Abstract
Respiratory infections are associated with wheezing illnesses in all ages and may also impact the development and severity of asthma. Respiratory tract infections caused by viruses, Chlamydophila or Mycoplasma have been hypothesized to have significant roles in the pathogenesis of asthma. Progress is being made toward establishing the mechanisms by which these agents can cause acute wheezing and impact the pathophysiology of asthma. Host factors probably contribute to the risk of asthma inception and exacerbation, and these contributions may also vary with respect to early- versus adult-onset disease. This review discusses these various associations as they pertain to the development and exacerbation of asthma.
Keywords: asthma, asthma inception, exacerbation, respiratory infection, virus
Respiratory infections are associated with wheezing illnesses at all ages and may also impact the development and severity of asthma. Respiratory tract infections caused by viruses [1–3], Chlamydophila [4–7] or Mycoplasma [5,8–10] have been hypothesized to have significant roles in the pathogenesis of asthma. Of these respiratory pathogens, viruses have been shown to be epidemiologically associated with asthma in several ways. First, particular viruses associated with infantile wheezing have been theorized to lead to the inception of the asthmatic phenotype [11,12]. Second, children who experience severe viral respiratory infections in early life are more likely to have asthma later in childhood [3,11,13]. Furthermore, in children and adults with established asthma, viral upper respiratory tract infections (URIs) play a key role in producing acute exacerbations that may lead to healthcare utilization [14–16]. Several host factors, such as allergic sensitization [14,17] and virus-induced interferon responses [18–20], modify the risk of virus-induced wheezing. For infections with other microbes, interest has focused on Chlamydophila and Mycoplasma as possible contributors to both acute exacerbations and the severity of chronic asthma [5,7]. Finally, colonization of the upper airways in infancy with common bacterial pathogens has been demonstrated to increase the risk of subsequent asthma [21]. We review these various associations as they pertain to the development and exacerbation of asthma.
Epidemiology
Incidence, noninfectious risk factors & remission: are childhood & adult-onset asthma the same disease?
Several studies have documented that the incidence of asthma peaks in early childhood with rates as high as five new cases per 1000 population per year, more commonly in boys [22–24]. Incidence declines in adolescence and then climbs again in early adulthood, with a reversal of gender predilection favoring women after the onset of puberty [22–25]. Whereas eczema and a family history of asthma are the dominant noninfectious risk factors for pediatric asthma, the triggers of adult-onset disease are less well defined [26–28]. Occupational exposures and a personal history of smoking contribute in a minority of cases, whereas either allergic or nonatopic rhinitis has been associated with adult onset disease in multiple studies [29–32]. With respect to remission rates, there is up to a 63% chance of remission of asthma in patients who develop disease before 10 years of age, but only 5–15% for adult-onset disease [24,33]. This is consistent with the notion that pediatric and adult asthma may have some important clinical differences, with the latter having a higher incidence of steroid resistance and more complicated management [34,35]. Nonetheless, respiratory infection is the most common acute inflammatory trigger in incident asthma [36].
Relationship of virus-induced wheezing in early life to childhood asthma
Two of the most common viral illnesses leading to lower respiratory tract infection (LRI) and wheezing in infancy are those caused by respiratory syncytial virus (RSV) and human rhinoviruses (HRVs). Using multiple virus detection methods, Jartti and colleagues determined the etiology of wheezing illness in 293 hospitalized children [37]. Out of the 76 infants with virus detected, 54% had RSV, 42% had picornavirus (HRV and enterovirus) and 1% had human metapneumovirus (hMPV). From 1980 to 1996, the rates of hospitalization of infants with bronchiolitis increased substantially [38] and RSV was the etiology in approximately 70% of these episodes. In older children, respiratory picornaviruses dominated (65% of children aged 1–2 years and 82% of children aged ≥3 years) [37].
However, bronchiolitis is a severe form of RSV infection that occurs in a minority of children. By 1 year of age, 50–65% of children will have been infected with this virus, and by 2 years of age nearly 100% have been infected [39]. Children 4 months of age and born close to the onset of the viral season have a higher likelihood of developing lower respiratory tract symptoms [40,41], and this is likely due to an airway, lung parenchyma and/or immunologic maturation [39]. Furthermore, a child born 4 months before the winter virus peak predicts an increased risk of childhood asthma [40]. Additional risk factors for bronchiolitis include antiviral immune responses (both innate [42] and adaptive [43]), gender, lung size and passive smoke exposure [13].
Several large, prospective studies of children have demonstrated that RSV bronchiolitis is an important risk factor for recurrent wheezing and asthma during the first decade of life [12,44]. A longitudinal, population-based cohort study has shown that the association between RSV LRIs and both frequent (> three episodes) and infrequent (< three episodes) wheezing diminish markedly with age and becomes nonsignificant by 13 years of age, whereas the early RSV negative, other infectious agent group continued to confer risk of frequent wheezing [12]. This decreased association between wheezing and RSV LRI with increasing age has also been observed by other investigators [45,46]. These data suggest that although RSV infections increase the risk of recurrent wheezing and asthma in early childhood, other genetic, environmental and developmental factors modify the expression of the asthma phenotype over time.
Although bronchiolitis during infancy is associated with an approximately twofold increased risk of early childhood asthma, this risk differs by season of bronchiolitis. Bronchiolitis occurring during HRV-predominant months (Spring and Fall) was associated with an estimated 25% increased likelihood of early childhood asthma compared with RSV-predominant (Winter) months. However, the proportion of associated asthma after winter season bronchiolitis is greater than HRV-predominant months owing to the larger numbers of children affected by RSV bronchiolitis [47]. Similarly, it has been shown that 16.6% of children with non-RSV bronchiolitis develop recurrent wheezing, compared with 2.5% of children with RSV bronchiolitis [48]. Another study by Kusel and coworkers demonstrated that, although RSV was associated with more severe LRI requiring hospitalization, HRV was associated with more than three-times the number of both wheezing and nonwheezing LRI in infancy [3]. In addition, these authors found that acute severe LRI caused by HRV or RSV in the first year of life were strongly associated with the diagnosis of current asthma and persistent wheeze in 5-year-old children, particularly in those who are sensitized during infancy. Jackson and coworkers also demonstrated that in a large, high-risk cohort, children had an increased risk of asthma at 6 years of age if they wheezed in the first 3 years of life with RSV (odds ratio [OR]: 2.6), HRV (OR: 9.8), or both HRV and RSV (OR: 10) [11]. By 3 years of age, wheezing with HRV (OR: 25.6) was more strongly associated with asthma at 6 years of age than aeroallergen sensitization (OR: 3.4). Similarly, this strong association of early-life HRV wheezing and increased risk of recurrent wheeze [49,50] and asthma [51] has been demonstrated in several studies. These findings support the concept that HRV is an important cause of bronchiolitis and is strongly associated with the risk of asthma development.
Host factors such as allergic sensitization or decreased lung function in infancy may also influence the development of recurrent wheeze and/or asthma. Premorbid measurements of lung function indicate that children with reduced levels of lung function in infancy appear to be at an increased risk of the development of chronic lower respiratory tract sequelae after viral infections [52] and an obstructive pattern of lung function into adulthood [53]. Whether reduced lung function alone is responsible for these developments is presently unknown. Children with early allergic sensitization (<2 years of age) were more likely to be diagnosed with current wheeze or asthma if they had an LRI with RSV or HRV in infancy [3]. Thus, viral infections act synergistically with allergic sensitization and reduced lung function in infancy, leading to asthma in later life.
Viral respiratory infections & acute exacerbations of asthma
The association between viral infections and asthma exacerbations has been illuminated by the development of sensitive diagnostic tests based on PCR and/or microarray technology, for viruses that are difficult to culture, such as HRV, hMPV and bocaviruses. With the advent of these more sensitive diagnostic tools, information linking common cold infections with exacerbations of asthma has come from a number of sources. Prospective studies of subjects with asthma have demonstrated that up to 85–95% of exacerbations of wheezing or asthma in children are caused by viral infection [16,54]. This rate is as high as 60% for adults with seasonal trends that occur 1–2 weeks later than in children, suggesting household transmission of the same strain [15,55–57]. HRVs are most often detected, especially during the Spring and Fall seasons. Indeed, the Spring and Fall peaks in asthma hospitalizations correlate with patterns of HRV detection within the community [15]. HRV infections are frequently found in children older than 2 years of age who present to emergency departments with acute wheezing [14] and in children hospitalized for acute asthma [58]. A newly discovered HRV species, HRV-C, is associated with asthma flares in children during the Fall and Winter [59–61], and new strains of HRV and coronaviruses have been identified by the Virochip method in adults [62]. RSV is more likely to trigger acute asthma symptoms in the wintertime but likely account for a smaller proportion of overall asthma exacerbations. Influenza infections are also associated with increased healthcare utilization among children with asthma compared with healthy children [63]. Bocaviruses have been associated with up to 19% of acute wheezing episodes in children [54]. Other viruses that are less frequently associated with exacerbations of asthma include metapneumovirus and coronaviruses [64]. Together, these studies provide evidence of a strong relationship between viral infections, particularly those due to HRV, and acute exacerbations of asthma.
Chronic infections & asthma development
It has been hypothesized that chronic viral and bacterial infections or colonization with pathogenic bacteria could lead to chronic lower airway inflammation, impaired mucociliary clearance, increased mucous production and eventually asthma [21,65]. Organisms implicated in this progression include adenoviruses [66], Chlamydophila pneumoniae [4,5,7], Mycoplasma pneumoniae [8–10], Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis [21]. With respect to the former, latent adenoviral infection can persist in the airway for many years [67], where a latent infection is defined by when a virus inserts part of the virus genome into the host cell DNA and continues to periodically express viral genes. It has been demonstrated that 94% of children with steroid-resistant asthma had detectable adenovirus antigens compared with 0% of controls [68]. In adults, both with and without asthma, evidence of adenoviral infection has been observed to be as high as 50% of the individuals tested [65]. There is more literature regarding the association between chronic Mycobacterium or Chlamydophila infection and asthma in children and adults; however, these studies have produced contradictory results, probably due to the limitations of current diagnostics. Findings of diagnostic tests in the upper and lower airways do not always agree, and the diagnosis of infection by serology can be imprecise.
Previously, the first relationship between asthma and C. pneumoniae was described in 1991 in 19 wheezing adult asthmatic patients, of whom nine were found to have serologic evidence of current or recent infection with this pathogen [6]. In school-age children with wheezing, a surprisingly high prevalence of low-grade C. pneumoniae infection in nasal aspirates has been observed [69]. The detection of C. pneumoniae infection by PCR and serology (secretory IgA) was similar during symptomatic and asymptomatic episodes (23 vs 28%, respectively). Children who experienced multiple episodes also tended to remain PCR positive for C. pneumoniae, suggestive of chronic infection. Moreover, C. pneumoniae-specific secretory IgA antibody levels were more than seven-times higher in subjects who reported four or more exacerbations in the study compared with those who reported just one. In a study of 70 pediatric patients by flexible fiber optic bronchoscopy, 40% of PCR C. pneumoniae-positive samples were from patients with asthma. Culture of the blood samples revealed that a significantly higher proportion of asthma subjects (34.3%) were positive for Chlamydia compared with matched nonrespiratory control subjects (11%) [4]. It is possible that chronic Chlamydophila infection promotes persistent airway inflammation that increases susceptibility to other stimuli such as viruses, allergens, or both associated with asthma exacerbations.
A comprehensive evaluation of the role of both Chlamydophila and Mycoplasma infections in chronic asthma was reported by Johnston et al. [70]. The authors concluded that although many studies investigating the association between asthma and these pathogens have been uncontrolled and have provided conflicting evidence, there are biological mechanisms that could account for such a link, and that there may be a role of antibacterial therapy in the management of asthma. Martin et al. evaluated 55 adult patients with chronic asthma (percent predicted forced expiratory volume in 1 s [FEV1] = 69.3 [2.1%]), and 11 controls for infection with Mycoplasma, C. pneumoniae and viruses (influenza A and B, parainfluenza 1, 2 and 3, RSV, HRV and adenovirus) were evaluated [65]. Bronchoalveolar lavage cell count and differential as well as tissue morphometry were also performed. Of the asthmatic patients, 56% had a positive PCR for M. pneumoniae (n = 25) or C. pneumoniae (n = 7), which was mainly found in lavage fluid or biopsy samples. Only one of 11 control subjects had a positive PCR for Mycoplasma. A significantly greater number of tissue mast cells were observed in the group of patients who were positive on PCR compared with those that were negative. Cultures for both organisms were negative in all patients, and serologic confirmation had poor correlation with PCR results. In another study by Biscardi et al., 119 children aged 2–15 years with asthma hospitalized for a severe asthma exacerbation were tested for acute infection due to M. pneumoniae or C. pneumoniae by serologic testing [9]. Nasopharyngeal aspirate PCR was also performed. Acute M. pneumoniae infection by positive serology was found in 20% and C. pneumoniae infection was found in 3.4% of the patients during the current exacerbation. Out of 51 patients experiencing their first asthma flare, acute M. pneumoniae infection was demonstrated in 50% and C. pneumoniae in 8.3% of the patients. Out of the children infected with M. pneumoniae or C. pneumonia and experiencing their first asthma exacerbation, 62% had recurrent asthma but only 27% without these infections had recurrent asthma [9]. Similar to the previous study, serologic confirmation correlated poorly with PCR results. Thus, chronic Chlamydophila infection may promote ongoing airway inflammation.
Some investigators have theorized as a mechanism that the development of C. pneumoniae-specific IgE antibody facilitates the release of mediators that cause bronchospasm, airway inflammation and airway reactivity [71]. Unless infection is treated, antigenic stimulation promoting specific IgE production would continue, thus producing the prolonged course of asthma in some patients that is unresponsive to bronchodilators and steroids [71]. The major C. pneumoniae antigen, heat-shock protein 60 (cHSP60), has been implicated in the stimulation of deleterious immune responses in human chlamydial infections. It has been observed to colocalize with infiltrating macrophages in atheromatous lesions. cHSP60 was reported to be a powerful inducer of macrophage inflammatory responses mediated through the innate immune receptor complex Toll-like receptor (TLR)4–MD2 [72]. Taken together, these findings imply that chronic asymptomatic chlamydial infections may affect persistent airway inflammatory responses through both innate and adaptive immune responses.
As stated previously, M. pneumoniae has also been associated with both acute and chronic asthma. Again, the results of trials have been contradictory and investigators have not been able to firmly establish a causal relationship between mycoplasmal infections and asthma exacerbations. Although some have reported infection in up to 25% of children with wheezing [73], others have not been able to confirm these observations [69]. Moreover, when the same group of children was evaluated for the relative contributions of mycoplasmal (and chlamydial) infections to acute exacerbations, viruses were a far more common etiology [12,69]. It is possible that this association may become more established as more sensitive and specific serologic diagnostic tests become available and/or culture techniques improve.
Unlike the inconsistent relationship between Mycoplasma species and acute asthma exacerbations, associations of this pathogen with chronic asthma have been more firmly established. As previously mentioned, using PCR techniques on bronchial biopsy specimens, Mycoplasma species have been detected in 25 out of 55 adult asthmatic subjects and in only one out of 11 controls [40, 65]. Case reports of chronic asthma commencing with M. pneumoniae infection suggest that this pathogen is a potentially causative agent in some patients [74]. Potential mechanisms of Mycoplasma-induced airway inflammation have been explored, including augmented Th2 responses and inflammatory neuropeptides. Children with acute M. pneumonia have an elevated IL-4/IFN-γ ratio compared with children with pneumococcal pneumonia or controls [75], and mice experimentally infected with M. pneumoniae develop airway hyperresponsiveness (AHR), which is associated with the diminished production of mRNA for IFN-γ [76]. Last, asthmatic patients with M. pneumoniae infection detected by PCR have increased levels of neurokinin-1, which decreases in response to treatment with a macrolide antibiotic [77].
The microbiome of the lower airway has not been thoroughly evaluated to date, but there is a growing sense that it is not a sterile compartment, and that its constituents may differ between healthy persons and patients with asthma. A recent study by Bisgaard and coworkers found that neonates colonized in the hypopharyngeal region with S. pneumoniae, H. influenzae or M. catarrhalis, or with a combination of these organisms, are at increased risk for recurrent, early-life wheezing and asthma at 5 years of age [21]. Although these preliminary studies are intriguing, additional data are needed to establish causality to asthma pathogenesis and to define the mechanisms contributing to these associations in both adult and pediatric patients. Another possible mechanism for these associations is that a person with immune function that is biased towards atopy may have both altered host defenses that increase susceptibility to bacterial and viral infections and an increased risk of developing asthma [3,78–82]. Collectively, these studies regarding chronic bacterial infection/colonization have formed the basis for randomized, placebo-controlled, double-blind clinical trials of prolonged courses of macrolide antibiotics on acute and chronic asthma control. The effect of these drugs has unfortunately been variable depending on the population [83–86].
Mechanisms of virus-induced wheezing & asthma
Several mechanisms have been proposed to elucidate how respiratory viruses lead to wheezing illnesses and exacerbations of asthma. First, viral infections injure airway epithelial cells and can initiate airway edema and leakage of serum proteins into the airway. Along with shedding of infected cells into the airway, the effects can lead to obstruction and wheezing. Furthermore, virus-induced immune responses are essential to clear the viral infection, but may also contribute to symptoms and airway hyperactivity by causing an influx of inflammatory cells that negatively impact lower airway physiology. Respiratory viruses can augment airway inflammation by directly infecting lower airway tissues, or perhaps by infecting the upper airway and then initiating a systemic immune response that promotes lower airway inflammation. Environmental factors may act synergistically with viral infections and other known triggers leading to acute exacerbations. Allergic sensitization increases the risk of the development of asthma after virus-induced wheezing episodes in infancy and is strongly related to virus-induced exacerbations of asthma in older children and adults with asthma. For example, effects of viral infection on the severity of an asthma exacerbation may be augmented by exposure to allergens to which an individual is sensitized [17] and by exposure to increased levels of air pollutants [87]. It is also possible that allergic sensitization alone may increase the susceptibility to viral infections such as HRVs [88].
Although a general cohort of adult patients with asthma does not appear to get more URIs compared with healthy cohabitants with similar exposures [57], observational studies in adults and children suggest that those with low vitamin D serum levels have a higher incidence of virus-induced asthma exacerbations [89–93]. The relationship between low vitamin D status and asthma is further supported by a number of observations. First, genetic polymorphisms in vitamin D receptor-dependent pathways are associated with the development of asthma [94–96]. Second, vitamin D deficiency may be related to the seasonal variation in symptomatic respiratory infections [97] even in southern climates where, despite greater potential for cutaneous synthesis, low vitamin D status is common [98–100]. Third, mechanistic studies have shown that vitamin D treatment increases β-defensin production [101,102], alters MHC, CD14 and TLR expression [103–105], and enhances T-regulatory cell suppression [106]. Finally, vitamin D treatment of airway epithelial cells promotes differentiation [107], and in this sense may promote the healing of epithelial barrier defenses, as undifferentiated basal cells are more easily infected [108]. These basic and clinical observations remain to determine whether vitamin D supplementation decreases the rate of infectious illnesses and/or asthma exacerbations.
Several groups have postulated that the airway epithelium regulates the immune response to environmental injury (including those induced by respiratory infection) and that there are key differences in these responses that distinguish healthy controls from patients with asthma and may even contribute to asthma inception [109,110]. Repeated asthma exacerbations damage the airway epithelium and contribute to the loss of lung function [111]. Some studies describe patches of denuded airway in asthmatic patients at baseline with good symptom control, which are not observed in healthy controls, although these differences are not universally found [112–114]. Epithelial experiments in vitro suggest that relative to healthy controls, the asthmatic epithelial cells are slower to differentiate and form tight junctions for barrier defense, and produce numerous inflammatory mediators important for innate immunity to a variety of insults [115,116]. Denuded airways are also associated with vascular leak and cellular inflammation, contributing to worse symptom control [117]. In addition, viral exposure may promote dysfunctional cilia-to-goblet cell transition that depends on both the EGF receptor and exposure to IL-13 [118].
In terms of epithelial repair mechanisms, IL-13 appears to be a key Th2 cytokine that distinguishes asthma patients in a way that may prolong the course toward restoring normal barrier function [119]. Its production in the airway is increased in asthmatics, in part due to well-established genetic variants [120–122]. IL-13 promotes goblet cell formation with hypersecretion of mucin [123], at the relative expense of tight junction-forming ciliated epithelium [124]. It is important in the epithelial cell-initiated recruitment of eosinophils via eotaxin production [125], and there is a general sense that this cytokine can enhance profibrotic repair [126]. From a phenotyping perspective, a genome-wide airway epithelial cell expression array study identified three IL-13-induced genes forming a signature that distinguished asthma patients from healthy controls with the repression of these genes by inhaled corticosteroids (ICS) in a clinical trial [127]. As the presence of IL-13 can also downregulate IFN responses, stimulation of this pathway may contribute to selective susceptibility to prolonged infections in patients with asthma [128].
Much attention has been focused lately on the role of IFNs produced during viral infection. After binding to intercellular adhesion molecule (ICAM)-1 or the low density lipoprotein (LDL) receptor, for major and minor group HRVs respectively, entry and uncoating occurs. IFNs are then rapidly induced by HRV-stimulated signaling by TLR3 via the transcription factor NF-κB, as well as by activating RNA helicases such as retinoic acid-induced gene (RIG)-1 or melanoma differentiation antigen (MDA)-5-induced activation of the transcription factor, interferon regulatory factor 3 [129]. Epithelial cell production of IFN-β in particular is responsible for the stimulation of many of the genes subsequently induced by HRVs [130]. Of significant interest, Johnston and colleagues have shown that the undifferentiated airway epithelial cell production of IFN-β and IFN-γ in response to HRVs discerns healthy subjects from asthmatics [131, 132]; however, these differences were not observed using a genome-wide expression array or candidate gene quantitative PCR [133]. In summary, there is wide recognition that the airway epithelial cell is a key component of innate defense, that asthma-specific defects in epithelial-directed airway repair and infection response may be regulated by IL-13, and that the responses of epithelial cells to HRV infection are important to asthma; however, the mechanisms warrant further study.
The cellular inflammatory response in the upper airway may hold a key determinant to HRV control that is different in asthmatic patients. In a classic longitudinal cohort study, patients with asthma get a similar number of colds as their healthy cohabitants, but those with asthma have an approximately twofold greater risk of a lower airway infection with a greater duration and more intense symptoms [57], suggesting that asthmatics possess an inability to efficiently contain the virus in the initial upper airway inflammatory response. Regarding potential mechanisms, upper airway epithelial cells are more resistant to rhinoviral infection than bronchial cells; however, this occurs for both healthy and asthma subjects [134]. Nasal chemokines have been associated with neutrophilic influx and cold symptoms [135]. Interestingly in subjects with allergic rhinitis, three nasal challenges with allergen sufficient to promote nasal eosinophilia the week before an experimental HRV-16 cold had a delayed onset and shorter duration of cold symptoms, compared with a placebo-primed, HRV-challenged group [136]. Upper airway neutrophil influx during a naturally acquired asthmatic viral cold has been shown to be inversely associated with the subsequent development of lower airway symptoms, although in a multivariate model a novel asthma biomarker, the pore function of the nucleotide receptor, P2X7, was more predictive of loss of control [137]. P2X7 is a leukocyte/epithelial cell trimeric cation channel/pore that amplifies TLR-dependent immune responses in the presence of danger signals such as ATP, present in the airway at higher concentrations during allergic or viral challenges [138–141]. To summarize, the initial epithelial cell response to infection in the upper airway may be modified by several host factors important to asthma pathophysiology, with attenuated microbial clearance contributing to a greater risk of lower airway infection and asthma symptoms.
Viral infections of the lower airway
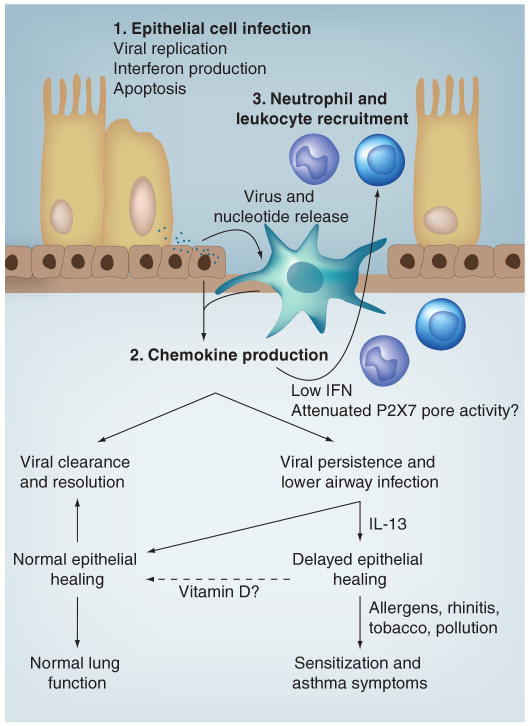
Respiratory syncytial virus and influenza infections are well-recognized causes of bronchitis, bronchiolitis and pneumonia [73,142,143]. Historically, HRV was considered to be solely an upper airway pathogen because of its association with common cold symptoms and the observation that HRV replicates best at 33–35°C, thought consistent only with the temperature of the upper airway. In fact, lower airway temperatures have been recorded using a bronchoscope equipped with a small thermistor [144]. During quiet breathing at room temperature, airway temperatures are favorable to HRV replication down to fourth-generation bronchi, and surpass 35°C only in the periphery of the lung. Furthermore, HRV appears to replicate equally well in cultured epithelial cells derived from either upper or lower airway epithelium [145], although this finding is not universal [134]. Finally, HRV has been observed in lower airway cells and secretions by several techniques after experimental inoculation [146–148]. In support of the experimental infection model data, HRV is frequently found in infants and children with lower respiratory signs and symptoms, including children hospitalized for pneumonia [149]. In infants with recurrent respiratory symptoms, HRV was detected in lower airways by bronchial biopsy in 45%, and the majority of these HRV-positive infants also showed increased airway resistance [150]. Viral clearance may also be affected in asthma, in that two studies with adults under conditions of stable disease have demonstrated evidence of viral pathogens in sputum samples or transbronchial lung biopsies [151,152]. Together, these findings imply that respiratory viruses, including HRV, are likely to promote wheezing illnesses and exacerbations of asthma largely by infecting lower airways and causing or augmenting lower airway inflammation, and that these pathogens may be incompletely cleared in select patients. These features and a proposed model for the epithelial cell-dependent promotion of asthma symptoms are reviewed in Figure 1.
Figure 1. Model of infection-triggered airway epithelial cell injury with variable responses that may contribute to asthma symptoms.
After upper airway infection and viral replication, epithelial cell lysis occurs, releasing virus particles and danger signals (including ATP), which act to stimulate resident antigen-presenting cells such as dendritic cells. Defense mechanisms include the epithelial cell production of interferons and initiation of apoptosis to limit viral productivity. Dendritic cell-derived chemokines initiate the recruitment of granulocytes and lymphocytes to assist with viral clearance. Attenuated IFN production and/or P2X7 pore function are correlated with less upper airway inflammation and a greater risk of acute asthma symptoms. This may result in lower airway infection with subsequent epithelial cell injury. The presence of IL-13 has been shown to delay epithelial tight junction formation, which may render the airway more susceptible to chronic asthmagenic environmental stimuli. Vitamin D has been shown to promote epithelial cell differentiation and has numerous innate immune effects; whether supplementation strategies affect asthma outcomes has not been evaluated.
IFN: Interferon.
Interactions between viral infections & allergy
The effect of allergic sensitization on the asthmatic airway response to viral infection has been a topic of much research. The relationship between these two factors appear to be bidirectional, as the atopic state can alter the lower airway response to viral infections [13,153], viral infections can affect the development of allergen sensitization [154,155], and synergistic interactions can emerge when individuals are exposed concurrently to both allergens and viruses [11,156,157]. As previously suggested, atopic individuals may have both altered host defenses that increase susceptibility to bacterial and viral infections, and an increased risk of developing asthma [3,78–82].
Atopy is a risk factor for the development of childhood asthma after virus-induced wheezing illnesses and many investigative groups have explored the mechanisms of this relationship [158]. It has also been proposed that atopy may be an important predisposing factor for the development of acute bronchiolitis during RSV epidemics [159]. Some investigators have reported that atopic parents increase the likelihood of children developing persistent wheezing [159–161], whereas others have not observed this [162,163]. Similarly, there is controversy over whether personal atopy is more prevalent after bronchiolitis [12,154,163,164]. Albeit, it is clear that children who wheeze in early life and have atopic features such as allergic sensitization, atopic dermatitis and either blood eosinophilia or allergen-specific IgE are at the highest risk for later asthma. These findings have led to the development of predictive indices to estimate the risk of asthma after wheezing in infancy [165,166].
There are data to support that allergic sensitization is a risk factor for wheezing with common cold infections in later childhood [17,167]. In an emergency department setting, risk factors associated with acute wheezing episodes were reported [14]. These included the detection of a respiratory virus, most commonly HRV, positive allergen-specific IgE, and the presence of eosinophilia. Notably, viral infections and allergic inflammation synergistically augmented the risk of wheezing. Moreover, experimental inoculation with HRV is more likely to increase airway responsiveness in allergic individuals compared with nonallergic individuals [168]. Finally, the risk of hospitalization among virus-infected individuals is amplified in patients who are both sensitized and exposed to respiratory allergens [17]. These results imply that individuals with respiratory allergies or eosinophilic airway inflammation are at increased risk for viral-induced wheezing. However, this theory has not been confirmed with experimentally induced colds, as allergen administration before viral inoculation did not increase cold symptoms [136,169].
Viral infections are proposed to interact with allergic inflammation, leading to airway dysfunction through several mechanisms. Viral infections could potentially damage the barrier function of the airway epithelium, leading to an enhanced absorption of aeroallergens across the airway wall and subsequent inflammation [170]. Moreover, the production of various cytokines (TNF-α, IL-1β and IL-6), chemokines (CCL3/MIP-1α, CCL5/RANTES, CCL2/MCP-1 and CXCL8/IL-8), leukotrienes and adhesion molecules (ICAM-1) may further upregulate cellular recruitment, cell activation and the ongoing inflammatory response [171]. Several studies have also demonstrated that HRV infection increases airway hyperresponsiveness in patients with atopy and asthma [156,167,168,172]. Finally, a recent study has demonstrated that the use of prednisolone was associated with less recurrent wheezing in young children with HRV infection but not those with RSV or non-HRV/RSV infections. Thus, HRV infection may be a marker for wheezing children that will respond favorably to corticosteroid treatment [50].
Expert commentary
Respiratory infections, and particularly those caused by viruses, are significant causes of wheezing illnesses in all ages, and progress is being made toward establishing the mechanisms by which these agents can cause acute wheezing and impact the pathophysiology of asthma. Whether there are true asthmagenic strains of these viruses will require additional epidemiological study over several cold seasons, with sampling from diverse geographic regions. Host factors likely contribute to the risk of asthma inception and exacerbation, and these contributions may also vary with respect to early- versus adult-onset disease. These include epithelial barrier defense, IL-13, interferons and/or danger receptors such as P2X7. Once these mechanisms are understood, it may be possible to identify patients who are at the greatest risk for wheezing with viral infections, or those whose virus-induced wheezing heralds the onset of asthma. This would be a key step as preventive therapy could then be focused to the groups with the greatest need.
Five-year view
In the next 5 years, more gene-by-environment interactions should be elucidated, particularly the way in which innate immune system defects impact the severity of viral infections and the inception of asthma. In addition, we will have an improved understanding of whether host factors such as epithelial cell function or immune response versus virulence of virus strain are important to the inception of asthma after viral infection. Identification of a pre-asthma phenotype using novel biomarkers will facilitate the improved identification of at-risk populations and the use of more effective treatment strategies.
Key issues.
Respiratory tract infections caused by viruses, Chlamydophila or Mycoplasma have been hypothesized to have significant roles in the pathogenesis of asthma.
Particular viruses associated with infantile wheezing have been theorized to lead to the inception of the asthmatic phenotype and those children who experience severe viral respiratory infections in early life are more likely to have asthma later in childhood.
In children and adults with established asthma, viral upper respiratory tract infections play a key role in producing acute exacerbations that may lead to healthcare utilization.
Both Chlamydophila and Mycoplasma infection may contribute to both acute exacerbations and the severity of chronic asthma.
Several host factors such as allergic sensitization, epithelial barrier defense and virus-induced interferon responses modify the risk of asthma inception and virus-induced wheezing, and these contributions may vary with respect to early- versus adult-onset disease.
Acknowledgments
Loren C Denlinger is supported by NHLBI grants, K23 HL081492 and P01 HL088594. Theresa W Guilbert is supported by NHLBI grants, U10 HL64305 and P01 HL070831-06A1
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Theresa W Guilbert, Email: tguilbert@wisc.edu, The Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, K4/944, CSC-4108, Madison, WI 53792, USA, Tel.: +1 608 263 9608, Fax: +1 608 265 0510.
Loren C Denlinger, Email: ldenling@wisc.edu, The Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, K4/912, CSC-9988, Madison, WI 53792, USA, Tel.: +1 608 261 1552, Fax: +1 608 263 3104.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Gern JE. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27:S97–S103. doi: 10.1097/INF.0b013e318168b718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gern JE, Bacharier LB, Lemanske RFJ. Infections and asthma. In: Leung DYM, Sampson HA, Geha RS, Szefler SJ, editors. Pediatric Allergy. Mosby; St Louis, Missouri, USA: 2003. pp. 366–378. [Google Scholar]
- 3.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Important study that demonstrated that acute severe lower respiratory tract infection caused by human rhinovirus (HRV) or respiratory syncytial virus (RSV) in the first year of life is strongly associated with the diagnosis of current asthma and persistent wheeze in 5-year-old children, particularly in those who are sensitized during infancy.
- 4.Webley WC, Salva PS, Andrzejewski C, et al. The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med. 2005;171:1083–1088. doi: 10.1164/rccm.200407-917OC. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland ER, Brandorff JM, Martin RJ. Atypical bacterial pneumonia and asthma risk. J Asthma. 2004;41:863–868. doi: 10.1081/jas-200038477. [DOI] [PubMed] [Google Scholar]
- 6.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 7.von HL. Role of persistent infection in the control and severity of asthma: focus on Chlamydia pneumoniae. Eur Respir J. 2002;19:546–556. doi: 10.1183/09031936.02.00254402. [DOI] [PubMed] [Google Scholar]
- 8.Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]; •• Perhaps the first documentation of lower airway infection by Mycoplasma pneumoniae using a PCR test of endobronchial samples from stable patients with moderate, persistent asthma not exhibiting signs of acute infection for at least 3 months, providing direct evidence to support the notion of chronic lower airway infection in select patients.
- 9.Biscardi S, Lorrot M, Marc E, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 10.Esposito S, Blasi F, Arosio C, et al. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–1146. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Large, high-risk cohort study, which found that children had a high risk of asthma at 6 years of age if they wheezed with HRV in the first 3 years of life, and a very high risk if they wheezed at 3 years of age with HRV.
- 12.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 14.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 15.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time–trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important study demonstrating that the large majority of wheezing or asthma exacerbations are caused by viral infections.
- 17.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case–control study. Br Med J. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copenhaver CC, Gern JE, Li Z, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 19.Ly NP, Rifas-Shiman SL, Litonjua AA, et al. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics. 2007;119:e171–e178. doi: 10.1542/peds.2006-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gern JE, Brooks GD, Meyer P, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]; • Novel study that observed that infants colonized with bacteria in the hypopharyngeal region are at increased risk for recurrent, early-life wheezing and asthma at 5 years of age.
- 22.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980;122:567–575. doi: 10.1164/arrd.1980.122.4.567. [DOI] [PubMed] [Google Scholar]
- 23.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146:888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 24.De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 25.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 26.Lemanske RF, Jr, Busse WW. 6. Asthma: factors underlying inception, exacerbation, and disease progression. J Allergy Clin Immunol. 2006;117:S456–S461. doi: 10.1016/j.jaci.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel SE. Asthma: defining of the persistent adult phenotypes Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 29.Toren K, Olin AC, Hellgren J, Hermansson BA. Rhinitis increase the risk for adult-onset asthma – a Swedish population-based case–control study (MAP-study) 2002;96(8):635–641. doi: 10.1053/rmed.2002.1319. [DOI] [PubMed] [Google Scholar]
- 30.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–425. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 31.Polosa R, Knoke JD, Russo C, et al. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J Allergy Clin Immunol. 2008;121:1428–1434. doi: 10.1016/j.jaci.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–1057. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 33.Ronmark E, Lindberg A, Watson L, Lundback B. Outcome and severity of adult onset asthma – report from the obstructive lung disease in northern Sweden studies (OLIN) Respir Med. 2007;101:2370–2377. doi: 10.1016/j.rmed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Barnes N. Most difficult asthma originates primarily in adult life. Paediatr Respir Rev. 2006;7:141–144. doi: 10.1016/j.prrv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Gelfand EW. Pediatric asthma: a different disease. Proc Am Thorac Soc. 2009;6:278–282. doi: 10.1513/pats.200808-090RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sama SR, Hunt PR, Cirillo CI, et al. A longitudinal study of adult-onset asthma incidence among HMO members. Environ Health. 2003;2:10. doi: 10.1186/1476-069X-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jartti T, Lehtinen P, Vuorinen T, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This large study of hospitalized children with wheezing illnesses determined that these children had RSV, HRV, enterovirus and human metapneumovirus, with RSV being more common in infants and HRV in children 1 year of age or more.
- 38.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 39.Openshaw PJ. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Semin Immunopathol. 1995;17:187–201. doi: 10.1007/BF00196165. [DOI] [PubMed] [Google Scholar]
- 40.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 42.Smyth RL. Innate immunity in respiratory syncytial virus bronchiolitis. Exp Lung Res. 2007;33:543–547. doi: 10.1080/01902140701756711. [DOI] [PubMed] [Google Scholar]
- 43.Welliver RC., Sr The immune response to respiratory syncytial virus infection: friend or foe? Clin Rev Allergy Immunol. 2008;34:163–173. doi: 10.1007/s12016-007-8033-2. [DOI] [PubMed] [Google Scholar]
- 44.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 45.Kneyber MCJ, Steyerberg EW, de Groot R, Moll HA. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatrica. 2000;89:654–660. doi: 10.1080/080352500750043945. [DOI] [PubMed] [Google Scholar]
- 46.Wennergren G, Kristjansson S. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. Eur Respir J. 2001;18:1044–1058. doi: 10.1183/09031936.01.00254101. [DOI] [PubMed] [Google Scholar]
- 47.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123(5):1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valkonen H, Waris M, Ruohola A, Ruuskanen O, Heikkinen T. Recurrent wheezing after respiratory syncytial virus or non-respiratory syncytial virus bronchiolitis in infancy: a 3-year follow-up. Allergy. 2009;64:1359–1365. doi: 10.1111/j.1398-9995.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 49.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 50.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy – the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 53.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendley JO, Gwaltney JM, Jr, Jordan WS., Jr Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969;89:184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 56.Dales RE, Schweitzer I, Toogood JH, et al. Respiratory infections and the autumn increase in asthma morbidity. Eur Respir J. 1996;9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 57.Corne JM, Marshall C, Smith S. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]; • Classic cohort study demonstrating that patients with asthma have viral upper respiratory infections at the same frequency as healthy controls, but are twice as likely to have lower respiratory symptoms.
- 58.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau SK, Yip CC, Tsoi HW, et al. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104 e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kistler A, Avila PC, Rouskin S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller EK, Griffin MR, Edwards KM, et al. Influenza burden for children with asthma. Pediatrics. 2008;121:1–8. doi: 10.1542/peds.2007-1053. [DOI] [PubMed] [Google Scholar]
- 64.Calvo C, Garcia-Garcia ML, Pozo F, Carvajal O, Perez-Brena P, Casas I. Clinical characteristics of human bocavirus infections compared with other respiratory viruses in Spanish children. Pediatr Infect Dis J. 2008;27:677–680. doi: 10.1097/INF.0b013e31816be052. [DOI] [PubMed] [Google Scholar]
- 65.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 66.Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2001;164:S71–S75. doi: 10.1164/ajrccm.164.supplement_2.2106063. [DOI] [PubMed] [Google Scholar]
- 67.Matsuse T, Hayashi S, Kuwano K, Keunecke H, Jefferies WA, Hogg JC. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1992;146:177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 68.Macek V, Sorli J, Kopriva S, Marin J. Persistent adenoviral infection and chronic airway obstruction in children. Am J Respir Crit Care Med. 1994;150:7–10. doi: 10.1164/ajrccm.150.1.8025775. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham AF, Johnston SL, Julious SA, Lampe FC, Ward ME. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11:345–349. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 70.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172:1078–1089. doi: 10.1164/rccm.200412-1743PP. [DOI] [PubMed] [Google Scholar]
- 71.Emre U, Sokolovskaya N, Roblin PM, Schachter J, Hammerschlag MR. Detection of anti-Chlamydia pneumoniae IgE in children with reactive airway disease. J Infect Dis. 1995;172:265–267. doi: 10.1093/infdis/172.1.265. [DOI] [PubMed] [Google Scholar]
- 72.Bulut Y, Faure E, Thomas L, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 73.Henderson FW, Clyde WA, Jr, Collier AM, et al. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979;95:183–190. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- 74.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 75.Koh YY, Park Y, Lee HJ, Kim CK. Levels of interleukin-2, interferon-γ, and interleukin-4 in bronchoalveolar lavage fluid from patients with Mycoplasma pneumonia: implication of tendency toward increased immunoglobulin E production. Pediatrics. 2001;107:E39. doi: 10.1542/peds.107.3.e39. [DOI] [PubMed] [Google Scholar]
- 76.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- 77.Chu HW, Kraft M, Krause JE, Rex MD, Martin RJ. Substance P and its receptor neurokinin 1 expression in asthmatic airways. J Allergy Clin Immunol. 2000;106:713–722. doi: 10.1067/mai.2000.109829. [DOI] [PubMed] [Google Scholar]
- 78.Korppi M, Kotaniemi-Syrjanen A, Waris M, Vainionpaa R, Reijonen TM. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995–999. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 79.Jartti T, Korppi M, Ruuskanen O. The clinical importance of rhinovirus-associated early wheezing. Eur Respir J. 2009;33:706–707. 707–708. doi: 10.1183/09031936.00155808. author reply. [DOI] [PubMed] [Google Scholar]
- 80.Hartert TV. Are persons with asthma at increased risk of pneumococcal infections, and can we prevent them? J Allergy Clin Immunol. 2008;122:724–725. doi: 10.1016/j.jaci.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 82.Juhn YJ, Kita H, Yawn BP, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122:719–723. doi: 10.1016/j.jaci.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 84.Black PN, Blasi F, Jenkins CR, et al. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med. 2001;164:536–541. doi: 10.1164/ajrccm.164.4.2011040. [DOI] [PubMed] [Google Scholar]
- 85.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 86.Strunk RC, Bacharier LB, Phillips BR, et al. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J Allergy Clin Immunol. 2008;122(6):1138–1144 e4. doi: 10.1016/j.jaci.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarlo SM, Broder I, Corey P, et al. The role of symptomatic colds in asthma exacerbations: influence of outdoor allergens and air pollutants. J Allergy Clin Immunol. 2001;108:52–58. doi: 10.1067/mai.2001.116574. [DOI] [PubMed] [Google Scholar]
- 88.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. 83–84. doi: 10.1016/j.jaci.2008.08.013. quiz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/l with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]; • This cross-sectional study evaluating service-related health records of military recruits in Finland showed that there is a 60% increased risk of respiratory tract infections in individuals with the lowest levels of 25-hydroxyvitamin D.
- 90.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2007;63(4):473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 91.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50:364–368. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 92.Muhe L, Lulseged S, Mason KE, Simoes EA. Case–control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 93.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 94.Wjst M, Altmuller J, Faus-Kessler T, Braig C, Bahnweg M, Andre E. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res. 2006;7:60. doi: 10.1186/1465-9921-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raby BA, Lazarus R, Silverman EK, et al. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 96.Poon AH, Laprise C, Lemire M, et al. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 97.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27:853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 98.Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 99.Levis S, Gomez A, Jimenez C, et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab. 2005;90:1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 100.Jacobs ET, Alberts DS, Foote JA, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 102.Bastian A, Schafer H. Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–161. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 103.Scherberich JE, Kellermeyer M, Ried C, Hartinger A. 1-α-calcidol modulates major human monocyte antigens and Toll-like receptors TLR2 and TLR4 in vitro. Eur J Med Res. 2005;10:179–182. [PubMed] [Google Scholar]
- 104.Do JE, Kwon SY, Park S, Lee ES. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet's disease. Rheumatology (Oxford) 2008;47:840–848. doi: 10.1093/rheumatology/ken109. [DOI] [PubMed] [Google Scholar]
- 105.Schauber J, Oda Y, Buchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 106.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 107.Sakurai R, Shin E, Fonseca S, et al. 1α,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial–mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. quiz 226. [DOI] [PubMed] [Google Scholar]; • Summarizes evidence supporting the epithelial–mesenchymal interaction model of the remodeling process that occurs in select patients with asthma, which has been extended to suggest that epithelial responses to environmental injury distinguish patients with asthma from normal controls.
- 110.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]; • Secondary analysis of a large clinical trial enrolling patients within the first 2 years of asthma diagnosis suggests that severe exacerbations are a biomarker identifying patients who exhibit a rapid decline in lung function.
- 112.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- 113.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 114.Ordonez C, Ferrando R, Hyde DM, Wong HH, Fahy JV. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- 115.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. quiz 1245–1246. [DOI] [PubMed] [Google Scholar]
- 116.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7:63–68. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
- 118.Tyner JW, Kim EY, Ide K, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study involving transgenic mice overexpressing IL-13 was pivotal in establishing the role of this cytokine in airway hyperplasia and the potential for subepithelial fibrosis.
- 120.DeMeo DL, Lange C, Silverman EK, et al. Univariate and multivariate family-based association analysis of the IL-13 ARG130GLN polymorphism in the Childhood Asthma Management Program. Genet Epidemiol. 2002;23:335–348. doi: 10.1002/gepi.10182. [DOI] [PubMed] [Google Scholar]
- 121.Graves PE, Kabesch M, Halonen M, et al. A cluster of seven tightly linked polymorphisms in the IL-13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol. 2000;105:506–513. doi: 10.1067/mai.2000.104940. [DOI] [PubMed] [Google Scholar]
- 122.Howard TD, Whittaker PA, Zaiman AL, et al. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am J Respir Cell Mol Biol. 2001;25:377–384. doi: 10.1165/ajrcmb.25.3.4483. [DOI] [PubMed] [Google Scholar]
- 123.Zhen G, Park SW, Nguyenvu LT, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 125.Matsukura S, Stellato C, Georas SN, et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24:755–761. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 126.Chu HW, Balzar S, Seedorf GJ, et al. Transforming growth factor-β2 induces bronchial epithelial mucin expression in asthma. Am J Pathol. 2004;165:1097–1106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-α- and IFN-γ-induced β-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179:984–992. doi: 10.4049/jimmunol.179.2.984. [DOI] [PubMed] [Google Scholar]
- 129.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Hamati E, Lee PK, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]; • Evaluated both type III interferon responses to RV16 infection of primary epithelial cells from a small number of patients with asthma compared with healthy controls.
- 132.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.109. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lopez-Souza N, Favoreto S, Wong H, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390 e2. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]; • Demonstrates that during rhinovirus infection, nasal chemokines are associated with neutrophilic influx and cold symptoms.
- 136.Avila PC, Abisheganaden JA, Wong H, et al. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. J Allergy Clin Immunol. 2000;105:923–932. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 137.Denlinger LC, Shi L, Guadarrama A, et al. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009;179:265–270. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 139.Idzko M, Hammad H, van Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 140.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 141.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39:12–19. doi: 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 142.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Staat MA. Respiratory syncytial virus infections in children. Semin Respir Infect. 2002;17:15–20. doi: 10.1053/srin.2002.31688. [DOI] [PubMed] [Google Scholar]
- 144.McFadden ER., Jr Improper patient techniques with metered dose inhalers: clinical consequences and solutions to misuse. J Allergy Clin Immunol. 1995;96:278–283. doi: 10.1016/s0091-6749(95)70206-7. [DOI] [PubMed] [Google Scholar]
- 145.Mosser AG, Brockman-Schneider R, Amineva S, et al. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 146.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 147.Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 148.Mosser AG, Vrtis R, Burchell L, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 149.Simons E, Schroth MK, Gern JE. Analysis of tracheal secretions for rhinovirus during natural colds. Pediatr Allergy Immunol. 2005;16:276–278. doi: 10.1111/j.1399-3038.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 150.Malmstrom K, Pitkaranta A, Carpen O, et al. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J Allergy Clin Immunol. 2006;118:591–596. doi: 10.1016/j.jaci.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 151.Harju TH, Leinonen M, Nokso-Koivisto J, et al. Pathogenic bacteria and viruses in induced sputum or pharyngeal secretions of adults with stable asthma. Thorax. 2006;61:579–584. doi: 10.1136/thx.2005.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008;177:1082–1089. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bardin PG, Fraenkel DJ, Sanderson G, et al. Amplified rhinovirus colds in atopic subjects. Clin Exp Allergy. 1994;24:457–464. doi: 10.1111/j.1365-2222.1994.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 155.Frick OL. Effect of respiratory and other virus infections on IgE immunoregulation. J Allergy Clin Immunol. 1986;78:1013–1018. doi: 10.1016/0091-6749(86)90295-2. [DOI] [PubMed] [Google Scholar]
- 156.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sly PD, Boner AL, Bjorksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Laing I, Reidel F, Yap PL, Simpson H. Atopy predisposing to acute bronchiolitis during an epidemic of respiratory syncytial virus. Br Med J (Clin Res Ed) 1982;284:1070–1072. doi: 10.1136/bmj.284.6322.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rooney JC, Williams HE. The relationship between proved viral bronchiolitis and subsequent wheezing. J Pediatr. 1971;79:744–747. doi: 10.1016/s0022-3476(71)80385-2. [DOI] [PubMed] [Google Scholar]
- 161.Zweiman B, Schoenwetter WF, Pappano JE, Jr, Tempest B, Hildreth EA. Patterns of allergic respiratory disease in children with a past history of bronchiolitis. J Allergy Clin Immunol. 1971;48:283–289. doi: 10.1016/0091-6749(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 162.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murray M, Webb MS, O'Callaghan C, Swarbrick AS, Milner AD. Respiratory status and allergy after bronchiolitis. Arch Dis Child. 1992;67:482–487. doi: 10.1136/adc.67.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Noma T, Mori A, Yoshizawa I. Induction of allergen-specific IL-2 responsiveness of lymphocytes after respiratory syncytial virus infection and prediction of onset of recurrent wheezing and bronchial asthma. J Allergy Clin Immunol. 1996;98:816–826. doi: 10.1016/s0091-6749(96)70131-8. [DOI] [PubMed] [Google Scholar]
- 165.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 166.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 167.Murray CS, Poletti G, Kebadze T, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gern JE, Calhoun W, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 169.de Kluijver J, Evertse CE, Sont JK, et al. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med. 2003;168:1174–1180. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- 170.Sakamoto M, Ida S, Takishima T. Effect of influenza virus infection on allergic sensitization to aerosolized ovalbumin in mice. J Immunol. 1984;132:2614–2617. [PubMed] [Google Scholar]
- 171.Papadopoulos NG, Xepapadaki P, Mallia P, et al. Mechanisms of virus-induced asthma exacerbations: state-of-the-art A GA2LEN and InterAirways document. Allergy. 2007;62:457–470. doi: 10.1111/j.1398-9995.2007.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-γ and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]