan was interpreted as positive if greater activity was present within the sites of tumor uptake of FDG than the activity present in adjacent soft-tissue sites.
Treatment plan
The patients were treated with either two to three cycles of cisplatin (100 mg/m2) or 5-fluorouracil (600 mg/m2) and carboplatin (70mg/m2), typically every 3 weeks, plus intensity-modulated RT (IMRT). Cisplatin was the preferred chemotherapy regimen (Table 1). Patients who had hearing or renal impairment were offered 5-fluorouracil/carboplatin. The GTV was defined by the tumor visible on all imaging and physical examinations. Depending on the clinical scenario, the clinical target volume (CTV) was divided into high- and low-risk CTVs. A 3–5-mm margin was applied to all of the target volumes to account for organ motion and patient setup errors. Therefore, several separate planning target volumes (PTVs) were defined: PTVGTV; PTVhigh-risk CTV, and PTVlow-risk CTV. A total dose of 70 Gy within 33 fractions was prescribed to PTVGTV simultaneously with 59.4 Gy to the PTVhigh-risk CTV; and, when clinically indicated, simultaneously with 54 Gy to the PTVlow-risk CTV. Compliance with full-dose radiation was 100%.
Table 1.
Patient characteristics (n = 20)
| Characteristic | Value |
|---|---|
| Age (y) | |
| Median | 58.5 |
| Range | 46–79 |
| History of tobacco use* | |
| Yes | 14 (70) |
| No | 6 (30) |
| Primary site | |
| Oropharynx | 19 (95) |
| Larynx | 1 (5) |
| T stage | |
| 1 | 5 (25) |
| 2 | 5 (25) |
| 3 | 5 (25) |
| 4 | 5 (25) |
| N stage | |
| 1 | 5 (25) |
| 2 | 13 (65) |
| 3 | 2 (10) |
| Stage group | |
| III | 3 (15) |
| IV | 17 (85) |
| Baseline hypoxia | |
| Yes | 18 (90) |
| No | 2 (10) |
| Chemotherapy regimen | |
| Cisplatin | |
| 2 Cycles | 8 (40) |
| 3 Cycles | 5 (25) |
| 5-FU/carboplatin | |
| 2 Cycles | 5 (25) |
| 3 Cycles | 2 (10) |
Abbreviation: 5-FU = 5-fluorouracil.
Data presented as number of patients, with percentages in parentheses, unless noted otherwise.
See Table 2 for details.
Follow-up and statistical analysis
The patients were evaluated weekly during IMRT and followed up every 1–2 months for the first 2 years and every 4–6 months thereafter. A post-treatment FDG PET/CT scan was performed 2–4 months after therapy completion. Descriptive statistics were calculated for the patient and disease characteristics and treatment. The 3-year estimates for local progression-free, regional progression-free, distant metastases-free, and overall survival rates were calculated using the Kaplan-Meier product-limit method. Freedom from local, regional, or distant progression was defined as the absence of demonstrable tumor on physical and imaging studies. The durations were calculated from the start of RT.
RESULTS
Patient characteristics
All patients had Stage III–IV squamous cell carcinoma of the oropharynx, except for 2 patients with laryngeal primaries (Table 1). The median follow-up of surviving patients was 36 months (range, 33–48). Table 2 contains the primary site, stage, and tobacco use. Table 3 records the tumor vs. nodal staging.
Table 2.
Primary site, stage, and tobacco use
| Pt. No. | Primary site | Stage | Daily tobacco use |
Approximate time (y) |
|---|---|---|---|---|
| 1 | Tonsil | T2N2a | 2 packs | 45 |
| 2 | Tonsil | T1N1 | None | NA |
| 3 | BOT | T3N1 | 3 packs | 26 |
| 4 | Tonsil | T3N2b | 1 pack | 20 |
| 5 | Tonsil | T2N2b | None | NA |
| 6 | Tonsil | T2N1 | 1.5 packs | 12 |
| 7 | BOT | T4N2b | 3.5 packs | 40 |
| 8 | BOT | T3N2b | 2 packs | 40 |
| 9 | BOT | T2N2c | None | NA |
| 10 | Tonsil | T1N1 | 0.5–0.75 pack | 25 |
| 11 | BOT | T4N2c | None | NA |
| 12 | BOT | T3N1 | 2 packs | 5 |
| 13 | BOT | T4N2c | None | NA |
| 14 | Tonsil | T3N2b | 0.5 pack | 30 |
| 15 | BOT | T1N3 | 1 pack | 10 |
| 16 | Larynx | T2N2b | None | NA |
| 17 | BOT | T1N3 | 1 pack | 8 |
| 18 | BOT | T1N2b | 1 pack | 25 |
| 19 | Tonsil | T4N2b | 2 cigars | 10 |
| 20 | BOT | T4N2c | 5–6 cigars | 46 |
Abbreviations: Pt. No. = patient number; NA = not applicable; BOT = base of tongue.
Table 3.
Tumor vs. nodal stage
| T stage | ||||
|---|---|---|---|---|
| N stage | T1 | T2 | T3 | T4 |
| N1 | 2 | 1 | 2 | 0 |
| N2a | 0 | 1 | 0 | 0 |
| N2b | 1 | 2 | 3 | 2 |
| N2c | 0 | 1 | 0 | 3 |
| N3 | 2 | 0 | 0 | 0 |
18F-FMISO PET imaging
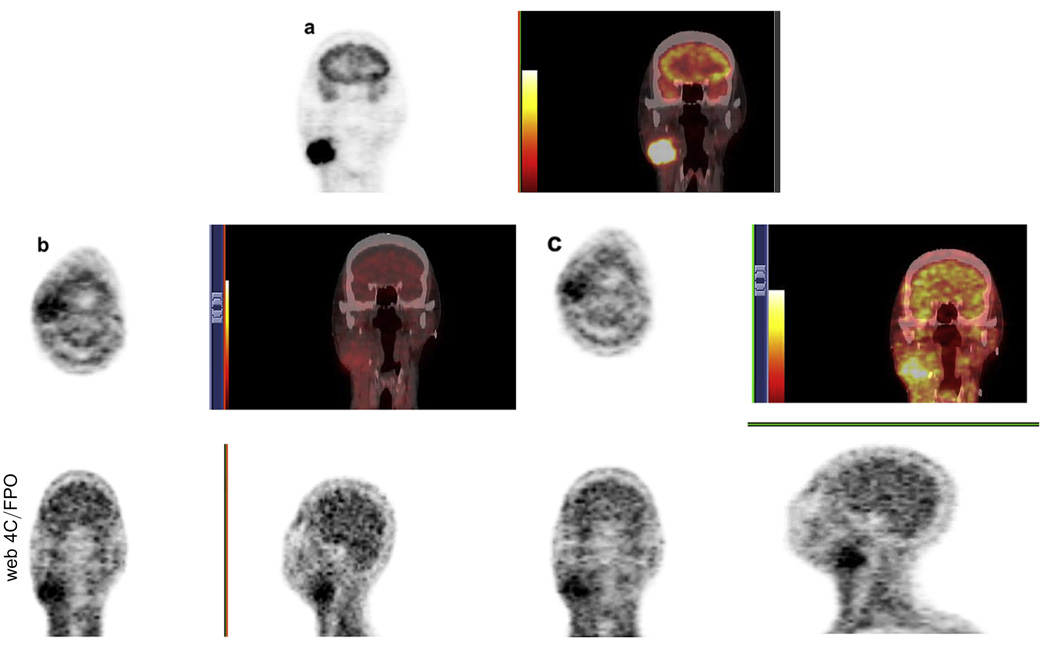
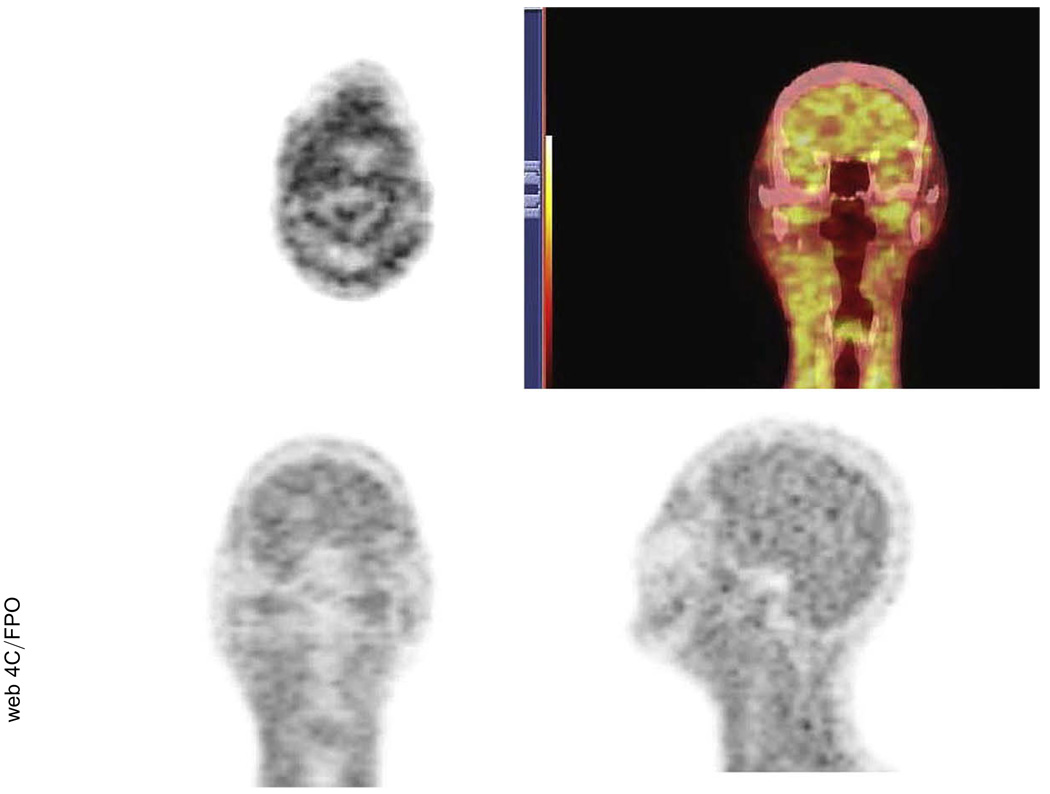
The distribution of 18F-FMISO showed that the severity of hypoxia varied throughout the tumor, with 90% of patients having detectable hypoxia in the primary tumor and/or nodes (Table 4). Two patients did not have any uptake (Stage T1N1 tonsil, Stage T1N2b base of tongue). Figure 1 is an example of a complete set of baseline PET images from a patient with Stage IV oropharyngeal cancer, including the 18F-FDG PET/CT scan and the first and second pretreatment 18F-FMISO PET/CT scans. The 18F-FMISO PET images of this patient demonstrated good, but not perfect, co-localization of the tracer between the first two scans. The cause of the difference in radiotracer localization between injections was not known exactly but could have been attributed to changes resulting from acute or transient hypoxia plus the inherent variability associated with the imaging statistics between scans. For details regarding the co-localization of the two 18F-FMISO PET images, along with a detailed voxel analysis of the statistical variation between serial baseline 18F-FMISO scans can be found in an earlier publication (28). Approximately 4 weeks into treatment, after receiving one cycle of 5-fluororacil and carboplatin and 40 Gy of IMRT, this patient underwent a third 18F-FMISO PET scan (Fig. 2).
Table 4.
Hypoxia in primary tumor vs. nodal regions
| Primary tumor hypoxia | ||
|---|---|---|
| Nodal hypoxia | Yes | No |
| Yes | 9 (45) | 7 (35) |
| No | 2 (10) | 2 (10) |
Fig. 1.
Examples from typical patient of three baseline positron emission tomography scans performed: (a) 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) at Day 0, (b) first [18F]-misonidazole (18F-FMISO) PET scan at Day 1, and (c) second pretreatment 18F-FMISO PET scan at Day 4. Note, general similarity of hypoxia tracer distribution between two pretreatment 18F-FMISO PET/CT scans.
Fig. 2.
Example of mid-treatment [18F]-misonidazole positron emission tomography/computed tomography scan with no residual detectable hypoxia.
Of the 20 patients, 18 successfully underwent all three 18F-FMISO PET scans. Of these 18 patients, 16 had complete resolution of the hypoxia tracer uptake as evidenced by the third 18F-FMISO PET/CT scan. Only 2 of the patients exhibited apparent residual hypoxia in the mid-treatment as identified by positive 18F-FMISO tumor uptake. Neither of these patients had treatment failure, but 1 did develop osteoradionecrosis. Two of the patients with positive baseline 18F-FMISO findings but negative mid-treatment PET scans were later found to have a second primary (hepatocellular and renal cell carcinoma). Table 5 records the information for all patients for all PET scans, along with the disease status.
Table 5.
Results of 18F-FMISO scans 1–3 and disease status
| Pt. No. |
FMISO 1 | FMISO 2 | FMISO 3 | Disease status |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finding | Tumor | Intensity | Nodal | Intensity | Finding | Tumor | Intensity | Nodal | Intensity | Finding | Intensity | ||
| 1 | P | Primary | None | Neck | 3 | P | Primary | None | Neck | 3 | N | NA | NED |
| 2 | N | NA | NA | NA | NA | N | NA | NA | NA | NA | N | NA | NED |
| 3 | P | Primary | 3 | Neck | None | P | Primary | 3 | Neck | None | N | NA | NED |
| 4 | P | Primary | 2 | Neck | 2 | P | Primary | 2 | Neck | 2 | N | NA | NED |
| 5 | P | Primary | 3 | Neck | 3 | P | Primary | 3 | Neck | 3 | N | NA | NED |
| 6 | P | Primary | 3 | Neck | 3 | P | Primary | 3 | Neck | 3 | N | NA | NED |
| 7 | P | Primary | 3 | Neck | 3 | P | Primary | 3 | Neck | 3 | N | NA | NED |
| 8 | P | Primary | 2 | Neck | 2 | P | Primary | 2 | Neck | 2 | N | NA | NED |
| 9 | P | Primary | None | Neck | 3 | P | Primary | None | Neck | 3 | N | NA | NED |
| 10 | P | Primary | None | Neck | 2 | P | Primary | None | Neck | 2 | N | NA | NED |
| 11 | P | Primary | 3 | Neck | 3 | P | Primary | None | Neck | 3 | P | Neck | NED |
| 12 | P | Primary | 3 | Neck | 3 | NA | NA | NA | NA | NA | NA | NA | NED |
| 13 | P | Primary | 3 | Neck | 3 | P | Primary | 3 | Neck | 3 | N | NA | NR+M |
| 14 | P | Primary | None | Neck | 2 | P | Primary | None | Neck | 2 | N | NA | NED |
| 15 | P | Primary | None | Neck | 2 | P | Primary | None | Neck | 2 | N | NA | NED |
| 16 | P | Primary | None | Neck | 2 | P | Primary | None | Neck | 2 | P | Neck | NED |
| 17 | P | Primary | None | Neck | 2 | P | Primary | None | Neck | 2 | NA | NA | NED |
| 18 | N | NA | NA | NA | NA | N | NA | NA | NA | NA | N | NA | NED |
| 19 | P | Primary/neck | 2 | Primary/neck | 2 | P | Primary | 2 | Neck | 2 | N | NA | NED |
| 20 | P | Primary | 3 | Primary | 3 | P | Primary | 3 | Neck | 3 | N | NA | NED |
Abbreviations: 18F-FMISO = [18F]-misonidazole; Pt. No. = patient number; P = positive (hypoxia identified); N = negative (no hypoxia identified); NA = not applicable (no FMISO scan performed); NED = no evidence of disease; NR+M = nodal recurrence plus metastases.
Local, regional, and distant failure
None of the patients experienced local failure. The 3-year local progression-free rate was 100%. One patient experienced regional failure 5 months after treatment completion. The 3-year regional progression-free rate was 95%. The same patient who experienced neck recurrence subsequently developed distant metastases, and the 3-year distant metastases-free rate was 95%. Two patients ultimately died, one of his disease and the other of hepatocellular carcinoma after having control of his head-and-neck cancer. The 3-year overall survival rate was 90%. The 1 patient who did experience failure both regionally and distantly was not 1 of the patients with positive 18F-FMISO uptake on their third PET scan.
DISCUSSION
Improving cure and quality of life are important goals in the management of head-and-neck cancer. RT has been shown to result in decreased complications compared with surgery without compromising overall cure (31). The addition of chemotherapy to RT has further improved patient outcomes, as evidenced by recent meta-analyses, with the largest benefits seen with platinum-based chemotherapy (32, 33). In addition, improvement of quality of life has been observed with the refinement of radiation techniques such as IMRT (34–39).
Hypoxia portending a more aggressive phenotype has been well recognized for various tumors (7, 8). Some have shown that hypoxia significantly decreased locoregional control, compromising both disease-free and overall survival. A retrospective multi-institutional study showed tumors with a greater percentage of partial pressure of oxygen values of <2.5 mm Hg was associated with worse survival, with no patients alive at 5 years for the most hypoxic tumors (7). Although it is not questioned that hypoxia results in worse outcomes, it is hard to compare the patient outcomes across series because different treatment approaches were used. Some series treated patients with RT alone and some with combination therapy. Single-institution preferences in choosing one chemotherapy agent over another and the different radiation techniques used can all affect the treatment outcome. It is possible that with certain combinations of chemotherapy and RT, improved treatment outcomes can be observed despite the presence of hypoxia.
The use of tirapazamine in combination with RT with or without chemotherapy has shown great promise in targeting hypoxia, although no single agent activity has been observed in Phase I trials (40). Recent publications have shown great results with concurrent tirapazamine and RT with or without chemotherapy (14, 15, 41–43). One study showed that >90% of the patients had evidence of tumor hypoxia on 18F-FMISO PET (14). That study also reported that >90% of the patients with initial positive 18F-MISO PET findings showed complete resolution of any abnormality within 4–5 weeks of treatment. The investigators concluded that the rapid normalization of the hypoxia tracer 18F-FMISO suggested successful treatment of the hypoxic component (14). Subsequent trials, including one focusing on hypoxia imaging from the same center, have demonstrated that the identification of hypoxia on 18F-FMISO PET scans in patients receiving a non–tirapazamine-containing regimen was associated with a greater risk of locoregional failure (15). The results of the present study have confirmed these findings. We have also shown that most of our patients (18 of 20 patients) despite being treated with different chemotherapy and RT regimens had resolution of the hypoxia tracer at 4–5 weeks into treatment.
On the basis of the results of these Phase II studies, 2 prospective multi-institutional Phase III randomized trials were initiated. The results of the larger randomized trial known as the HEADSTART trial, which included 860 locoregionally advanced head-and-neck cancer patients, were recently presented at the 2008 American Society of Clinical Oncology. No significant difference in overall survival, failure-free survival, or locoregional failure was observed between the standard cisplatin and RT arm vs. the tirapazamine, cisplatin, and RT arm (16). Because of an excess of early deaths in the tirapazamine arm, the second randomized trial, known as the TRACE trial, was closed early. This was an unexpected finding, because no difference was found in the incidence of early deaths or major toxicity between the two arms in the HEADSTART trial.
Because of our center’s own interest in hypoxia research, we also conducted a prospective trial incorporating pre-/mid-treatment hypoxia imaging for head-and-neck cancer. The patients (90% with oropharyngeal carcinoma) were treated with two to three cycles of platinum-based chemotherapy plus IMRT. Of the 20 patients, 18 (90%) had evidence of pretreatment hypoxia on 18F-FMISO PET/CT. Our study was unique in that the patients underwent two pretreatment 18F-FMISO PET/CT scans, done 3 days apart, before the initiation of therapy to verify the presence of detectable hypoxia using two independent pretreatment scans (30). Of the 20 patients, only 1 had persistent disease in the neck at adjuvant neck dissection. This patient ultimately died of distant metastases. Another interesting finding was that this patient did not have residual detectable hypoxia on his mid-treatment PET scan. The overall 3-year local control, regional control, and distant metastasis-free rate was 100%, 95%, and 95%, respectively.
Several reasons can account for our excellent results in patients with hypoxic tumors despite the lack of treatment with hypoxia-targeting gents. First, the third 18F-FMISO PET scan indicated resolution of tumor hypoxia by mid-treatment. This is consistent with the concept of reoxygenation during fractionated RT, which is expected after ~40 Gy of external beam RT (44). The possibility of efficient reoxygenation in these patients may have diminished the effect of hypoxia resistance on the outcome of a multifraction regimen. It is unclear why 2 of the 20 patients exhibited persistent 18F-FMISOuptake, suggestive of residual tumor hypoxia, so late in the treatment regimen; however, the results of their scans did not translate to a poor treatment outcome for these individuals.
Second, some evidence has shown that cisplatin is more toxic to hypoxic vs. aerated cells (45). Although not as powerful as the nitroimidazoles, evidence has shown that cisplatin can act as a hypoxia cell radiosensitizer (44, 46–50). In addition, platinum-based chemotherapy is also able to enhance the RT effects by possibly reducing the tumor volume, which can lead to increased oxygenation and, hence, improved local control (44). It is also possible that this combination of high-dose platinum-based chemoradiotherapy contributed to our excellent results. The treatment is similar to the control arm of the HEADSTART trial, in which no differences in overall survival, failure-free survival, or locoregional failure were observed when patients were stratified by treatment with or without tirapazamine.
Third, per the study by Rischin et al. (41), patients treated with the tirapazamine regimen were also treated with three cycles of greater cisplatin doses (75 mg/m2) compared with the non-tirapazamine regimen (two cycles of cisplatin 50 mg/m2 plus 5-fluorouracil 360 mg/m2/d) (41). It is possible the greater chemotherapy doses used in the tirapazamine regimen contributed to the superior results. The investigators stated that although the result were promising, as evidenced by the rapid normalization of 18F-FMISO PET at mid-treatment, it was difficult to determine the specific contribution of tirapazamine, because no studies have been reported of serial 18F-FMISO PET scans in head-and-neck cancer patients treated by RT with or without chemotherapy in the absence of tirapazamine. Our results showed that rapid normalization had occurred by the mid-treatment FMISO PET scans in head-and-neck cancer patients treated with concurrent chemoradiotherapy without tirapazamine, although 90% of our patient population had oropharyngeal carcinoma compared with 60% in the study by Rischen et al. (41).
Finally, human papillomavirus (HPV) can play a significant role in the pathogenesis of head-and-neck squamous cell carcinoma. HPV-positive patients are clinically and molecularly distinct from HPV-negative patients, with HPV-positive patients having improved treatment outcomes (51–57). Oropharyngeal tumors are more likely to be HPV-positive, and, in our cohort, 90% of the tumors were of oropharynx origin. Although we did not routinely stain for HPV status, because the protocol was initiated before the knowledge of the role HPV plays, ~60% of the patients had a history of smoking. Nonetheless, it is possible that some of these patients were HPV-positive, because 90% of our patient population presented with oropharyngeal carcinoma, resulting in an excellent treatment response to chemoradiotherapy despite evidence of hypoxia on the pretreatment 18F-FMISO PET/CT scans.
CONCLUSION
The results of this clinical study have suggested that high-dose platinum-based chemotherapy concurrent with definitive IMRT can effectively treat hypoxia in a cohort of locoregionally advanced head-and-neck cancer patients. This information has provided important data to suggest that agents specifically targeting hypoxia might not be necessary, particularly for those with oropharyngeal carcinoma. These data are consistent with the findings from the recently reported Phase III HEADSTART trial in which tirapazamine was not found to add a significant benefit to patients’ overall survival, failure-free survival, or locoregional control. On the basis of the results from this pilot study, a larger hypoxia imaging study with more patients and a slightly different design is ongoing at our institution.
Acknowledgments
Supported by the American Society for Therapeutic Radiation Oncology Junior Investigator Award.
Footnotes
Note—An online CME test for this article can be taken at http://asro.astro.org under Continuing Education.
Conflict of interest: none.
REFERENCES
- 1.Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 2.Bussink J, Kaanders JH, van der Kogel AJ. Tumor hypoxia at the micro-regional level: Clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol. 2003;67:3–15. doi: 10.1016/s0167-8140(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Knoop C, Schlenger K, et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Hansgen G, Bloching M, et al. Oxygenation of squamous cell carcinoma of the head and neck: comparison of primary tumors, neck node metastases, and normal tissue. Int J Radiat Oncol Biol Phys. 1998;42:35–41. doi: 10.1016/s0360-3016(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 5.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 6.Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: Changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 7.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy: An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 9.Isa AY, Ward TH, West CM, et al. Hypoxia in head and neck cancer. Br J Radiol. 2006;79:791–798. doi: 10.1259/bjr/17904358. [DOI] [PubMed] [Google Scholar]
- 10.Corry J, Rischin D. Strategies to overcome accelerated repopulation and hypoxia—What have we learned from clinical trials? Semin Oncol. 2004;31:802–808. doi: 10.1053/j.seminoncol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Brown JM, Siim BG. Hypoxia-specific cytotoxins in cancer therapy. Semin Radiat Oncol. 1996;6:22–36. doi: 10.1053/SRAO0060022. [DOI] [PubMed] [Google Scholar]
- 12.Dorie MJ, Brown JM. Tumor-specific, schedule-dependent interaction between tirapazamine (SR 4233) and cisplatin. Cancer Res. 1993;53:4633–4636. [PubMed] [Google Scholar]
- 13.Dorie MJ, Menke D, Brown JM. Comparison of the enhancement of tumor responses to fractionated irradiation by SR 4233 (tirapazamine) and by nicotinamide with carbogen. Int J Radiat Oncol Biol Phys. 1994;28:145–150. doi: 10.1016/0360-3016(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 14.Rischin D, Peters L, Hicks R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:535–542. doi: 10.1200/JCO.2001.19.2.535. [DOI] [PubMed] [Google Scholar]
- 15.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 16.Rischin D, Peters L, O’Sullivan B, et al. Phase III study of tirapazamine, cisplatin and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck [Abstract] J Clin Oncol. 2008:26. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 17.Rischin D, Fisher R, Peters L, et al. Hypoxia in head and neck cancer: Studies with hypoxic positron emission tomography imaging and hypoxic cytotoxins. Int J Radiat Oncol Biol Phys. 2007;69:S61–S63. doi: 10.1016/j.ijrobp.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 18.Brizel DM, Rosner GL, Harrelson J, et al. Pretreatment oxygenation profiles of human soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1994;30:635–642. doi: 10.1016/0360-3016(92)90950-m. [DOI] [PubMed] [Google Scholar]
- 19.Nordsmark M. Direct measurements of tumor-tissue pO2: A way of selecting patients for hyperoxic treatment. Strahlenther Onkol. 1996;172 Suppl. 2:8–9. [PubMed] [Google Scholar]
- 20.Apisarnthanarax S, Chao KS. Current imaging paradigms in radiation oncology. Radiat Res. 2005;163:1–25. doi: 10.1667/rr3279. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen L, Keiding S, Nordsmark M, et al. Tumour oxygenation assessed by 18F-fluoromisonidazole PET and polaro-graphic needle electrodes in human soft tissue tumours. Radiother Oncol. 2003;67:339–344. doi: 10.1016/s0167-8140(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 22.Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–428. doi: 10.1016/s0360-3016(96)00325-2. [DOI] [PubMed] [Google Scholar]
- 23.Hicks RJ, Rischin D, Fisher R, et al. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur J Nucl Med Mol Imaging. 2005;32:1384–1391. doi: 10.1007/s00259-005-1880-2. [DOI] [PubMed] [Google Scholar]
- 24.Rajendran JG, Schwartz DL, O’Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donoghue JA, Zanzonico P, Pugachev A, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys. 2005;61:1493–1502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 26.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med. 2007;37:451–461. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: A feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nehmeh SA, Lee NY, Schroder H, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–242. doi: 10.1016/j.ijrobp.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamoto Y, Osman M, Cohade C, et al. PET/CT: Comparison of quantitative tracer uptake between germanium and CT transmission attenuation-corrected images. J Nucl Med. 2002;43:1137–1143. [PubMed] [Google Scholar]
- 30.Nehmeh S, Hossam EZ, Yusuf E, et al. An iterative technique for lesion segmentation in PET images. J Nucl Med. 2006;47:364P. [Google Scholar]
- 31.Soo KC, Tan EH, Wee J, et al. Surgery and adjuvant radiotherapy vs concurrent chemoradiotherapy in stage III/IV nonmetastatic squamous cell head and neck cancer: A randomised comparison. Br J Cancer. 2005;93:279–286. doi: 10.1038/sj.bjc.6602696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pignon JP, Bourhis J, Domenge C, et al. for the Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC) Collaborative Group. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 33.Pignon JP, le Maitre A, Bourhis J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An update. Int J Radiat Oncol Biol Phys. 2007;69:S112–S114. doi: 10.1016/j.ijrobp.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 34.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: The Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64:363–373. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Clark CH, Miles EA, Bidmead AM, et al. In regard to Lee, et al., Int J Radiat Oncol Bio Phys 2002;53:630–637. Int J Radiat Oncol Biol Phys. 2003;55:1150–1151. doi: 10.1016/s0360-3016(02)04481-4. [DOI] [PubMed] [Google Scholar]
- 36.Barker JL, Jr, Glisson BS, Garden AS, et al. Management of nonsinonasal neuroendocrine carcinomas of the head and neck. Head Neck. 2003;98:2322–2328. doi: 10.1002/cncr.11795. [DOI] [PubMed] [Google Scholar]
- 37.Alber M, Paulsen F, Eschmann SM, et al. On biologically conformal boost dose optimization. Phys Med Biol. 2003;48:N31–N35. doi: 10.1088/0031-9155/48/2/404. [DOI] [PubMed] [Google Scholar]
- 38.Yao M, Karnell LH, Funk GF, et al. Health-related quality-of-life outcomes following IMRT versus conventional radiotherapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:1354–1360. doi: 10.1016/j.ijrobp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:966–974. doi: 10.1016/j.ijrobp.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 40.Senan S, Rampling R, Graham MA, et al. Phase I and pharmacokinetic study of tirapazamine (SR 4233) administered every three weeks. Clin Cancer Res. 1997;3:31–38. [PubMed] [Google Scholar]
- 41.Rischin D, Peters L, Fisher R, et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: A randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02) J Clin Oncol. 2005;23:79–87. doi: 10.1200/JCO.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 42.von Pawel J, von Roemeling R, Gatzemeier U, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group—Cisplatin and tirapazamine in subjects with advanced previously untreated non-small-cell lung tumors. J Clin Oncol. 2000;18:1351–1359. doi: 10.1200/JCO.2000.18.6.1351. [DOI] [PubMed] [Google Scholar]
- 43.Lee DJ, Trotti A, Spencer S, et al. Concurrent tirapazamine and radiotherapy for advanced head and neck carcinomas: A phase II study. Int J Radiat Oncol Biol Phys. 1998;42:811–815. doi: 10.1016/s0360-3016(98)00310-1. [DOI] [PubMed] [Google Scholar]
- 44.Hall E. Radiobiology for the radiologist. 4th ed. Philadelphia: JB Lippincott; 1994. [Google Scholar]
- 45.Richmond RC, Zimbrick JD, Hykes DL. Radiation-induced DNA damage and lethality in E. coli as modified by the antitumor agent cis-dichlorodiammineplatinum (II) Radiat Res. 1977;71:447–460. [PubMed] [Google Scholar]
- 46.Korbelik M, Skov KA. Inactivation of hypoxic cells by cisplatin and radiation at clinically relevant doses. Radiat Res. 1989;119:145–156. [PubMed] [Google Scholar]
- 47.Skov K, MacPhail S. Interaction of platinum drugs with clinically relevant x-ray doses in mammalian cells: A comparison of cisplatin, carboplatin, iproplatin, and tetraplatin. Int J Radiat Oncol Biol Phys. 1991;20:221–225. doi: 10.1016/0360-3016(91)90094-k. [DOI] [PubMed] [Google Scholar]
- 48.Yan RD, Durand RE. The response of hypoxic cells in SCCVII murine tumors to treatment with cisplatin and x rays. Int J Radiat Oncol Biol Phys. 1991;20:271–274. doi: 10.1016/0360-3016(91)90103-b. [DOI] [PubMed] [Google Scholar]
- 49.Skov KA, MacPhail HS, Marples B. The effect of radiosensitizers on the survival response of hypoxic mammalian cells: The low x-ray dose region, hypersensitivity and induced radio-resistance. Radiat Res. 1994;138:S113–S116. [PubMed] [Google Scholar]
- 50.Douple EB, Richmond RC. Platinum complexes as radiosensitizers of hypoxic mammalian cells. Br J Cancer Suppl. 1978;3:98–102. [PMC free article] [PubMed] [Google Scholar]
- 51.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 53.Gillison ML, Koch WM, Shah KV. Human papillomavirus in head and neck squamous cell carcinoma: Are some head and neck cancers a sexually transmitted disease? Curr Opin Oncol. 1999;11:191–199. doi: 10.1097/00001622-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363:1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 55.Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: Mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol. 2001;13:183–188. doi: 10.1097/00001622-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]