Abstract
As new disease threats arise and existing pathogens grow resistant to conventional interventions, attention increasingly focuses on the development of vaccines to induce protective immune responses. Given their admirable safety records, protein subunit vaccines are attractive for widespread immunization, but their disadvantages include poor immunogenicity and expensive manufacture. We show here that engineered Escherichia coli outer membrane vesicles (OMVs) are an easily purified vaccine-delivery system capable of greatly enhancing the immunogenicity of a low-immunogenicity protein antigen without added adjuvants. Using green-fluorescent protein (GFP) as the model subunit antigen, genetic fusion of GFP with the bacterial hemolysin ClyA resulted in a chimeric protein that elicited strong anti-GFP antibody titers in immunized mice, whereas immunization with GFP alone did not elicit such titers. Harnessing the specific secretion of ClyA to OMVs, the ClyA-GFP fusion was found localized in OMVs, resulting in engineered recombinant OMVs. The anti-GFP humoral response in mice immunized with the engineered OMV formulations was indistinguishable from the response to the purified ClyA-GFP fusion protein alone and equal to purified proteins absorbed to aluminum hydroxide, a standard adjuvant. In a major improvement over current practice, engineered OMVs containing ClyA-GFP were easily isolated by ultracentrifugation, effectively eliminating the need for laborious antigen purification from cell-culture expression systems. With the diverse collection of heterologous proteins that can be functionally localized with OMVs when fused with ClyA, this work signals the possibility of OMVs as a robust and tunable technology platform for a new generation of prophylactic and therapeutic vaccines.
Keywords: protein subunit, adjuvant
Vaccines have widespread impact on human health by conferring protection against infectious diseases. Among traditional vaccine designs, protein subunit vaccines are particularly attractive, because they induce a protective immune response and avoid the safety limitations of attenuated or killed whole organisms. Despite their advantages in safety and purity, a major limitation of protein subunit antigens is their inability to stimulate strong immune responses in vivo when administered alone. To improve their immunogenicity, subunit antigens are often combined with adjuvants (1), conjugated to polysaccharide or protein carriers (2, 3), or formulated in controlled-release systems.
Controlled-release technologies have emerged as promising strategies for antigen delivery, because they are similar in geometry to naturally occurring pathogens and are readily internalized by antigen-presenting cells. These delivery systems primarily comprise polymer particles (4), immune-stimulating complexes (5), and liposomes, proteosomes, and related vesicles (6–9). Although these formulations seem to be technically appealing solutions, the complex manufacturing steps required to purify and encapsulate antigens in particulate delivery systems can render these approaches economically nonviable (10).
There are currently two vaccines for serogroup B meningococcal disease that are formulations consisting of bacterial surface antigens naturally incorporated in outer membrane vesicles (OMVs) (11, 12). OMVs are nanoscale (∼100 nm) proteoliposomes that constitutively bud from the outer membrane of Gram-negative bacteria (13, 14), and they contain components derived from the bacterial outer membrane and periplasm. In contrast to synthetic particulate systems, these OMV vaccines represent a unique system where the antigen and delivery vehicle together are derived from the Neisseria meningitidis pathogen itself (15, 16). The proven safety and efficacy records of these OMV vaccines, particularly in The Netherlands (17) and in Norway (12, 18), together with the knowledge that vesicles are produced by nearly all species of Gram-negative bacteria (including Escherichia coli), present the possibility of employing OMVs for the delivery of recombinant protein antigens. To date, however, antigens that are foreign to the parental bacteria remain notably absent from OMVs largely because of challenges associated with the transport of heterologous proteins to the vesicles (19).
ClyA (also referred to as SheA or HlyE) is a proteinaceous bacterial hemolysin that is specifically enriched in OMVs (20, 21). Recent work by our group showed that genetic fusion with ClyA results in the efficient translocation of heterologous proteins to OMVs (22). Additionally, Galen et al. (23) showed that antigens fused to ClyA exhibit immunostimulatory activity when secreted from a live Salmonella vaccine.
Building on observations regarding ClyA functionality, this study examines the ability of ClyA to serve as a platform to enhance the immunogenicity of a recombinant subunit antigen and to simplify antigen purification from cell-culture expression systems through secretion to OMVs. In this first demonstration of an engineered OMV vaccine, delivery of a poorly immunogenic green-fluorescent protein (GFP) antigen fused with ClyA in engineered OMVs elicited strong and sustained humoral responses in immunized mice, which were equivalent to the responses elicited by the standard adjuvant aluminum hydroxide. With the diversity of heterologous proteins that are able to be functionally localized in OMVs (22), this report illustrates the potential of engineered OMVs to deliver poorly immunogenic subunit antigens without the lengthy and expensive antigen-purification schemes necessary for traditional subunit vaccines or antigen-delivery systems.
Results
The Immunogenicity of GFP in Mice Is Significantly Enhanced when Administered in Fusion with ClyA.
ClyA, GFP, and ClyA-GFP were overexpressed in E. coli DH5α and purified from the soluble fractions of the bacteria (SI Text and Fig. S1). The immunostimulatory effects of these soluble proteins were then tested in mice; s.c. immunization of BALB/c mice with ClyA-GFP elicited GFP-reactive antibody responses that were significantly higher than immunization with ClyA mixed with GFP (Fig. 1). A GFP-specific IgG response was detected beginning 2 weeks after priming in mice immunized with ClyA-GFP; this response was augmented by booster vaccination and sustained for 4 weeks after the booster injection. No detectable anti-GFP IgG antibodies were observed until after boosting in any of the other treatment groups. Interestingly, immunization with GFP elicited little to no detectable response at any time during the study period, whereas in two mice, immunization with ClyA alone triggered fluctuating levels of GFP cross-reactive antibody species after the booster. Antibody titers in the ClyA-GFP immunization group (group III) were significantly higher (P < 0.05) than antibody titers in mice vaccinated with unfused proteins (group IV). The difference was statistically significant beginning at day 14 of the study and remained so through the conclusion of the study period. GFP-specific antibody levels in the treatment group immunized with ClyA and GFP separately (group IV) were statistically similar to the antibody levels generated by ClyA immunization alone (group II) at all time points throughout the study.
Fig. 1.
The immunogenicity of model antigen GFP in mice is significantly enhanced when administered in fusion with ClyA. Scatter dot plots (with median lines) show individual host anti-GFP IgG responses in serum diluted 1:12,800. Groups of five BALB/c mice were s.c. immunized with 2.5 μg GFP (group I), 2.5 μg ClyA (group II), 5 μg ClyA-GFP fusion (group III), and 2.5 μg ClyA mixed with 2.5 μg GFP (group IV). Mice were immunized on day 0 and day 28, which is marked by the arrowheads in each chart. The daggers (†) represent statistical significance (P < 0.05; Wilcoxon rank-sum test) of antibody titers in group III compared with titers in groups II and IV.
ClyA-GFP Is Localized in Engineered OMV Vaccines.
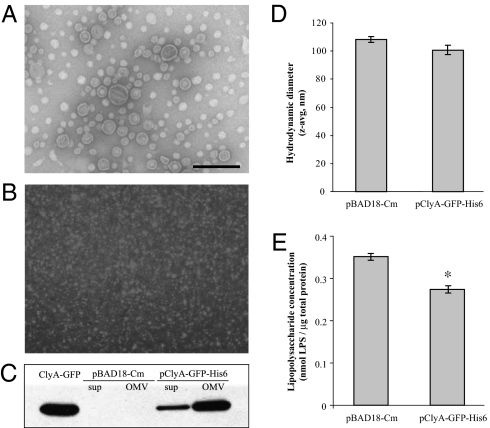
OMVs were prepared from vesicle hyper-producing E. coli strain JC8031 transformed with plasmid pClyA-GFP-His6 (engineered OMVs) or the empty pBAD18-Cm cloning vector (empty, wild-type OMVs). Electron microscopic analysis after negative staining showed the spherical bilayered structure of OMVs (Fig. 2A), which have a diameter of ∼100 nm. ClyA-GFP fluorescence was observed in association with the engineered OMVs (Fig. 2B), a finding confirmed by Western blotting with anti-GFP antibodies (Fig. 2C). Fluorescence-intensity measurements and SDS/PAGE gel-band densitometry indicated that ClyA-GFP comprises ∼5% of the total protein content in OMVs. Because expression of clyA in E. coli is strongly repressed under normal laboratory conditions (24), no free ClyA was detected in the engineered or empty OMVs preparations. Consistent with earlier observations (22), the association of ClyA-GFP in engineered OMVs had no apparent effect on the size of vesicles (Fig. 2D). Bacterial lipopolysaccharide (LPS) content in empty and engineered OMVs was measured by a colorimetric assay to detect 2-keto-3-deoxyoctonate (KDO), a core sugar component of LPS. The KDO assay indicated that empty OMVs contain a slightly higher concentration of LPS, normalized by total protein content, than engineered OMVs (Fig. 2E).
Fig. 2.
The model antigen GFP is functionally localized in engineered OMVs when fused with ClyA. (A) Electron micrograph of empty OMVs stained by uranyl acetate. (Scale bar: 200 nm.) This image is also representative of engineered OMVs containing ClyA-GFP (cf. reference 22). (B) Fluorescence micrograph (100× objective) of ClyA-GFP in association with engineered OMVs. (C) Western blot with anti-GFP antibodies of cell-free culture supernatants and OMV suspensions from cultures of E. coli expressing the empty plasmid vector or ClyA-GFP. (D) Z-average hydrodynamic diameter of empty and engineered OMV suspensions in PBS, as measured by dynamic light scattering. (E) LPS content in empty and engineered OMV suspensions is normalized by total protein content. The asterisk (*) denotes statistical significance (P < 0.05; Student’s t test).
The Immunogenicity of ClyA-GFP in Engineered OMV Vaccines Is Equivalent to the Immunogenicity of the Purified ClyA-GFP Fusion Protein Alone.
To examine the immunogenicity of ClyA-GFP in outer membrane vesicles, BALB/c mice were immunized with empty OMVs mixed with ClyA-GFP or engineered OMVs containing the ClyA-GFP fusion protein (Fig. 3). The effective dose of ClyA-GFP in the OMV formulations (2.5 μg) was one-half the amount used in these purified protein immunizations to observe if ClyA-GFP association with OMVs contributes any additional immunostimulatory effect. Immunization with empty OMVs mixed with soluble ClyA-GFP (group V) resulted in a GFP-specific response beginning at 2 weeks after priming and continuing for 4 weeks after the booster. Immunization with ClyA-GFP in engineered OMVs (group VI) elicited similar GFP-reactive IgG responses starting 2 weeks after the initial dose that then markedly increased after booster immunization at day 28. At all time points throughout the study, antibody titers between group V and group VI remained statistically indistinguishable. Additionally, antibody titers in OMV-immunized mice (both groups V and VI) were statistically equivalent to those in group III with an exception on day 56 for group V and on day 35 for group VI, when titers were significantly higher in group III (P < 0.05). Despite the presence of LPS detected in the OMV formulations, no significant inflammatory responses were observed in animal subjects. In addition, local reactions to vaccination, as determined by histological analysis of excised tissue, were indistinguishable between groups treated with the formulations with or without depletion of LPS from vaccine preparations, indicating that the adjuvant effect was a function of the OMV and not LPS (SI Text and Table S1 and Figs. S2 ‒ S5).
Fig. 3.
ClyA-GFP localized in engineered OMVs retains its immunogenicity in mice. Scatter dot plots (with median lines) show individual host anti-GFP IgG titers in serum diluted 1:12,800. Groups of five BALB/c mice were immunized with purified ClyA-GFP fusion protein mixed with empty OMVs (group V) and ClyA-GFP fusion in association with engineered OMVs (group VI). The effective dose of ClyA-GFP in both treatment groups was 2.5 μg. Mice were immunized on day 0 and day 28, which is marked by the arrowheads in each chart. The asterisks (*) denote statistically significant differences (P < 0.05; Wilcoxon rank-sum test) compared with antibody titers in the purified ClyA-GFP treatment group (group III) on the corresponding day.
Antibody Titers Elicited by ClyA-GFP in Engineered OMV Vaccines Are Equivalent to Those Elicited by the Adjuvant Aluminum Hydroxide (AlOH3, alum).
To directly compare the level of immune response elicited by the engineered OMV vaccines with a standard adjuvant, mice were immunized with the OMV formulations or with GFP or ClyA-GFP absorbed to aluminum hydroxide. As shown in Fig. 4, the antibody responses to all four preparations were statistically equivalent, suggesting that engineered OMV preparations are potentially viable candidates for further clinical development and evaluation.
Fig. 4.
Head-to-head comparison of engineered OMVs with the clinical standard adjuvant, aluminum hydroxide (alum). Scatter dot plots show individual host anti-GFP IgG titers in serum diluted 1:1.600 at day 56. Groups of five BALB/c mice were immunized with purified GFP absorbed to alum, ClyA-GFP absorbed to alum, ClyA-GFP mixed with empty OMVs, or ClyA-GFP fusion in engineered OMVs. The effective dose for each group was 2.5 μg. Mice were immunized on day 0 and 28. No statistical differences in antibody titers were observed among the groups (Wilcoxon rank-sum test).
Discussion
Vaccination remains one of the most cost-effective strategies for preventing infectious disease (25, 26). The safety of protein subunit vaccines makes them particularly attractive for administration to wide swaths of the human population, healthy and immunocompromised individuals alike. A major limiting factor in the further development of subunit vaccines, however, remains the poor immunogenicity of purified antigens when administered alone. Despite ongoing research to novel immune potentiators, the only compounds currently licensed for human use in North America remain aluminum salts (27). In addition to increasing the cost of vaccine production, these aluminum adjuvants may sometimes result in severe adverse events postadministration (28, 29). With ongoing disease threats and the promise of emerging immunotherapies on the horizon, there clearly is strong demand for new vaccine technologies.
Owing largely to their size, plasticity, and safety profile in humans, OMVs are attractive vehicles for vaccine delivery. After the first reports of OMV vaccines for serogroup B meningococcal disease, vesicles from Salmonella thyphimurium and Pseudomonas aeruginosa containing surface antigens native to the pathogens have also been shown to exhibit immunogenic properties (30, 31). The prospects of using OMVs to deliver antigens that are nonnative to the parental bacteria are not yet realized, largely because of challenges in transporting heterologous proteins to vesicles. In a recent demonstration of the remarkable tunability of OMVs by our group, heterologous proteins fused with the native bacterial protein ClyA are efficiently transported in their native functional forms to vesicles (22). Previous work also points to the utility of ClyA in enhancing the immunogenicity of antigens secreted from a live attenuated Salmonella vector, further suggesting the possibility that antigens may be exported from live vectors in the form of OMVs (23).
The work presented here illustrates the potential of engineered OMVs to act as a potent adjuvant and carrier system for poorly immunogenic subunit antigens. Specifically, an OMV vaccine engineered to contain a model GFP antigen elicited strong GFP-specific humoral responses in immunized mice, whereas immunization with GFP alone failed to result in any significant antibody responses. GFP was chosen as the model antigen, because it constitutes a relatively weak antigen in and by itself (3). A salient advantage in the production of recombinant OMV overpurified proteins lies in vesicle purification by facile ultracentrifugation, which effectively eliminates the need for costly infrastructure to support a priori antigen purification. The results from this study introduce engineered OMVs as a versatile strategy for delivering poorly immunogenic protein antigens, while simultaneously sidestepping the complex purification processes of traditional subunit vaccines.
Expression of the engineered ClyA-GFP fusion protein in E. coli resulted in a 61-kDa product that retained the native biological activities of ClyA and GFP (Fig. S1). The specific fluorescence and hemolysis activities of ClyA-GFP under the specified assay conditions were diminished relative to the unfused constituent proteins, which were similar to previous observations made with other ClyA fusion partners (32). The retention of protein activity indicates that ClyA-antigen fusions maintain their conformations and suggests that ClyA may be an amenable carrier for both linear epitopes and conformational antigens.
Naïve mice were immunized with ClyA, GFP, the soluble ClyA-GFP fusion protein, and ClyA mixed with GFP. The results in Fig. 1, depicting antibody titers in mice immunized with the purified proteins, show ClyA to be an effective carrier protein for enhancing the immunogenicity of GFP. The immunogenicity of ClyA fusion proteins was first shown in a study by Galen et al. (23), where the observed enhancement in the immune response to the antigens was postulated to be largely dependent on export from a live Salmonella vector. Here, the results show that antigen fusion with ClyA is directly responsible for enhanced immunogenicity. Residual LPS was not detectable with the KDO assay in any of the purified protein preparations, suggesting that the enhanced immune response to GFP was a result of fusion with the ClyA moiety. As a bacterial cytolytic protein from enterobacteria (33), ClyA joins the ranks of other known toxins with adjuvant properties such as diphtheria toxin or heat-labile enterotoxin (34). Although no pathological effects were observed in mice immunized with ClyA in this study, detoxification of ClyA through mutation (35), truncation, or chemical methods may attenuate any possible toxicity while retaining its immunomodulatory capabilities.
Unlike other known bacterial toxin adjuvants, however, enhancement in the immune response to GFP was not observed when mice were immunized with a mixture of soluble ClyA and soluble GFP. Whereas the specific mechanisms that drive the immunostimulatory behavior of ClyA remain to be investigated, physical or chemical conjugation to protein carriers has frequently improved the immunogenicity of otherwise poorly immunogenic antigens by increasing the antigen size or improving antigen organization to optimize the B cell response (2, 3, 36). In general, the ability for antigens to be presented in the proper structure to activate B cell proliferation requires efficient antigen processing by-antigen presenting cells and costimulation by T helper cells. Further studies on ClyA and its interaction with the immune system are necessary to more fully characterize the immune-enhancing activity of ClyA and to explore the potential to simulate T cell, especially cytotoxic, responses.
Leveraging the secretion of ClyA to OMVs, engineered OMVs were purified from the nonpathogenic E. coli strain JC8031 expressing ClyA-GFP (Fig. 2). Fluorescence microscopy and Western blotting with anti-GFP antibodies verified the presence of ClyA-GFP in engineered OMVs; association of the active fusion protein with vesicles occurred through secretion and thus required no separate antigen purification, unlike the cases of other established particulate-delivery systems.
To examine the humoral immune response to ClyA-GFP when administered with OMVs, mice were immunized with empty (wild-type) OMVs mixed with soluble ClyA-GFP (Fig. 3, group V) or with the engineered OMV formulation containing ClyA-GFP (Fig. 3, group VI). It is generally observed that particulate antigens are more efficiently recognized by the immune system than soluble proteins (37). Therefore, to examine if ClyA-GFP association with OMVs contributes additional immunostimulatory effect over the purified protein alone, the effective dose of ClyA-GFP in the OMV formulations (2.5 μg) was one-half the amount used in the purified protein immunizations. The results show that the immunostimulatory behavior of ClyA-GFP is retained when delivered in vesicles. On day 35 of the study, anti-GFP titers in the recombinant OMV group (Fig. 3, group VI) were lower than in the group immunized with purified ClyA-GFP (Fig. 1, group III); however, the titers in these two treatment groups were statistically indistinguishable from each other beginning at day 42. The decreased ClyA-GFP dosage in the OMV vaccine formulation may be a factor in the slightly delayed immune response to engineered OMVs (at day 35) compared with the purified fusion protein.
The anti-GFP antibody titers observed in response to the engineered OMV immunizations are remarkable, especially when taking into account that the effective dose of the ClyA-GFP fusion protein in the OMV formulations was one-half that of the purified protein treatments. The results suggest that OMVs contribute additional immune-stimulating activities. As judged from the effect of the OMV preparations in inoculated mice, the low levels of residual LPS in OMVs at the administered doses did not cause a local inflammatory response (SI Text, Table S1 and Figs. S2 ‒ S5). However, because LPS can be counterproductive in vaccines, future work will examine the immunogenic effect of total LPS depletion from vesicles (38). As the immune-potentiation capabilities of ClyA become better understood, the combination of engineered OMVs with additional adjuvants or immunostimulatory compounds may also be of considerable interest in directing a specific immune response.
Comparisons between animals treated with the wild-type and engineered OMV formulations (Fig. 3) suggest that anti-GFP titers are largely independent of the direct association of ClyA-GFP with OMVs. There remains a possibility that the nonspecific association of purified ClyA-GFP with empty vesicles may render the two tested OMV formulations virtually indistinguishable to the immune system. An exception may be that the engineered OMV treatments seemed to result in a prolonged antibody response. At day 56, the antibody titers in group V (empty OMVs mixed with ClyA-GFP) were statistically decreased relative to group III (purified ClyA-GFP), whereas the titers in the engineered OMV treatment group exhibited no such decrease. Analogous to other particulate-delivery systems, the colocalization of antigens in the lumen of OMVs may protect antigens from protease degradation in vivo, resulting in more efficient B cell activation.
Importantly, a direct comparison of the engineered OMV preparations to a standard adjuvant, aluminum hydroxide (AlOH3 or alum), revealed that the antibody responses generated in both cases were equivalent. Given that alum is the basis of the only FDA-approved adjuvants and that new adjuvants continuously are sought, we conclude that the concept of engineered OMV vaccines may hold clinical promise. Work testing the broader applicability of OMV vaccines is currently ongoing in our laboratories.
The results here show that engineered OMVs are unique particulate vaccine-delivery vectors with adjuvant and carrier activity that combine features borrowed from both synthetic and living antigen-delivery systems. Whereas OMVs are derived from live bacterial cultures, vesicles themselves are nonreplicating entities that avoid the potential safety concerns associated with attenuated living bacteria or viruses. As nanoscale spherical structures comprising mainly protein and lipid, OMVs may share some structural or compositional similarity with liposome or proteosome carriers; but in the most significant departure from these particulate systems or other subunit vaccines, where as many as three components—antigen, adjuvant, and delivery system—are produced separately before formulated into a final product, engineered OMVs may not require these extensive purification and formulation procedures.
This work illuminates the potential of engineered OMV technology to overcome the significant safety and economic limitations that often arise in the course of vaccine development. A model green-fluorescent protein fused with the bacterial hemolysin ClyA was secreted in OMVs of E. coli, while maintaining the biological activity of both components. These engineered vesicles were administered to mice and found to be highly immunogenic, eliciting high anti-GFP antibody titers, whereas immunization with GFP alone failed to elicit any significant humoral response. Additionally, the engineered OMV formulation elicited a humoral response that is on par with the standard adjuvant aluminum hydroxide. Combining adjuvant and carrier activity, engineered OMVs enhance the response to an otherwise poorly immunogenic antigen and circumvent the protein-purification requirements of traditional subunit vaccines and particulate antigen-delivery modalities. With the wide assortment of heterologous proteins that are functionally localized to OMVs by fusion with ClyA, including enzymes, fluorescent proteins, and single-chain antibody fragments (22), and with additional possibilities of further engineering of vesicle luminal or surface features, OMVs are a uniquely tunable platform with ease of manufacture for the delivery of a wide spectrum of poorly immunogenic subunit antigens for vaccines against infectious disease or other therapeutic targets.
Materials and Methods
Recombinant Protein Purification.
Plasmid construction was performed as described in SI Text. Cultures of E. coli DH5α were grown in 100 mL of LB medium containing chloramphenicol. Protein expression was induced by the addition of L-arabinose to a final concentration of 0.2% after the OD600 reached ∼0.5. Bacterial cultures were harvested 4 hours after induction and lysed by treatment with lysozyme (1 mg/mL) and Triton X-100 (1% vol/vol). The polyhistidine-tagged proteins in the soluble fraction were purified by immobilized-metal affinity chromatography (Ni-NTA Agarose; Qiagen) according to the manufacturer’s instructions. The proteins were eluted with 200 mM imidazole in a buffer containing 50 mM NaH2PO4 and 300 mM NaCl (pH 8.0), and they were subsequently desalted into PBS using PD-10 size exclusion chromatography columns (Amersham Biosciences).
Preparation of OMVs.
OMVs were purified in accordance with a previously established procedure (39). Plasmids pClyA-GFP-His6 and pBAD18-Cm were transformed into E. coli vesicle-overproducing strain JC8031 (40), and they were selected in LB-chloramphenicol medium. Flasks containing 250 mL of medium were inoculated with overnight culture and allowed to grow until the OD600 reached ∼0.5. Protein expression was induced by addition of L-arabinose to a final concentration of 0.2%. Cell-free culture supernatants were collected 12 hours after induction and filtered through a 0.45-μm filter. Vesicles were isolated by ultracentrifugation (Beckman-Coulter; Ti SW28 rotor, 141,000 × g, 3 hours, 4 °C) and resuspended in PBS. For removal of LPS from OMV preparations, samples were passed through Detoxi-Gel columns (Pierce) according to the manufacturer’s instructions.
Protein Analyses.
Protein concentrations in OMV and purified recombinant protein preparations were quantified by the bicinchoninic-acid assay (BCA Protein Assay; Pierce) with BSA as the protein standard. Fluorescence activity of GFP in protein or OMV samples was measured in a microplate spectrofluorometer (Gemini EM; Molecular Devices) using excitation and emission wavelengths of 481 nm and 507 nm, respectively (41). For SDS/PAGE, samples were prepared in sample-loading buffer containing β-mercaptoethanol and heated at 100 °C for 5 min before electrophoresis on 10% polyacrylamide gels. Proteins were transferred to polyvinylidine difluoride membranes for Western blot analysis and probed with monoclonal mouse anti-GFP IgG (Invitrogen; 1:2,000) or monoclonal mouse anti-polyHistidine IgG (1:3000; Sigma) primary antibodies, and horseradish peroxidase conjugated goat anti-mouse IgG (1:10,000; Jackson Immunoresearch) secondary antibody. Membranes were developed by autoradiography with ECL detection reagents (GE Healthcare).
Liquid Hemolysis Assay.
The hemolytic activity of ClyA and ClyA-GFP was measured by a liquid hemolysis assay adopted from Rowe and Welch (42). Sheep erythrocytes (Becton Dickinson) were washed and diluted 1:100 in PBS. Aliquots of washed erythrocytes were transferred to microcentrifuge tubes, and ClyA or ClyA-GFP was added to reach the appropriate concentration in a final volume of 1 mL in PBS. The samples were incubated at 37 °C for 30 min with gentle rotation. Cells and debris were sedimented in a microcentrifuge (4,000 × g, 1.5 min), and the released hemoglobin in the supernatant was quantified by spectrophotometric detection at a wavelength of 540 nm. Hemolysis activity was reported relative to erythrocytes lysed in deionized water for 30 min at 37 °C, which was considered 100% lysis.
Dynamic Light Scattering.
Dynamic light-scattering measurements were performed with the Nanosizer Nano ZS (Malvern Instruments) using Dispersion Technology Software version 4.20 for data acquisition and analysis. OMV samples contained 60 μg/mL total protein in 1 mL of PBS. The refractive index and viscosity of water were used as parameter inputs.
Microscopy.
For electron microscopy, vesicles were negatively stained with 2% uranyl acetate on 400-mesh Formvar carbon-coated copper grids and viewed in a FEI Tecnai F20 transmission electron microscope. For fluorescence microscopy, vesicles were placed on a glass slide, sealed with a coverslip, and examined using an Olympus BX41 microscope with GFP filter set.
LPS Analyses.
Bacterial LPS concentrations were determined by measuring the presence of KDO according to a previously described colorimetric assay (43, 44). KDO forms part of the oligosaccharide core of the bacterial LPS molecule, and E. coli LPS contains two reactive KDO moieties per molecule (45). OMV samples (45 μL) in PBS were combined with 0.2 N H2SO4 (5 μL) and heated at 100 °C for 20 min. The samples were cooled to room temperature for 5 min, and then 25 μL of 0.04 M NaIO4 was added to the mixture. After 20 min of incubation at room temperature, 2% NaAsO2 (65 μL) was added to the sample tubes, and they were vortexed until the characteristic yellow color disappeared. Thiobarbituric acid (0.3%, 250 μL) was added, and the samples were returned to the boiling-water bath for 10 min, followed by the immediate addition of dimethyl sulfoxide (125 μL). The samples were cooled to room temperature for 5 min, and the absorbance was quantified at 550 nm in a microplate spectrophotometer. Calibration standards were prepared from KDO ammonium salt (Sigma-Aldrich).
Immunization.
Six groups of five BALB/c mice (Charles River Laboratories) each were immunized s.c. with 100 μL of PBS containing purified protein or OMV preparations as described. The six treatment groups were immunized with 2.5 μg GFP (group I), 2.5 μg ClyA (group II), 5 μg ClyA-GFP (group III), 2.5 μg ClyA mixed with 2.5 μg GFP (group IV), 2.5 μg ClyA-GFP mixed with empty OMVs (group V), and recombinant OMVs equivalent to 2.5 μg ClyA-GFP (group VI). Two doses of vaccine were administered 4 weeks apart. Blood was collected from the mandibular sinus immediately before and 2 weeks after the first immunization, immediately before the booster dose, and at weekly intervals thereafter. The protocol for the animal studies was approved by the Institutional Animal Care and Use Committee at Cornell University (protocol number 2008–0092).
ELISA.
GFP-specific antibodies mounted in immunized mice were measured by indirect ELISA. Polystyrene microtiter 96-well plates (Maxisorp; Nunc Nalgene) were coated with GFP [5 μg/mL in carbonate buffer (pH 9.6)] and incubated overnight at 4 °C. Plates were blocked with 3% nonfat dry milk (Bio-Rad) in PBS containing 0.05% Tween-20 (PBST). Samples were serially diluted 2-fold in blocking buffer in a range of 1:200–204,800, added to the wells, and incubated for 1.5 hours in a humidified chamber at 37 °C. Plates were washed six times with PBST, and horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Jackson Immunoresearch) was added to the wells for 1 hour at 37 °C. After six additional washes with PBST, 3,3′,5,5′ tetramethylbenzidine substrate (1-Step Turbo TMB; Pierce) was added, and the enzyme reaction proceeded for 20 min. The reaction was stopped with 2 M H2SO4. The absorbance was quantified in a microplate spectrophotometer at a wavelength of 450 nm. Statistical significance between treatment groups was determined by the nonparametric Wilcoxon rank-sum test, and P < 0.05 was considered significant.
Comparison of Engineered OMVs to Alum.
Purified GFP and ClyA-GFP (10 μg) were absorbed to Alhydrogel (AlOH3, 25 μg) at either 4 °C (overnight) or room temperature (1 hour) in PBS and subjected to SDS/PAGE to ensure that no protein migrated into the gel and that it was fully absorbed to the adjuvant. Neither protein migrated into the gel under both incubation conditions. Four formulations were prepared and evaluated in BALB/c mice (Charles River Laboratories): (i) GFP absorbed to Alhydrogel, (ii) ClyA-GFP absorbed to Alhydrogel, (iii) ClyA-GFP mixed with empty wild-type OMVs, and (iv) engineered OMVs containing ClyA-GFP. Each preparation was formulated to provide 2.5 μg of injectable protein per mouse. Each Alhydrogel formulation contained 1.3% (mass:volume) aluminum hydroxide. Two doses of vaccine were administered 4 weeks apart. Blood was collected from the mandibular sinus on day 56 after the first immunization. Antibody titers were determined by ELISA as described above.
In Vivo Inflammation Study.
Two days after injection, subjects were euthanized, and formalin-fixed tissues were processed routinely for histopathology and stained with hematoxylin and eosin for light microscopic evaluation. Slides were read and graded in a blinded fashion by a board-certified pathologist. Changes were given an overall score based on the grade of inflammation, edema, and tissue damage as well as the presence or absence of vasculitis or vascular thrombi. Relative numbers of individual cell types of inflammatory cells were scored on a scale of 1–4. Sections scored as 1 had mild lesions, consisting mainly of edema, with mild infiltration of neutrophils and macrophages; sections scored as 4 had extensive effacement of normal architecture by degenerate and viable neutrophils, cellular debris, macrophages, pools of fibrin, and vasculitis with intravascular fibrin thrombi.
Supplementary Material
Acknowledgments
We thank Prof. Abraham Stroock for insightful discussions on this manuscript. Additionally, we thank Elizabeth Craig for initial histological evaluation of tissues and the staff of the Biomedical Sciences Histopathology laboratory for processing the samples from the inflammation study, Dr. Gillian Perkins for assistance with the animal experimentation, and Dr. Gerlinde Van de Walle and Dr. Jae-Young Kim for technical assistance. This work was supported by National Institutes of Health Grant R21EB005669-01 (to D.P. and M.P.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805532107/DCSupplemental.
References
- 1.O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu YM, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci USA. 2006;103:18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koser ML, et al. Rabies virus nucleoprotein as a carrier for foreign antigens. Proc Natl Acad Sci USA. 2004;101:9405–9410. doi: 10.1073/pnas.0403060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Chakrapani A, O’Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6:797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- 5.Morein B, Sundquist B, Höglund S, Dalsgaard K, Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature. 1984;308:457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- 6.Lowell GH, et al. Proteosome-lipopeptide vaccines: Enhancement of immunogenicity for malaria CS peptides. Science. 1988;240:800–802. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- 7.Lowell GH, Smith LF, Seid RC, Zollinger WD. Peptides bound to proteosomes via hydrophobic feet become highly immunogenic without adjuvants. J Exp Med. 1988;167:658–663. doi: 10.1084/jem.167.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felnerova D, Viret JF, Glück R, Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518–529. doi: 10.1016/j.copbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Copland MJ, Rades T, Davies NM, Baird MA. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Biol. 2005;83:97–105. doi: 10.1111/j.1440-1711.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 10.Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: Challenges and solutions. Nat Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 11.Oster P, et al. MeNZB: A safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–2196. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 12.Feiring B, et al. Persisting immune responses indicating long-term protection after booster dose with meningococcal group B outer membrane vesicle vaccine. Clin Vaccine Immunol. 2006;13:790–796. doi: 10.1128/CVI.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 15.Claassen I, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 16.Arigita C, et al. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine. 2004;22:629–642. doi: 10.1016/j.vaccine.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 17.de Kleijn ED, et al. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine. 2000;18:1456–1466. doi: 10.1016/s0264-410x(99)00423-5. [DOI] [PubMed] [Google Scholar]
- 18.Sandbu S, et al. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin Vaccine Immunol. 2007;14:1062–1069. doi: 10.1128/CVI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279:2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouokam JC, Wai SN. Outer membrane vesicle-mediated export of a pore-forming cytotoxin from Escherichia coli. Toxin Reviews. 2006;25:31–46. [Google Scholar]
- 21.Wai SN, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim J-Y, et al. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380:51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galen JE, et al. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004;72:7096–7106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westermark M, Oscarsson J, Mizunoe Y, Urbonaviciene J, Uhlin BE. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J Bacteriol. 2000;182:6347–6357. doi: 10.1128/jb.182.22.6347-6357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine MM, et al., editors. New Generation Vaccines. New York: Marcel Dekker, Inc.; 2004. [Google Scholar]
- 26.Rappuoli R, Miller HI, Falkow S. Medicine. The intangible value of vaccination. Science. 2002;297:937–939. doi: 10.1126/science.1075173. [DOI] [PubMed] [Google Scholar]
- 27.Schijns VEJC, Degen WGJ. Vaccine immunopotentiators of the future. Clin Pharmacol Ther. 2007;82:750–755. doi: 10.1038/sj.clpt.6100394. [DOI] [PubMed] [Google Scholar]
- 28.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff TC, Myers M. Workshop summary. Aluminum in vaccines. Vaccine. 2002;20(Suppl 3):S1–S4. doi: 10.1016/s0264-410x(02)00163-9. [DOI] [PubMed] [Google Scholar]
- 30.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 31.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Castillo FJ, Moreno F, del Castillo I. Secretion of the Escherichia coli K-12 SheA hemolysin is independent of its cytolytic activity. FEMS Microbiol Lett. 2001;204:281–285. doi: 10.1016/s0378-1097(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 33.Eifler N, et al. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 2006;25:2652–2661. doi: 10.1038/sj.emboj.7601130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavelle EC, Leavy O, Mills KHG. In: Vaccine Adjuvants: Immunological and Clinical Principles. Hackett CJ, Harn DA, editors. Totowa, NJ: Humana Press; 2006. pp. 111–154. [Google Scholar]
- 35.Wai SN, et al. Characterization of dominantly negative mutant ClyA cytotoxin proteins in Escherichia coli. J Bacteriol. 2003;185:5491–5499. doi: 10.1128/JB.185.18.5491-5499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmann MF, et al. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 37.Li M, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 38.Steeghs L, et al. Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: Influence of adjuvants on the immune response. Infect Immun. 1999;67:4988–4993. doi: 10.1128/iai.67.10.4988-4993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol. 1999;65:1843–1848. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubès R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 42.Rowe GE, Welch RA. Assays of hemolytic toxins. Methods Enzymol. 1994;235:657–667. doi: 10.1016/0076-6879(94)35179-1. [DOI] [PubMed] [Google Scholar]
- 43.Karkhanis YD, Zeltner JY, Jackson JJ, Carlo DJ. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 44.Herlax V, de Alaniz MJT, Bakás L. Role of lipopolysaccharide on the structure and function of alpha-hemolysin from Escherichia coli. Chem Phys Lipids. 2005;135:107–115. doi: 10.1016/j.chemphyslip.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Lee CH, Tsai CM. Quantification of bacterial lipopolysaccharides by the purpald assay: Measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal Biochem. 1999;267:161–168. doi: 10.1006/abio.1998.2961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.