Abstract
Human immunodeficiency virus type one (HIV-1) associated neurocognitive disorders (HAND) can affect < 50% of infected people during the disease course. While antiretroviral therapies have substantively increased the quality of life and reduced HIV-1 associated dementia, less severe minor cognitive and motor deficits continue. Trafficking of HIV-1 into the central nervous system (CNS), peripheral immune activation, dysregulated glial immunity, and diminished homeostatic responses are the disease-linked pathobiologic events. Monocyte-macrophage passage into the CNS remains an underlying force for disease severity. Monocyte phenotypic may change at an early stage of cell maturation and immune activation of hematopoietic stem cells. Activated monocytes are pulled into the brain in response to chemokines made as a result of glial inflammatory processes, which in turn cause secondary functional deficits in neurons. Current therapeutic approaches are focused on adjunctive and brain-penetrating antiretroviral therapies. These may attenuate virus-associated neuroinflammatory activities thereby decreasing the severity and frequency of HAND.
Keywords: HIV-1 associated neurocognitive disorders, neuroinflammation, microglia, cognitive dysfunction, blood brain barrier, chemokines, pro-inflammatory cytokines, adaptive immunity, innate immunity, hematopoietic stem cells, adjunctive therapies
Introduction
Human immunodeficiency virus-1 (HIV-1) targets CD4+ expressing cells that include a subset of lymphocytes and a broad range of mononuclear phagocytes (MP; monocytes, dendritic cells, tissue macrophages and microglia). Over time this leads to profound immunodeficiency and an increased host susceptibility to a broad range of opportunistic infections (Ong, 2008). Moreover, continuous viral replication can directly impact end-organ dysfunction, particularly in the lung and central nervous system (CNS) (Everall et al., 2009; Hull et al., 2008). The advent of antiretroviral therapy (ART), however, has significantly changed the landscape of HIV neuropathogenesis (Cysique and Brew, 2009). Disease is no longer a result of continuous productive virus-infection and activation of brain MP but rather a result of more limited infection and neuroinflammation. Although widespread ART usage in resource available settings has increased life expectancy for virus-infected individuals with a concomitant decrease in disease morbidities (Achmat and Simcock, 2007; Aracena-Genao et al., 2008), neurological complications continue to persist. This may be attributed to viral mutation and ART resistance; failure of drugs to access viral sanctuaries and toxicities or poor compliance to complex ART regimens (Battegay and Elzi, 2009; Blankson, 2006; Kiertiburanakul and Sungkanuparph, 2009; Krusi et al., 2009). Illicit drug usage (Cabral, 2006) and lack of ART availability (Cohen, 2007) may also influence neurological disease manifestations. Of these, the most feared long-term complication of HIV-1 disease is cognitive dysfunction.
During the disease course, it is estimated that the prevalence of disease may as many as 50% of HIV-1-infected individuals will suffer from some form of impairment if asymptomatic neurocognitive disorder is included (McArthur et al., 2005). Although HIV-1 associated dementia (HAD), the most severe form of CNS impairment, has been reduced significantly incidence, now affecting <7% of infected people following ART, a concomitant increase in minor cognitive impairments is emerging (Fischer-Smith and Rappaport, 2005). The spectrum of such neurocognitive impairment, now termed HIV-1 associated neurocognitive disorders (HAND), includes asymptomatic neurocognitive impairment and varying degrees of HIV-associated mild neurocognitive disorders (Antinori et al., 2007). HAND is associated with immune suppression. Chronic neuroinflammation causes a metabolic encephalopathy that is fueled by MP viral infection and immune activation (Langford, et al, 2003; Yadav and Collman, 2009; Zheng and Gendelman, 1997). In the pre-ART era, this often paralleled the development of HIV-1 encephalitis (HIVE), a pathological correlate of HAD. Neuropathologically, HIVE is characterized by formation of multinucleated giant cells (Sharer et al., 1985), myelin pallor (Petito et al., 1986), formation of microglial nodules, astrogliosis, productive viral replication, and neuronal dropout (Masliah et al., 1996; Ances and Ellis, 2007). While incidence of HIVE is now quite rare in the setting of ART, more subtle neuropathological alterations are common. These include blood-borne monocyte brain infiltration and limited gliosis (Everall et al., 2005). Indeed, it is uncertain whether or not ongoing viral replication in the brain is required for the development of milder forms of HAND. Limited histopathologic aberrations in the brain characterize mild cognitive dysfunction, leading to the speculation that glial activation may be a key determinant driving the process (Everall et al., 2005).
ART can also lead to a reversal of severe cognitive dysfunction (Gendelman et al., 1998). Nonetheless, and despite changes in disease severity, viral reservoirs within the CNS remain common and significant (Kramer-Hammerle et al., 2005). Virus can enter the brain as cell-free progeny, in monocyte-macrophages or in T cells (Banks et al., 2004). Restricted HIV-1 infection continues in circulating monocytes and resting CD4+ lymphocytes (Lambotte et al., 2003). Furthermore, both cell types can productively replicate virus following cell differentiation and activation (Alexaki et al., 2008) and, in this way enable viable cellular reservoirs for HIV-1 to ensue and evade ART (McGee et al., 2006). Intriguingly, HIV-1 can cross the blood brain barrier (BBB) through blood-borne monocytes, thereby escaping immune surveillance (Nottet et al., 1996; Persidsky et al., 1997). The specific mechanism(s) by which inflammatory cells are recruited into the CNS revolves around peripheral immune activation (push) and an established chemokine gradient (pull) established within the CNS as a result of viral infection and glial immune activation (Dhillon et al., 2008; Persidsky et al., 1999; Shacklett et al., 2004). There exists a carefully orchestrated cooperation between chemokine release from the CNS and chemotaxis and differentiation of monocyte progenitor cells from the bone marrow (Hasegawa et al., 2009; Soulas et al., 2009; Westhorpe et al., 2009). This cooperation, in turn, ultimately culminates into neuroimmune inflammatory responses and neuronal impairments (Coleman and Wu, 2009).
Crosstalk between the peripheral and CNS immunity
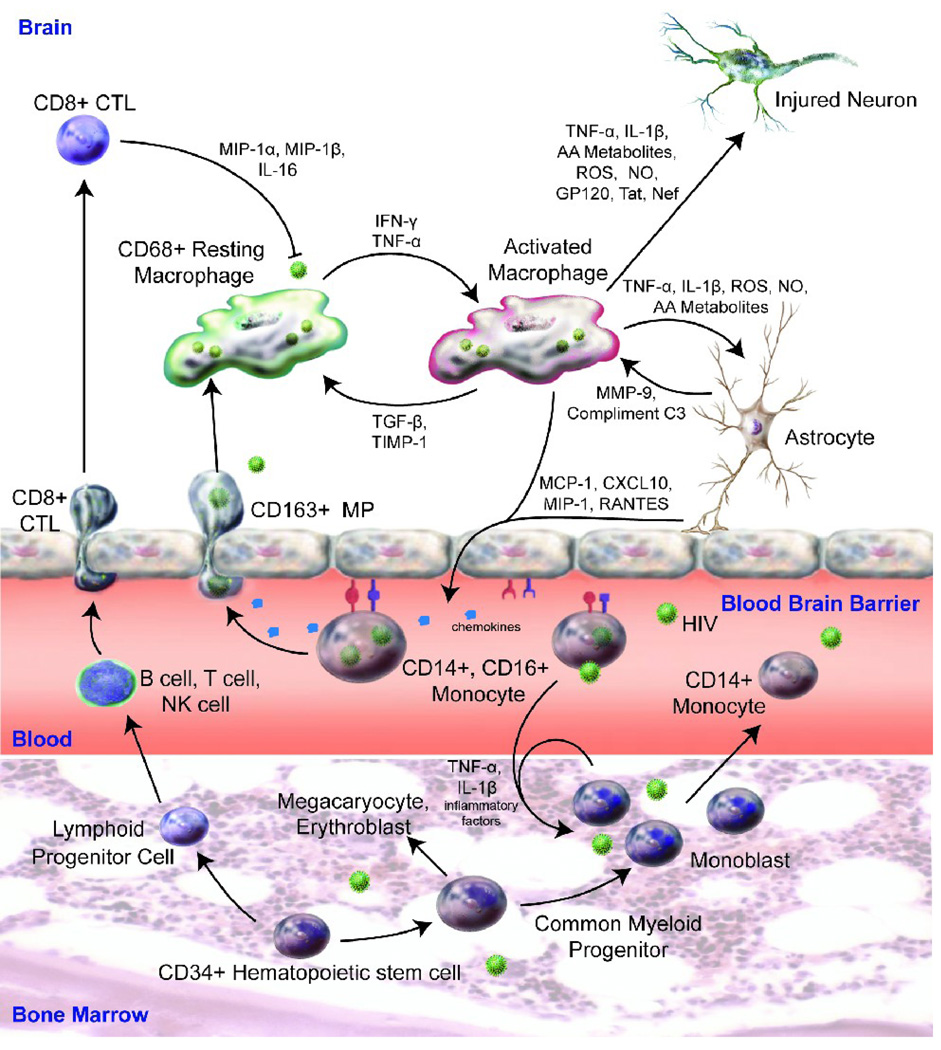
A Trojan horse cell model can explain how HIV-1 infected monocytes escape immune surveillance (Gendelman et al., 1985; Haase, 1986). The “pull” for viral entry is through CNS produced chemokines, such as monocyte chemoattractant protein (MCP)-1 and the IFN-γ inducible peptide, CXCL10, while the “push” is initiated by peripheral immune activation (Asensio et al., 2001; Fischer-Smith et al., 2008a; Yadav and Collman, 2009) (Figure).
Figure 1.
Immune competent peripheral blood monocytes derived from CD34+ hematopoietic stem cells show enhanced proliferation rates in response to HIV-1 infection and a pro-inflammatory environment. Cells are pushed into the CNS in response to activation signals. Simultaneously, infected monocytes are drawn into areas of the brain viral infection by the presence of chemokines and pro-inflammatory factors elicited as a result of HIV-1 infection and ongoing neuroinflammatory responses. Once in the brain, HIV-1 infected perivascular macrophages and microglia elicit neurotoxic effects on surrounding neurons and affect astrocyte immunity resulting in amplifications of ongoing inflammatory responses from endothelial cells, resident microglia and infiltrating blood borne monocyte-macrophages. Adaptive immunity serves in regulatory and effector functions (including HIV-1 specific CTL) in curtailing ongoing inflammatory responses and in anti-HIV-1 surveillance, respectively.
Once in the brain, HIV-1 infected blood-borne macrophages secrete pro-inflammatory cytokines such as, tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and viral proteins such as HIV-1gp120 and Tat. These all can affect neuronal function (Brabers and Nottet, 2006). While it is now known which of the viral and cellular factors most affect neuronal compromise prior reports suggest they work in synergy to drive M1 polarization for the brain macrophage and microglia (Kraft-Terry, Buch et al. 2009). Within the brain, astrocytes serve as a principal regulator for neural homeostasis (Eugenin and Berman, 2007; Wang et al., 2008a; Wang et al., 2008b). These cells, interestingly, exhibit conflicting roles in HIV-related neuronal function. For example, Genis et. al. found that astrocytes initially elicit a neurotoxic secretory response in macrophages during HIV-1 infection (Genis et al., 1992), leading to upregulation of arachidonic acid and its metabolites, quinolinic acid, chemokines and cytokine secretions. Dependent on viral infection and neuroimmune activation, astrocytes can modulate a macrophage phenotype resulting in either neurotoxicity or neuroprotection (Wang et al., 2008b). Astrocytes can also affect autocrine and paracrine inflammatory cascades contributing further to immune activation, viral infection and cellular ingress across the BBB (Nottet and Gendelman, 1995) (Figure).
Monocyte-Macrophage Subsets and CNS Entry
Monocytes restrict viral growth, and the viral life cycle only goes to completion after monocytes differentiate into macrophages (Coleman and Wu, 2009). A subset of CD16+ monocytes (<5% of total monocyte cell pool) may be linked to HIV-1 infection of the brain. CD16+ monocytes harbor HIV-1 and are readily infected by the virus (Jaworowski et al., 2007) (Figure). Cell-specific viral spread offers an explanation as to how monocytes can carry HIV-1 into the brain and become a source for viral dissemination (Ellery et al., 2007). Interestingly, HIV-1 infected monocytes are resistant to apoptosis thus permitting their roles as long-term viral reservoirs (Giri et al., 2009). Such resistance to apoptosis could also serve as a mechanism for host survival in an effort to sustain this cell subset despite ongoing viral infection.
CD163, a macrophage scavenger receptor, is also found on MP. It is linked to HIV-1 neuroinvasion and ongoing viral infection (Onofre et al., 2009). CD163 is localized primarily on perivascular brain macrophages and to a more limited extent in resident microglia (Kim et al., 2006; Roberts et al., 2004). CD163 is upregulated during both HIVE and simian immunodeficiency virus (SIVE) and has been linked to ongoing viral infection (Roberts et al., 2004). Productively infected cells in the brains demonstrate co-localization of both CD163+ and CD16+ (Fischer-Smith et al., 2008a). Targeting therapies to eliminate CD163+ and CD16+ double positive cells may be a unique avenue for developing therapeutics (Fischer-Smith et al., 2008b).
Pools of CD34+ hematopoietic stem cells have the potential to be infected and serve as viral reservoirs (Ruiz et al., 1998). Once incorporated, HIV-1 DNA can be transmitted to uninfected cells upon stem cell differentiation. CD34+ hematopoietic stem cells can renew as many as 70% of the pool of CD68+ brain perivascular macrophages (Soulas et al., 2009). Importantly, cell differentiation is disrupted during HIV-1 infection, therefore resulting in decreased cellular replenishment (Westhorpe et al., 2009). Importantly, monocyte turnover has been linked to disease progression during cellular recycling (Hasegawa et al., 2009).
Blood Brain Barrier (BBB)
The BBB plays a central role in the development of HAD serving as the conduit by which free virus and infected immune cells enter the brain from the circulatory system (Banks et al., 2000; Nottet et al., 1996; Persidsky et al., 1997). A number of laboratory animal models and human studies demonstrated BBB breakdown as a consequence of progressive HIV infection and immune compromise (Dallasta et al., 1999; Kanmogne et al., 2002; Persidsky et al., 2000). BBB dysfunction is more frequent in AIDS patients with dementia, as compared with AIDS patients without dementia or seronegative controls (Avison et al., 2004; Toborek et al., 2003). Such BBB breakdown may enhance entry of toxins, free virus, infected and/or activated monocytes, and lymphocytes into the CNS, thus spreading HIV-1 infection to brain macrophages and microglia. Structurally, the BBB is composed of specialized nonfenestrated human brain microvascular endothelial cells (HBMECs) connected by intercellular junctions in an impermeable monolayer devoid of transcellular pores. Infiltration of monocytes across the BBB has been a subject of excellent reviews. (Ivey et al., 2009; Persidsky et al., 2006).
Cellular products and viral proteins secreted by infected cells likely play an important role in BBB impairment and in the development of HAD. Increasing evidence suggests that the HIV-1 envelope protein gp120 and/or Tat can also cause BBB changes. Mechanism of HIV-1 gp120-mediated cytotoxicity to the brain endothelium (Kanmogne et al., 2002; Kanmogne et al., 2005) resulting in downregulation and rupture of tight junction proteins (TJPs) in HBMECs involves PKC signaling pathways and receptor mediated [Ca2+] release. Indeed, increased BBB microvascular permeability is due to degradation of TJPs by the proteasome induced by HIV-1. Similarly, intracranial injections of HIV Tat in rats and mice induce perivascular cuffing with monocyte infiltration and breaches in BBB integrity including the TJP (Andras et al., 2005; Jones et al., 1998). Tat-mediated alteration of tissue redox status has been shown to affect the integrity of the cell junction system through the ERK1/2 pathway, leading to the disruption of the BBB (Pu et al., 2005).
Adaptive Neuroimmunity
CD8+ cytotoxic T cells (CTL) can elicit the death of virus-infected cells (Yamamoto and Matano, 2008). Most CTLs express T-cell receptors that can recognize a specific antigenic peptide bound to Class I major histocompatability complex (MHC) molecules (Bangham, 2009). The affinity between CD8 and the MHC molecule keeps the CTL and the target cell bound closely during antigen-specific activation (Gulzar and Copeland, 2004). More recently, regulatory T cells (Treg) have been shown to exert cell killing as well as immunosuppressive effects (Kinter et al., 2007). Both CTLs and Tregs are important for immune surveillance and homeostasis and hence show potential for therapeutic applications. Importantly, the ability of CTLs to target HIV-1 infected cells has been exploited for the development of HIV-1 vaccines (Masopust, 2009). These cells also play critical roles in clearance of virus- infected cells during the course of infection (Gruters et al., 2002). In rhesus macaques infected with SIV and in humanized mouse models of HAD, CTLs have been shown to clear HIV-1 infected macrophages within the brain (Persidsky et al., 2006; Poluektova et al., 2002). Alternatively, CTLs present in the CNS may affect HIVE and ongoing neurodegeneration (Petito et al., 2006). Similarly, in patients with immune reconstitution inflammatory syndrome, increases in CTLs parallel poor disease outcomes (Gray et al., 2005).
In recent years, Tregs have become a pivotal area of study due to their immunosuppressive effects. These cells are CD4+, CD25+, FOXP3+ expressing cells that are known to also secrete IL-10 and transforming growth factor beta (TGF-β). In peripheral blood mononuclear cells from HIV-1 infected individuals, Tregs secreting IL-10 and IFN-γ appear to have a dual regulatory role in the disease (Torheim et al., 2009). HIV-1 resistant sex workers have decreased T cell activation and increased populations of Tregs compared with their uninfected counterparts (Card et al., 2009). In humanized mice wherein HIVE is induced by administration of virus-infected bone marrow-derived macrophages in the subcortex, adoptive transfer of activated Tregs attenuates astrogliosis and microglial activation leading to profound neuroprotective effects (Liu et al., 2009). These findings shed insights into the beneficial role of Tregs in the control of HIV-1 infection.
This being said, there is also evidence that increases in Treg populations are not always beneficial. In studies of chronically infected HIV-1 individuals, expanding Treg populations were found to accelerate HIV-1 disease and T cell activation. These findings provide evidence that the beneficial effects of Tregs may be limited and that the activation of these cells can be a double-edged sword (Cao et al., 2009). While Tregs have the potential to control neurodegeneration in HIV-1 infection, their role in systemic disease progression must also be considered. Taken together, the anti-inflammatory and immunosuppressive actions of Tregs may lead to overall beneficial outcomes in a variety of neurodegenerative diseases (Huang et al., 2009).
Chemokines
Monocyte and leukocyte passage into the CNS would not occur without the complex chemokine gradient that is established during HIV-1 infection. Chemokine involvement in HIV-1 neuropathogenesis is well-recognized because of their abilities to: (i) recruit HIV-1-infected immune cells into the brain, (ii) serve as mediators for inflammatory responses, and (iii) serve as ligands for HIV-1 co-receptors, specifically CXCR4 and CCR5 (Hesselgesser et al., 1998). Chemokines recruit monocytes/macrophages and lymphocytes into the brain (Conant et al., 1998). Cerebral expression of various chemokines and their receptors is increased in HIVE. MCP-1/CCL2, a member of the C–C subfamily of chemokines, is the most potent of a variety of monocyte chemoattractants with diverse immunological functions (Deshmane et al., 2009).
CCL2 is considered to be a critical factor involved in the infiltration of monocytes and lymphocytes across the BBB during CNS inflammation. Numerous studies strongly suggest that increased CCL2 expression in the CNS is associated with enhanced progression of HIVE (Dhillon et al., 2008). The chemokine is overexpressed during HIVE and accumulates in the CSF and brains of immunocompromised patients with HAD and HIVE and in macaques with SIVE (Mankowski et al., 2004; Monteiro de Almeida et al., 2006; Monteiro de Almeida et al., 2005). Chemokines can also promote virus replication and contribute to injury and eventual loss of neurons (Asensio and Campbell, 1999; Miller and Meucci, 1999).
In addition to CCL2, another chemokine, CXCL10 (interferon γ-inducible peptide) has also been detected in the CSF of individuals with HIV-1 infection (Kolb et al., 1999). CXCL10 is a secreted polypeptide of 10 kDa that was first identified as an early response gene induced after IFN-γ treatment in a variety of cells. CXCL10 is present in the CSF of all HIV-1 infected patients but is absent in uninfected individuals (Christo et al., 2009). In regards to mechanisms for how CXCL10 is regulated, it has been shown that the HIV-1 envelope glycoprotein gp120 can induce CXCL10 gene expression in astrocytes in vivo and in vitro, independent of IFN-γ (Asensio et al., 2001).
Immunohistochemical studies have revealed upregulation of some chemokines and chemokine receptors in the brains of patients with HIV and HIVE. In brain tissues from patients with HIVE, the presence of chemokines and chemokine receptors were found to be most abundant in microglial nodules and in astrocytes. The expression levels of CCR1, CCR3, CCR5 and CXCR4 were all increased in macrophages/microglia, especially in microglial nodules, with the presence of CCL2, MIP-1α/CCL3 and RANTES/CCL5 linked to histopathological signs of HIVE. Furthermore, CCR3 and/or CXCR4 were also highly expressed in pyramidal neurons within the hippocampus. Increased CXCR3 expression was typically found in areas of the brain that were pathologically abnormal. Understanding the underlying mechanisms that lead to the migration of HIV-1 infected monocytes from the periphery into the CNS can be considered in therapeutic strategies. If monocyte infiltration to the brain can be controlled disease may be attenuated.
In the brains of individuals with HAND, upregulation of chemokines in the CNS is often considered a correlate of neuroinflammation. However, recent evidence raises the possibility that, in addition to their role as chemoattractants, chemokines might also act as neurotransmitters or neuromodulators (Rostene et al., 2007). One example of this is the chemokine fractalkine that serves to attract peripheral macrophages into the brain and as a neuroprotective factor (Eugenin et al., 2003; Tong et al., 2000). Moreover, it can also function to regulate neuronal survival through its antiapoptotic effects (Meucci et al., 1998; Meucci et al., 2000).
Although CCL2 has been linked to the neuropathogenesis of HIV-1 infection (Ansari et al., 2007) it also possesses beneficial functions. These include its neurotrophic (Bolin et al., 1998), neuromodulatory (Gosselin et al., 2005), and neurohormonal actions (Melik-Parsadaniantz and Rostene, 2008). CCL2 has been shown to protect neurons against HIV-1 Tat toxicity (Eugenin et al., 2003; Yao et al., 2009). Additionally, processing of chemokines resulting in alteration of their functions has recently been reported for the chemokine stromal cell-derived factor 1 (SDF-1α). This is an apparent regulatory mechanism that modulates many neuropeptide systems. Herein, the proteolytic cleavage of SDF-1α results in a highly immunogenic peptide, that, in turn, affects HIV neurodegeneration through engagement of the chemokine receptor, CXCR3 (Vergote et al., 2006). This demonstrates a unique mechanism by which proteins can acquire neuropathogenic properties following proteolytic processing.
In response to cellular damage, the host is also capable of producing trophic growth factors [platelet-derived growth factor (PDGF), fibroblast growth factor, nerve growth factor or brain derived neurotropic factor] that may protect neuronal, glial and other resident cells of the brain. These neurotrophic growth factors also play somewhat paradoxically diverse roles during the progression of CNS infection by promoting increased viral replication or cooperating with viral and/or host factors that promote neuronal degeneration. Many of these trophic factors also provide protection to neurons against neurotoxic factors (cytokines, chemokines and viral products). Previously, it was demonstrated that while PDGF expression in activated MP was closely associated with SIV or chimeric SHIV-neuropathogenesis in macaques (Potula et al., 2004), its expression in neurons correlated with neuronal fitness (Peng et al., 2008). Thus, depending on the cell type within the tissue, the same host factor can manifest diverse activation responses. The ultimate outcome of infection in the CNS (neuronal survival versus damage) is thus a result of the ensuing shift in balance between the neurotrophic versus neurotoxic products manifested over time following infection.
Therapeutic Developments
Harnessing MP Function for Therapeutic Benefit
Drug penetration past the BBB into the CNS has long been an obstacle in treating HIV-1. HIV-1 protease inhibitors are known to have poor CNS penetration, while other HIV-1 therapies such as zidovudine (AZT) have very efficient BBB penetration (Letendre et al., 2008; Varatharajan and Thomas, 2009). This being said, BBB permeability is only beneficial for controlling CNS HIV-1 infection if HIV-1 therapies themselves are not neurotoxic.
More recently, efforts are being made to develop nanoparticulate-antiretroviral therapy (nanoART). Nanoparticles (NP) are being investigated for medicinal application in a variety of fields. Much effort has been placed in finding long-acting forms of injectable antiretrovirals to circumvent the challenges of therapy adherence currently faced by HIV-1 infected individuals (Baert et al., 2009; Govender et al., 2008). To specifically target the CNS, NP are synthesized with various combinations of ART therapies to be taken up by monocytes and carried into the CNS for release at sites of HIV-1 infection. These therapies will provide sustained release of ART within the CNS for days to weeks. For example, Dou et al. demonstrated that in an HIV-1 rodent model, injection of indinavir nanoART reduced HIV-1 replication within the HIVE brain (Dou et al., 2009). Some groups have begun to coat particles with antibodies (Beduneau et al., 2009) or proteins such as HIV-1 Tat (Berry, 2008) for better uptake and cellular localization. Others have used p24 loaded and coated NP to elicit a systemic CTL response, which has proven effective in mice (Aline et al., 2009). A comprehensive review of nanoART and other CNS penetrating therapies has recently been published (Nowacek and Gendelman, 2009). Such approaches are providing new avenues for treating or even preventing the spread of HIV-1 in the brain.
Adjunctive Therapeutics
ART is becoming increasingly effective as efforts are made to decrease side effects while improving drug combinations to combat virus mutation. With ever decreasing levels of HAD, more minor cognitive impairment must not be ignored. Many efforts are being made to improve penetrability of ART past the BBB, but efforts must proceed with caution. Recent studies have found that improved CNS penetration of ART can (Cysique et al., 2009) but does not always correlate with a decrease in the prevalence of cognitive impairment (Marra et al., 2009). In fact, studies by these authors demonstrated that increases in CNS penetrence of HIV-1 drugs resulted in decreased cognitive performance. This highlights the importance of considering drug toxicity and elicited cellular response for various ART regiments before working on methods to enhance CNS entry. Many therapies being developed in animal models of neurodegenerative disorders, like Parkinson’s and Alzheimer’s disease, are being applied toward HAND treatments.
One such adjunctive therapy is a platelet activating factor (PAF) antagonist, PMS-601, that has been demonstrated to reduce many of the classic signs of HAND, including: microgliosis and multinucleated giant cell formation (Eggert et al., 2009b). PAF is a downstream metabolite of arachidonic acid, known to play roles in inflammation and regulation of the immune response by controlling platelet aggregation. PAF receptors are increased in neurons during HAND and contribute to PAF neurotoxicity (Bellizzi et al., 2005). ART is also known to decrease PAF activity (Tsoupras et al., 2008). When used in combination with PAF antagonists, HIV-1-associated neurodegeneration could be substantially decreased.
Other therapies being explored include sodium valproate (Schifitto et al., 2006), those that regulate mixed-lineage kinase pathways such as CEP-1347 (Eggert et al., 2009a), N-methyl-D-aspartic acid antagonists such as memantine (Anderson et al., 2004), and lithium (Schifitto et al., 2009). All of these are also being developed as therapies for other neurodegenerative disorders and may prove successful in improving disease outcomes for HAND. These and other adjunctive therapies have recently been reviewed in the context of neuroAIDS (Crews et al., 2009) (Perry et al., 2005). While adjunctive therapies are proving mildly successful, there has been no drastic decrease in HAND as a result of therapy. This provides an open area for future research. A successful approach can combine CNS penetrating nanoART with adjunctive therapies to positively affect neurocognitive symptoms.
Conclusions
CNS complications of HIV-1 infection have evolved considerably since the widespread use of ART. Reduced severity of disease has paralleled lowered viral replication and reduced overt neuropathology. What remains are neuroinflammatory responses heralded by low levels of viral replication, disordered glial crosstalk and monocyte transmigration into the CNS. With antiretroviral treatments that specifically target the CNS and adjunctive therapies now becoming available, eliminating virus (and virus-mediated activation) in the brain is a realistic goal. Understanding the signals that lead to their passage into the brain and in attenuating viral growth and inflammatory responses within the CNS will ultimately lead to the elimination of HAND.
Acknowledgments
We thank Ms. Robin Taylor for outstanding administrative and computer support. This work was supported by National Institutes of Health grants P01 NS43985, P20RR15635, R37 NS36126, PO1 NS31492, and R01NS034239.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achmat Z, Simcock J. Combining prevention, treatment and care: lessons from South Africa. Aids. 2007;21(Suppl 4):S11–20. doi: 10.1097/01.aids.0000279702.71062.52. [DOI] [PubMed] [Google Scholar]
- Alexaki A, et al. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aline F, et al. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic PLA nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27:5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Anderson ER, et al. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004;24:7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras IE, et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Ansari AW, et al. Dichotomous effects of C–C chemokines in HIV-1 pathogenesis. Immunol Lett. 2007;110:1–5. doi: 10.1016/j.imlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracena-Genao B, et al. Costs and benefits of HAART for patients with HIV in a public hospital in Mexico. Aids. 2008;22(Suppl 1):S141–S148. doi: 10.1097/01.aids.0000327635.74919.fd. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- Asensio VC, et al. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avison MJ, et al. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol. 2004;157:140–146. doi: 10.1016/j.jneuroim.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Baert L, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Bangham CR. CTL quality and the control of human retroviral infections. Eur J Immunol. 2009;39:1700–1712. doi: 10.1002/eji.200939451. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Binding, internalization, and membrane incorporation of human immunodeficiency virus-1 at the blood-brain barrier is differentially regulated. Neuroscience. 2004;128:143–153. doi: 10.1016/j.neuroscience.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Battegay M, Elzi L. Morbidity and mortality in HIV-infected individuals - a shift towards comorbidities. Swiss Med Wkly. 2009;139:564–570. doi: 10.4414/smw.2009.12662. [DOI] [PubMed] [Google Scholar]
- Beduneau A, et al. Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS ONE. 2009;4:e4343. doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi MJ, et al. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry CC. Intracellular delivery of nanoparticles via the HIV-1 tat peptide. Nanomed. 2008;3:357–365. doi: 10.2217/17435889.3.3.357. [DOI] [PubMed] [Google Scholar]
- Blankson JN. Viral reservoirs and HIV-specific immunity. Curr Opin HIV AIDS. 2006;1:147–151. doi: 10.1097/01.COH.0000203831.78479.05. [DOI] [PubMed] [Google Scholar]
- Bolin LM, et al. Primary sensory neurons migrate in response to the chemokine RANTES. J Neuroimmunol. 1998;81:49–57. doi: 10.1016/s0165-5728(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1:280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Cao W, et al. Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res Hum Retroviruses. 2009;25:183–191. doi: 10.1089/aid.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card CM, et al. Decreased Immune Activation in Resistance to HIV-1 Infection Is Associated with an Elevated Frequency of CD4(+)CD25(+)FOXP3(+) Regulatory T Cells. J Infect Dis. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- Christo PP, et al. Cerebrospinal fluid levels of chemokines in HIV infected patients with and without opportunistic infection of the central nervous system. J Neurol Sci. 2009 doi: 10.1016/j.jns.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Access to diagnostics in support of HIV/AIDS and tuberculosis treatment in developing countries. Aids. 2007;21(Suppl 4):S81–S87. doi: 10.1097/01.aids.0000279710.47298.5c. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, et al. Molecular Pathology of Neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10:1045–1063. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Cysique LA, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallasta LM, et al. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, et al. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183:661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, et al. Neuroprotective activities of CEP-1346 in models of neuroAIDS. Journal of Immunology. 2009a doi: 10.4049/jimmunol.0902962. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, et al. Development of a platelet-activating factor antagonist for HIV-1 associated neurocognitive disorders. J Neuroimmunol. 2009b;213:47–59. doi: 10.1016/j.jneuroim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellery PJ, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, et al. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Everall I, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009:1–11. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, et al. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, et al. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008a;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, et al. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008b;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, et al. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985;82:7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, et al. Suppression of inflammatory neurotoxins by highly active antiretroviral therapy in human immunodeficiency virus-associated dementia. J Infect Dis. 1998;178:1000–1007. doi: 10.1086/515693. [DOI] [PubMed] [Google Scholar]
- Genis P, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri MS, et al. Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J Immunol. 2009;182:4459–4470. doi: 10.4049/jimmunol.0801450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Govender T, et al. Polymeric nanoparticles for enhancing antiretroviral drug therapy. Drug Deliv. 2008;15:493–501. doi: 10.1080/10717540802321776. [DOI] [PubMed] [Google Scholar]
- Gray F, et al. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005;11(Suppl 3):16–22. doi: 10.1080/13550280500511741. [DOI] [PubMed] [Google Scholar]
- Gruters RA, et al. The advantage of early recognition of HIV-infected cells by cytotoxic T-lymphocytes. Vaccine. 2002;20:2011–2015. doi: 10.1016/s0264-410x(02)00089-0. [DOI] [PubMed] [Google Scholar]
- Gulzar N, Copeland KF. CD8+ T-cells: function and response to HIV infection. Curr HIV Res. 2004;2:23–37. doi: 10.2174/1570162043485077. [DOI] [PubMed] [Google Scholar]
- Haase AT. Pathogenesis of lentivirus infections. Nature. 1986;322:130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009 doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- Huang X, et al. CD 4+ T cells in the pathobiology of neurodegenerative disorders. J Neuroimmunol. 2009;211:3–15. doi: 10.1016/j.jneuroim.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MW, et al. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest. 2008;134:1287–1298. doi: 10.1378/chest.08-0364. [DOI] [PubMed] [Google Scholar]
- Ivey NS, et al. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol. 2009;15:111–22. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- Jones M, et al. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, et al. HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia. J Neuropathol Exp Neurol. 2002;61:992–1000. doi: 10.1093/jnen/61.11.992. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, et al. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kiertiburanakul S, Sungkanuparph S. Emerging of HIV drug resistance: epidemiology, diagnosis, treatment and prevention. Curr HIV Res. 2009;7:273–278. doi: 10.2174/157016209788347976. [DOI] [PubMed] [Google Scholar]
- Kim WK, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter AL, et al. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res Hum Retroviruses. 2007;23:438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- Kolb SA, et al. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, et al. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, et al. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;65:133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusi A, et al. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2009 doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lambotte O, et al. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2003;13:95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, et al. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 2003;13:195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182:3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, et al. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Marra CM, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. Aids. 2009 doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, et al. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- McArthur JC, et al. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McGee B, et al. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7:142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- Melik-Parsadaniantz S, Rostene W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198:62–68. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Meucci O, et al. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, et al. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Meucci O. AIDS and the brain: is there a chemokine connection? Trends Neurosci. 1999;22:471–479. doi: 10.1016/s0166-2236(99)01408-3. [DOI] [PubMed] [Google Scholar]
- Monteiro de Almeida S, et al. Relationship of CSF leukocytosis to compartmentalized changes in MCP-1/CCL2 in the CSF of HIV-infected patients undergoing interruption of antiretroviral therapy. J Neuroimmunol. 2006;179:180–5. doi: 10.1016/j.jneuroim.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Monteiro de Almeida S, et al. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J Neuroimmunol. 2005;169:144–152. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Gendelman HE. Unraveling the neuroimmune mechanisms for the HIV-1-associated cognitive/motor complex. Immunol Today. 1995;16:441–448. doi: 10.1016/0167-5699(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Nottet HS, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomed. 2009;4:557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong EL. Common AIDS-associated opportunistic infections. Clin Med. 2008;8:539–543. doi: 10.7861/clinmedicine.8-5-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofre G, et al. Scavenger receptor CD163 and its biological functions. Acta Medica (Hradec Kralove) 2009;52:57–61. [PubMed] [Google Scholar]
- Peng F, et al. Mechanisms of platelet-derived growth factor-mediated neuroprotection--implications in HIV dementia. Eur J Neurosci. 2008;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, et al. Adjunctive therapies for HIV-1 associated neurologic disease. Neurotox Res. 2005;8:161–166. doi: 10.1007/BF03033827. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, et al. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–3510. [PubMed] [Google Scholar]
- Persidsky Y, et al. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000;68:413–422. [PubMed] [Google Scholar]
- Petito CK, et al. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- Petito CK, et al. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. Journal of Neurovirology. 2006;12:272–283. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- Poluektova LY, et al. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- Potula R, et al. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165:815–824. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu H, et al. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab. 2005;25:1325–1335. doi: 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- Roberts ES, et al. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE) J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- Rostene W, et al. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Ruiz ME, et al. Peripheral blood-derived CD34+ progenitor cells: CXC chemokine receptor 4 and CC chemokine receptor 5 expression and infection by HIV. J Immunol. 1998;161:4169–4176. [PubMed] [Google Scholar]
- Schifitto G, et al. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66:919–921. doi: 10.1212/01.wnl.0000204294.28189.03. [DOI] [PubMed] [Google Scholar]
- Schifitto G, et al. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J Neurovirol. 2009;15:176–186. doi: 10.1080/13550280902758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklett BL, et al. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis. 2004;189:2202–2212. doi: 10.1086/421244. [DOI] [PubMed] [Google Scholar]
- Sharer LR, et al. Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol. 1985;16:760. doi: 10.1016/s0046-8177(85)80245-8. [DOI] [PubMed] [Google Scholar]
- Soulas C, et al. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem. 2003;84:169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- Tong N, et al. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Torheim EA, et al. Interleukin-10-secreting T cells define a suppressive subset within the HIV-1-specific T-cell population. Eur J Immunol. 2009;39:1280–1287. doi: 10.1002/eji.200839002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoupras AB, et al. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): a new insight in the drug therapy of HIV infection? AIDS Res Hum Retroviruses. 2008;24:1079–1086. doi: 10.1089/aid.2007.0263. [DOI] [PubMed] [Google Scholar]
- Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote D, et al. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. Proteomic modeling for HIV-1 infected microglia-astrocyte crosstalk. PLoS ONE. 2008a;3:e2507. doi: 10.1371/journal.pone.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. HIV-1-infected astrocytes and the microglial proteome. J Neuroimmune Pharmacol. 2008b;3:173–186. doi: 10.1007/s11481-008-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhorpe CL, et al. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J Leukoc Biol. 2009;85:1027–1035. doi: 10.1189/jlb.0808501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS Inflammation and Macrophage/Microglial Biology Associated with HIV-1 Infection. J Neuroimmune Pharmacol. 2009 doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Matano T. Anti-HIV adaptive immunity: determinants for viral persistence. Rev Med Virol. 2008;18:293–303. doi: 10.1002/rmv.577. [DOI] [PubMed] [Google Scholar]
- Yao H, et al. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Gendelman HE. The HIV-1 associated dementia complex: a metabolic encephalopathy fueled by viral replication in mononuclear phagocytes. Curr Opin Neurol. 1997;10:319–325. [PubMed] [Google Scholar]