Abstract
Inducible DNA repair via the base-excision repair pathway is an important prosurvival mechanism activated in response to oxidative DNA damage. Elevated levels of the essential base-excision repair enzyme apurinic/apyrimidinic endonuclease 1 (APE1)/redox effector factor-1 correlate closely with neuronal survival against ischemic insults, depending on the CNS region, protective treatments, and degree of insult. However, the precise mechanisms by which this multifunctional protein affords protection and is activated by upstream signaling pathways in postischemic neurons are not well delineated. Here we show that intracerebral administration of pituitary adenylate cyclase-activating polypeptide (PACAP), an endogenously occurring small neuropeptide, induces expression of APE1 in hippocampal neurons. Induction of APE1 expression requires PKA- and p38-dependent phosphorylation of cAMP response-element binding and activating transcription factor 2, which leads to transactivation of the APE1 promoter. We further show that PACAP markedly reduces oxidative DNA stress and hippocampal CA1 neuronal death following transient global ischemia. These effects occurred, at least in part, via enhanced APE1 expression. Furthermore, the DNA repair function of APE1 was required for PACAP-mediated neuroprotection. Thus, induction of DNA repair enzymes may be a unique strategy for neuroprotection against hippocampal injury.
Keywords: activating transcription factor 2, cAMP response-element binding, delayed neurodegeneration, DNA repair, oxidative stress
Oxidative stress is a hallmark of neurological disorders, including cerebral ischemia. Ischemic injury rapidly elicits oxidative DNA damage either directly via reactive oxygen species attack or indirectly via oxidization of lipids or proteins (1–3). These actions induce DNA lesions, involving base modifications and damage to the sugar moiety in the DNA, formation of abasic (apurinic/apyrimidinic, AP) sites, single strand breaks, and DNA intra- or interstrand crosslinks. Of these lesions, AP sites and single strand breaks are highly prevalent types of DNA damage in insulted neurons (4). Whereas the accumulation of unrepaired DNA ultimately induces cell death, active repair of DNA promotes cell survival (4–6).
In neurons, base-excision repair (BER) is the predominant mechanism for repair of oxidative DNA lesions (7). BER is a three-step process. First, 5′-acting AP endonuclease 1 (APE1, also known as redox-effector factor 1) cleaves the phosphodiester backbone, then β-polymerase or alternative repair proteins, such as FEN1 or PCNA, fill in the gap with newly synthesized nucleotides, and finally a DNA ligase seals the nick. In several models of ischemia, oxidative DNA damage is reversible in regions that survive (4, 6, 8), raising the possibility that repair of DNA damage via BER is required for cell survival following ischemia.
APE1 is an attractive target for neuroprotection, as it is a critical component of the BER process (9) and as also has redox and transcription-regulation activities. Following cerebral ischemia, APE1 expression and activity are closely correlated with cellular survival comparing different brain regions and in response to different experimental interventions. APE1 expression and BER activity are significantly depressed before the onset of cell death in regions severely damaged by cerebral ischemia (6, 10). In contrast, APE1 activity is enhanced by sublethal insults (6). APE1 expression and activity are associated with several neuroprotective paradigms, including ischemic preconditioning and overexpression of copper/zinc superoxide dismutase (4, 11), and overexpression of APE1 protects against oxidative cell death in cultured neurons (12). However, a direct contribution of APE1 to brain neuroprotection has yet to be established.
The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) was first identified by its adenylate cyclase-stimulating activity (13), and is well known to attenuate glutamate- or oxidative stress-induced neuronal death (for review see ref. 14). PACAP confers neuroprotection against both global (15) and focal (16) ischemia. However, the mechanisms underlying PACAP-induced neuroprotection are, as yet, unclear.
The present study was undertaken to identify mechanisms by which PACAP induces APE1 in vulnerable neurons and to document a role for APE1 in protection by PACAP against ischemic injury. We show that PACAP acts via cAMP response-element binding (CREB) and activating transcription factor 2 (ATF2) to markedly up-regulate APE1 expression and that the DNA repair capacity of APE1 is essential for PACAP-induced neuroprotection. These results establish the primary mechanism by which APE1 affords neuroprotection against cell death induced by global ischemia.
Results
PACAP Increases APE1 Activity and Decreases DNA Damage and Neuronal Death.
Global ischemia reduced APE1 mRNA and protein expression and DNA repair activity in neurons destined to die, evident 3 to 24 h after reperfusion (Fig. S1); the decrease in APE1 protein is in corroboration of others (10). In contrast, APE1 mRNA and protein expression and AP site-specific endonuclease activity were up-regulated in the resistant CA3 and DG (Fig. S1). PACAP is neuroprotective against ischemia-induced neuronal death (15), and we determined the dose of PACAP required to suppress DNA damage and protect postischemic CA1 neurons (Fig. S2). PACAP (0.2 nmol), administered starting 24 h before and 0.5, 6, and 24 h after ischemia suppressed DNA damage and protected CA1 pyramidal neurons. In addition, PACAP induced sustained increase in expression of APE1 mRNA (Fig. 1A), protein (Fig. 2A), and endonuclease activity (Fig. 2B) in the CA1 that lasted for more than 24 h after reperfusion. These findings indicate that the increases in APE1 expression and activity are associated with both relative resistance to ischemia and PACAP-mediated neuroprotection.
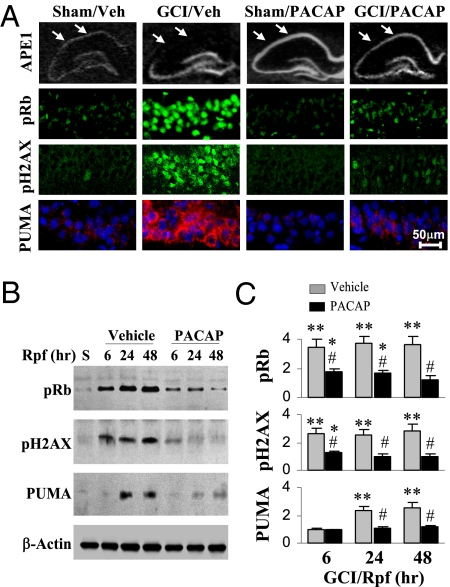
Fig. 1.
PACAP attenuates DNA-damage responsive signals after global cerebral ischemia (GCI). Rats received either vehicle (Veh) or PACAP infusions (0.2 nmol at −24, −12, and −6 h) and were then subjected to GCI or sham operation at 0 h. (A) In situ hybridization for APE1 mRNA (Top, arrows indicate the lateral portion of CA1) or immunostaining for retinoblastoma protein phosphorylated at Ser795 (pRb, green), histone H2AX phosphorylated at Ser139 (pH2AX, green), or PUMA (red, counterstained with Hoechst) 24 h after GCI on brain sections through the dorsal hippocampus. Arrows in the top panel represent the CA1 hippocampal region. Higher magnification of CA1 was used in the immunostained sections. (Scale bar, 50 μm). PACAP enhanced expression of APE1 mRNA but suppressed expression of DNA-damage responsive proteins in CA1 after GCI. (B) Representative Western blots show suppression by PACAP of expression of the same DNA-damage responsive proteins in CA1 at 6 to 48 h of reperfusion (Rpf) or 24 h after sham operation (S). (C) Graphs illustrate the semiquantitative results of B. Data are expressed as fold changes normalized to the sham group. *, P < 0.05; **, P < 0.01 vs. the sham group; #, P < 0.05 vs. vehicle controls; n = 5 per group.
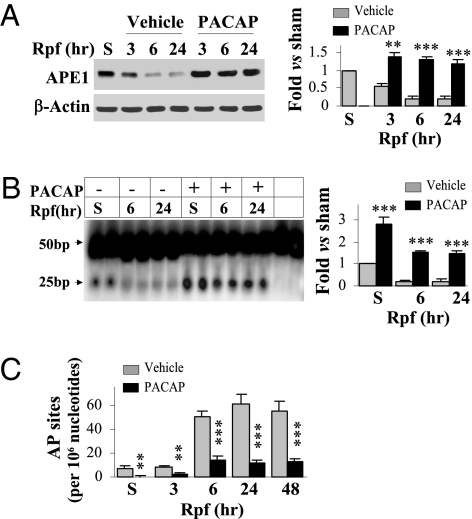
Fig. 2.
PACAP augments APE1 expression and activity in CA1 after GCI. Rats received either vehicle (Veh) or PACAP infusions (0.2 nmol at −24, −12, and −6 h) and were then subjected to GCI or sham operation at 0 h. Western blots for APE1 protein (A), the oligonucleotide incision assay for APE1 activity (B), and quantitative AP site measurement (C) were performed using CA1 extracts at the indicated time points after GCI or at 24 h after sham operation. **, P < 0.01; ***, P < 0.001 vs. vehicle controls; n = 5–6 per group. PACAP increased APE1 expression after GCI, augmented AP endonuclease activity, and reduced the levels of AP sites in sham-operated as well as GCI animals.
Because PACAP bolsters DNA repair and prevents neuronal death, PACAP would be expected to attenuate activation of DNA damage-responsive, prodeath molecules. Accordingly, PACAP reduced accumulation of ischemia-induced AP sites (Fig. 2C) and attenuated induction of the cell cycle protein retinoblastoma phosphorylated at Ser795, of histone H2AX phorphorylated at Ser139 (sensitive to DNA double-stranded breaks), and of the p53 mobilizing protein, PUMA, in postischemic CA1 (Fig. 1). In addition, PACAP improved the performance of postischemic rats in a water maze (Fig. S3 A and B), and protected a significant portion of CA1 neurons as late as 60 d following global ischemia (Fig. S3C). Thus, PACAP-elicited neuroprotection, assessed histologically and functionally, is associated with repression of prodeath signaling induced by DNA damage in postischemic CA1 neurons.
APE1 Knockdown Abrogates Neuroprotection Against Cerebral Ischemia.
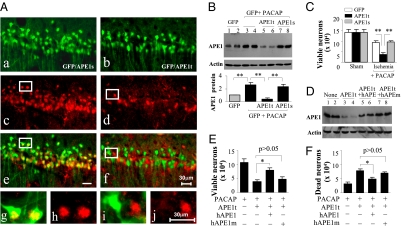
Given the robust increase in APE1 DNA repair activity induced by PACAP, we hypothesized that augmentation of DNA repair significantly contributes to PACAP-mediated neuroprotection. To address this concept directly, we transduced lentiviral vectors containing either shRNA targeted to APE1 (APE1t) or a scrambled control shRNA (APE1s) directly into CA1 of living rats along with a lentiviral vector for GFP. APE1t-transduced cells, as indicated by GFP expression, but not APE1s-transduced cells, exhibited significantly decreased APE1 expression in CA1 neurons (Fig. 3A) and Western blot showed reduction in APE1 by APE1t but not by APE1s (Fig. 3B). APE1t, but not APE1s, attenuated PACAP-elicited neuroprotection against ischemia-induced neuronal death (Fig. 3C). These data were confirmed by cotransduction of APE1t and the human APE1 (hAPE1), which differs from the rat cDNA and is resistant to APE1t-mediated knockdown (Fig. 3D). Increased APE1 expression by transduction of hAPE1 restored PACAP-elicited neuroprotection and suppressed DNA damage (Fig. 3 E and F).
Fig. 3.
Knockdown of APE1 abrogates PACAP neuroprotection of CA1 following GCI. (A) CA1 was cotransfected for 14 d with lentiviral vectors expressing GFP and the APE1-targeting sequence APE1t (b) or the scrambled sequence APE1s (a), and the sections were immunostained for APE1 (c and d). e (low magnification) and g and h (high magnification) show double-label immunofluorescence of APE1 (red) and GFP (green) in GFP/APE1s-transfected CA1 neurons. (Scale bar, 30 μm.) f (low magnification) and i and j (high magnification) show reduced APE1 expression (red) in GFP/APE1t-transfected CA1 neurons. (B) Western blots show the expression levels of APE1 in CA1, at 14 d after transfection with either GFP alone (Lanes 1–4) or GFP together with APE1t (Lanes 5 and 6) or APE1s (Lanes 7 and 8), without or with PACAP infusions (0.2 nmol, x 3 times, −24, −12, and −6 h). The graph illustrates the relative changes of APE1 expression in CA1 after PACAP treatment. **, P < 0.01; n = 6 per group. (C) Knockdown of APE1 expression attenuated CA1 cell survival after GCI. Viable CA1 neurons were quantified at 4 d after GCI or sham operation after PACAP infusions (0.2 nmol, 24, 12, and 6 h before and 0.5, 6, and 24 h after GCI). **, P < 0.01; n = 8–9 per group. (D) Western blots show the expression of APE1 in CA1, either nontransfected (Lanes 1 and 2) or at 14 d after transfection with APE1t (Lanes 3 and 4), cotransfection with APE1t/hAPE1 (Lanes 5 and 6), or cotransfection with APE1t/hAPE1m (Lanes 7 and 8). (E and F) Overexpression of human APE1, but not the DNA repair-incompetent hAPE1m, restored the prosurvival effect of PACAP for CA1 after GCI. Viable (E) and DNA-damaged CA1 neurons (F) were quantified, respectively, at 4 d after GCI. *, P < 0.05; n = 8–9 per group.
APE1 has multiple functions in addition to its DNA repair activity that could contribute to neuroprotection. To address the relation between the repair activity function and protection, we used a human APE1 construct containing a single point-mutation D210A (hAPE1m). This mutant lacks DNA repair activity but retains its redox/transcription-regulation function. When cotransduced with APE1t shRNA, hAPE1m allowed for mutant APE1 protein expression but did not restore PACAP-mediated neuroprotection or suppression of DNA damage (Fig. 3 D−F). The requirement of APE1 DNA repair activity for PACAP neuroprotection was also observed in primary cultures of hippocampal and cortical neurons subjected to oxygen/glucose deprivation (Fig. S4). These data provide convincing evidence that induction of APE1 DNA repair activity is a major contributor to neuroprotection by PACAP.
APE1 Promoter Is Activated via CREB and ATF2 Signaling Pathways.
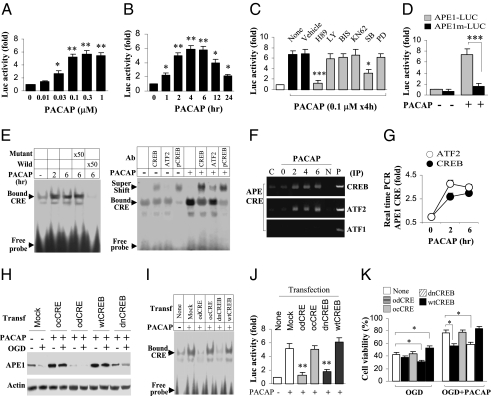
PACAP induced a robust increase in APE1 mRNA and protein expression in the hippocampal CA1 (Figs. 1 and 2). PACAP promotes cAMP-dependent activation of PKA (17), and the APE1 promoter contains an AP-1/ cAMP responsive element (CRE)-binding site that is implicated in transcriptional regulation of the APE1 gene (18). Thus, PACAP may act via CREB to stimulate transactivation of the APE1 promoter. Using a luciferase reporter under the control of the APE1 promoter, we found that PACAP induced APE1 promoter activity in a dose- and time-dependent manner in cultured hippocampal/cortical neurons (Fig. 4 A and B). Selecting 0.1 μM as the minimal dose to obtain a maximal effect, we examined upstream signaling pathways required for APE1 promoter activation. Both the PKA inhibitor H89 and the p38/MAPK inhibitor SB239063 (SB) attenuated PACAP activation of the APE1 promoter (Fig. 4C). In contrast, inhibitors of PI3K, PKC, CaMKII, and MEK1/2 (LY, BIS, KN62, and PD compounds, respectively) had little or no effect on PACAP-induced increase in APE1 promoter activity (Fig. 4C).
Fig. 4.
CREB-ATF2 signaling mediates PACAP-induced APE1 expression and neuroprotection in neuronal cultures. Primary hippocampal/cortical neurons were transfected for 24 h with a luciferase reporter construct containing 1.8 kb of the rat APE1 promoter, and then treated with PACAP. (A) Cultures were treated with one application of PACAP at the indicated concentrations; luciferase activity was measured 4 h after treatment. (B) Cultures were treated with one application of 0.1 μM of PACAP; luciferase activity was measured at the indicated time points after treatment. (C) Cultures were incubated with kinase inhibitors for 1 h and then with the inhibitors plus PACAP for 4 h before assays for luciferase activity. The PKA inhibitor H89 and the p38/MAPK inhibitor SB, but not any of other inhibitors tested, reduced PACAP activation of the APE1 promoter. (D) Cultures were transfected for 24 h with the reporter construct containing either the wild-type APE1 promoter (APE1-LUC), or the APE1 promoter mutated in the CRE sequence (APE1m-LUC, SI Materials and Methods), and assessed for luciferase activity 4 h after PACAP treatment. PACAP markedly activated APE1-LUC, but had no effect on APE1m-LUC. (E) EMSA was performed at 2 and 6 h after PACAP treatment using a radio-labeled APE1-CRE sequence. Competition assays using cold probes (50 times the labeled probe concentration) showed that the wild-type but not the mutant CRE inhibited the CRE binding activity (Left). Antibodies to CREB, ATF2, or pCREB caused supershifts in CRE binding (Right). (F and G) ChIP assays based on CREB- or ATF2-pull down products at 2 and 6 h after PACAP treatment detected the APE1-CRE DNA sequence by PCR (F) or real-time PCR (G) using primers for rat APE1-CRE promoter region (SI Materials and Methods). (H–K) Neurons were transfected with the CRE decoy oligo (odCRE) or control oligo (ocCRE) for 6 h or transfected with lentiviral vectors containing wild-type CREB (wtCREB) or dominant-negative CREB (dnCREB) for 48 h before PACAP treatment for 4 h. Western blots were performed using cell extracts from neurons without oxygen and glucose deprivation (OGD) or collected at 2 h after 1 h of OGD (H). EMSA was performed at 4 h after PACAP treatment (I). Luciferase activity was measured at 4 h after PACAP treatment in neurons cotransfected with APE1-LUC and odCRE, ocCRE, dnCREB, or wtCREB (J). Cell viability was measured at 24 h after OGD where PACAP was applied 4 h before, during, and after OGD (K). CREB was necessary for PACAP-induced APE1 expression (H), promoter binding (I), promoter transactivation (J), and neuroprotection against OGD (1 h) (K). *, P < 0.05; **, P < 0.01; ***, P < 0.001; all quantitative data are based on at least four independent experiments.
CREB and ATF2 are known downstream targets of PKA and p38, respectively. PACAP induced robust phosphorylation of CREB and ATF2 in cultured neurons. PACAP-induced phosphorylation of CREB was prevented by PKA inhibition and phosphorylation of ATF2 was reduced to below the basal level by p38MAPK inhibition (Fig. S5 A–D). PACAP also induced phosphorylation of CREB and ATF2 in neurons after oxygen and glucose deprivation (OGD) (1 h) (Fig. S5F). The APE1 promoter contains a canonical CRE binding site (18), and we explored whether the CRE site of the APE1 promoter mediates transactivation of APE1 by CREB and ATF2 in response to PACAP. Mutation of the CRE site in the APE1 promoter of the luciferase reporter construct greatly reduced the increase in luciferase activity induced by PACAP (Fig. 4D). Moreover, labeled wild-type APE1-CRE oligo bound to a more slowly moving band, but the APE1-CRE mutant did not; this binding was prevented by excess cold APE1-Cre oligo but not by mutant APE1-CRE oligo (Fig. 4E, Left). Antibodies to ATF2, CREB, or p-CREB (pCREB) induced a supershift; that is, further slowing of the APE1-CRE binding band (Fig. 4E). Finally, we confirmed that CREB and ATF2 physically associate with the endogenous APE1 promoter using ChIP with CREB or ATF2 antibody followed by amplification via PCR (Fig. 4F) or real-time PCR (Fig. 4G) of the CRE region of the APE1 promoter.
To examine a possible role for CREB in PACAP-induced APE1 expression in postischemic neurons, we used neuronal cultures and OGD. We applied a decoy CRE oligonucleotide (odCRE) or transfected neurons with dominant-negative CREB (dnCREB) before OGD. As with global ischemia in vivo, OGD (1 h) and reperfusion for 2 h decreased APE1 protein abundance, and PACAP prevented OGD-induced decrease in APE1 (Fig. 4H). Inhibition of CREB binding to APE1-CRE with either odCRE or dnCREB (Fig. 4H) or RNAi-mediated knockdown of ATF2 (Fig. S6 A–C) attenuated the PACAP-induced increase in APE1 expression and transactivation of the APE1 promoter in postischemic neurons. Moreover, knockdown of APE1 (Fig. S4), application of odCRE, expression of dnCREB (Fig. 4K), or knockdown of ATF2 (Fig. S5D) attenuated PACAP-mediated neuroprotection. These data strongly suggest that PACAP acts via CREB and ATF2 to drive APE1 expression and protect postischemic neurons.
CREB Signaling Induces APE1 Expression and Activity Necessary for PACAP-Mediated Neuroprotection Against GCI.
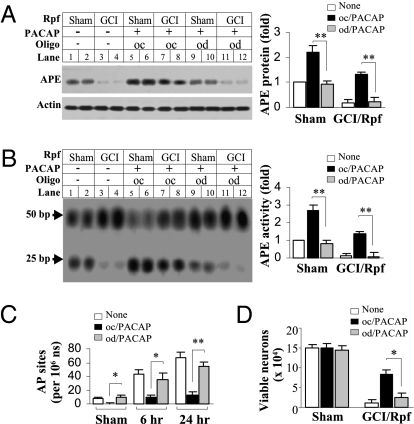
Having determined that CREB and its induction of APE1 are required for PACAP-induced neuroprotection in our in vitro model of ischemia, we examined whether the same processes occurred in a clinically relevant in vivo model of global ischemia. PACAP induced an increase in CREB and ATF2 phosphorylation (Fig. S7 A and B), and promoted a mobility shift of APE1-CRE binding (Fig. S7C). The binding was at least in part to pCREB and pATF2, as indicated by further slowing of migration (supershift) with antibodies to pCREB and pATF2 (Fig. S7C, Left). Furthermore, binding of CREB and ATF2 required the CRE regulatory element and pCREB activity, as administration of either the odCRE or H89 greatly reduced the density of the shifted band (Fig. S7C, Right). Intercerebral infusion of the odCRE significantly decreased PACAP-induced APE1 expression and endonuclease activity in both sham and postischemic CA1 (Fig. 5 A and B). Furthermore, infusion with the odCRE diminished PACAP-elicited protection of DNA integrity and promotion of neuronal survival in postischemic CA1 (Fig. 5 C and D). Thus, PACAP acts via CREB and ATF2 to promote APE1 expression and elicit protection of postischemic CA1 neurons.
Fig. 5.
CREB signaling mediates PACAP-induced APE1 expression, activity, and neuroprotection in CA1 after GCI. Rats received PACAP infusions (0.2 nmol at −24, −12, and −6 h) and were subjected to GCI or sham operation. The CRE decoy oligo (od) or the control oligo (oc) was infused into the right cerebral ventricle (5 μL in 10 μmol/L, three times, 10 min before each PACAP infusion). Western blots for APE1 protein (A) and the oligonucleotide incision assays for APE1 activity (B) were performed using CA1 extracts 6 h after GCI. AP sites were measured in CA1 at 6 and 24 h after GCI (C). *, P < 0.05; **, P < 0.01, n = 6/group. (D) Viable CA1 neurons were quantified 4 d after GCI or sham operation. *, P < 0.05, n = 8 per group.
Discussion
Regulation of critical DNA repair enzymes such as APE1 in postischemic neurons presents a logical approach to therapeutic intervention against ischemia-induced neuronal death; however, the functional significance of the DNA repair pathways in ischemia-induced neuronal death has not been directly tested. The present study reveals the unique finding that PACAP acts via induction of APE1 expression and DNA repair activity to afford neuroprotection in global ischemia. Furthermore, we identify CREB and ATF2 as downstream mediators of PACAP, leading via PKA and p38 to APE1 expression, activation, and neuroprotection. These important mechanistic findings present previously unexplored possibilities in targeting DNA repair pathways as therapeutic strategies.
APE1 is a critical cellular protein that performs multiple functions. In addition to its repair activity, APE1 acts as a transcriptional cofactor, as well as a suppressor of reactive oxygen species via a redox site (19). Many of these functions could lead to neuroprotection independent of DNA repair. However, we found that a single-point mutation (D210A) that abrogates the DNA repair capacity of APE1 greatly diminished PACAP neuroprotection. These data suggest that the function of APE1 in PACAP-mediated neuroprotection lies primarily in its capacity to repair DNA, rather than in redox activity and transcriptional coactivation. The redox-sensing site is essential for APE1 to regulate the DNA binding activity of many transcription factors, such as the AP-1 proteins, NF-κB, and Myb. However, our results indicate that the increased APE1 redox-regulating activity alone (in the absence of the DNA repair activity) does not confer neuroprotection. However, to completely exclude that the redox-regulating activity of APE1 contributes to the neuroprotection afforded by its endonuclease activity, future studies need to be performed using a mutant APE1 disabled in its redox site.
DNA repair activity, in particular activation of APE1, represents an interesting point of control for cell death. Long understood to lead to genetic mutations associated with spontaneous tumor formation, AP sites have also been found to induce cell death in yeast (5), and deficiency in APE1 expression and activity exacerbates oxidative injury in multiple models, including neurons (12, 20, 21). Unrepaired AP sites accumulate following cerebral ischemia (4, 6), and sustained decrease in APE1 protein expression has been observed to precede cell death in injured cell populations (10, 22, 23) (Fig. 2A and Fig. S1). Furthermore, APE1 protein expression and activity increase in association with neuroprotection (4, 6, 11, 24), but the relevance has been largely correlative rather than interventional. Our data are unique in showing that induction of APE1 expression and activity plays a significant role in neuroprotection against ischemia, as attenuation of APE1 expression or DNA repair activity significantly decreased PACAP-mediated neuroprotection against ischemia.
In addition to describing a functional role for DNA repair in conferring neuroprotection, we characterized the upstream signaling pathways required for APE1 induction and neuroprotection by PACAP. In other neuronal subtypes, PACAP is associated with CREB activation (25, 26). The APE1 gene contains a critical CRE site located proximal to the 5′ coding region (18). We demonstrate in the current study that the APE1-CRE region binds with CREB and ATF2 following PACAP stimulation; however, the transactivation of the APE1 promoter may also include other factors not identified in the context of this study. Interestingly, we found that both CREB and ATF2 significantly contributed to APE1 up-regulation following PACAP treatment, and that both PKA and p38 MAP kinase also contributed to neuronal survival against ischemic insults. Our findings illustrate that two distinct upstream kinase signaling cascades act independently to converge on the transactivation of a particular promoter. Thus, although activation of one arm may provide a limited level of neuroprotection, dual targeting of upstream pathways may better serve to maximize induction of APE1 expression and lead to maximal neuroprotection afforded by PACAP. Previously unexplored neuroprotective therapies aimed at synergistic signaling mechanisms may greatly improve efficacy against oxidative neuronal injury.
Although the current study was directed at exogenously restoring expression and activity of a gene that is specifically down-regulated in regions sensitive to ischemic injury, further studies examining the mechanism of endogenous suppression of the APE1 gene may also present therapeutic targets. For example, suppression of specific genes via the gene-silencing transcriptional repressor REST and REST-dependent epigenetic regulation are implicated in cell death in vulnerable CA1 neurons following global ischemia (27). Detailed knowledge of the mechanisms that regulate the APE1 gene may serve to better target therapeutic intervention.
Taken together, these data indicate that DNA damage and repair play essential roles in determining the balance between cell survival and cell death. Pharmacological agents that enhance DNA repair may provide a new protective strategy against postischemic neurodegeneration.
Materials and Methods
Details beyond the descriptions here are provided in SI Materials and Methods.
Models of Neuronal Ischemia.
All animal experiments were approved by the University of Pittsburgh and Fudan University Institutional Animal Care and Use Committees and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Global cerebral ischemia (GCI) was induced for 12 min in isoflurane-anesthetized male Sprague-Dawley rats using the four-vessel occlusion method. Hippocampal CA1 cell death was quantified at either 4 or 60 days after GCI by stereological cell counting throughout the dorsal hippocampal formation (28).
An in vitro model of neuronal ischemia was induced in primary cultures of hippocampal/cortical neurons by transient oxygen and glucose deprivation (OGD) for 1 h (28).
Construction of Small, Interfering RNA Expression Vectors.
Mammalian expression plasmids directing the transcription of short hairpin (sh) small interfering RNAs (shRNAs) against rat APE1 or ATF2 were constructed using the pcDNA3.1 (+) backbone containing the U6 promoter (28). For each of the targeted genes, the scrambled sequence served as a control.
Construction of Viral Vectors.
To construct lentiviral vectors for overexpression of human APE1 (hAPE1) or its repair-incompetent mutant (hAPE1m), wild-type CREB (wtCREB) or its dominant-negative mutant (dnCREB), the cDNA (also encoding a hemoagglutinin tag) was inserted into the lentiviral transfer vector FSW under the control of the neuron-specific, synapsin I promoter. To construct lentiviral vectors expressing shRNA against rat APE1 or ATF2, the gene-specific targeting sequence or its counterpart scrambled sequence was inserted into the transfer vector FSW under the control of the U6 promoter (28).
Intracerebral Infusion of Viral Vectors, Decoy Oligonucleotides, and PACAP.
Lentiviral vectors for gene knockdown of rat APE1 or overexpression of human APE1 were infused into the CA1 sector in the dorsal hippocampus at the rate of 0.1 μl/min over 50 min via a custom-made convection-enhanced microinfusion system linked to a UMP2 microsyringe pump (28). The animals were allowed to recover for up to 14 d to enable sufficient gene expression.
To inhibit CRE-mediated activation of APE1 promoter, a CRE decoy oligonucleotide based on the APE1 CRE sequence or a mismatch control sequence, similar to those previously described (29), was intracerebroventricularly infused into rat brain at 6 h before GCI.
PACAP was infused into the rat brain through a preimplanted 21-ga cannula in the left ventricle. For histochemical studies, each animal received repetitive ventricular infusions of the indicated doses at 24, 12, and 6 h before ischemia and 0.5, 6, and 24 h after ischemia, except as indicated otherwise.
Oxidative DNA Damage and Repair Activity.
Nuclear DNA was isolated from hippocampal CA1 tissues at 3, 6, 24, and 48 h after GCI or 24 h following sham operation. AP sites were measured using the biotin-labeled aldehyde reactive probe with a colorimetric assay (Dojindo Molecular Technologies). The oligonucleotide incision assay was performed at 6 and 24 h after GCI to estimate the ability of nuclear protein extracts to remove AP sites in damaged DNA (6). In this assay, an excess of radiolabeled 50-bp oligonucleotide containing a synthetic AP site at position 26 is incubated with cell lysates, and the resulting presence of a 25-bp product is reflective of AP endonuclease activity.
Electrophoretic Mobility Shift Assay.
EMSA was performed on nuclear extracts from cultured neurons or hippocampal CA1 tissues using a 32P-labeled oligonucleotide designed from the rat APE1 promoter sequence. Supershifts were performed with antibodies against pCREB, CREB (this antibody also reacts with ATF1), and ATF2, respectively, to determine the identity of proteins bound to DNA.
Chromatin Immunoprecipitation Assay.
ChIP was performed on nuclear extracts from cultured neurons by immunoprecipitation with anti-CREB or anti-ATF2 antibody, followed by PCR with primers specific to the APE1-CRE promoter region.
APE1 Promoter Activity Assay.
APE1 promoter activity was assessed in neuronal cultures transfected with a luciferase reporter pGL3 plasmid (Promega) under the control of the rat APE1 promoter. Lipofectamine 2000-assisted transfection was performed 24 h before PACAP treatment.
Statistical Analysis.
Results are reported as mean ± SE values. The significance of difference between means was assessed by Student’s t test (single comparisons) or by ANOVA and post hoc Bonferroni’s/Dunn’s tests, with P < 0.05 considered statistically significant.
Supplementary Material
Acknowledgments
The project was supported by National Institutes of Health Grants NS36736, NS43802, and NS45048 (to J.C.), NS46742 (to R.S.Z.), an American Heart Association fellowship grant (to R.A.S.), and the Chinese Natural Science Foundation Grants 30870794 and 30670642 (to Y.G.). R.S.Z is the F.M. Kirby Professor of Neural Repair and Protection. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000030107/DCSupplemental.
References
- 1.Burcham PC. Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis. 1998;13:287–305. doi: 10.1093/mutage/13.3.287. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 4.Li W, et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- 5.Guillet M, Boiteux S. Endogenous DNA abasic sites cause cell death in the absence of Apn1, Apn2 and Rad1/Rad10 in Saccharomyces cerevisiae. EMBO J. 2002;21:2833–2841. doi: 10.1093/emboj/21.11.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan J, et al. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2003;23:1324–1339. doi: 10.1097/01.WCB.0000091540.60196.F2. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava DK, et al. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J Biol Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 8.Nagayama T, et al. Activation of poly(ADP-ribose) polymerase in the rat hippocampus may contribute to cellular recovery following sublethal transient global ischemia. J Neurochem. 2000;74:1636–1645. doi: 10.1046/j.1471-4159.2000.0741636.x. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JL, Dianova II, Dianov GL. APE1 is the major 3′-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 2004;32:3531–3536. doi: 10.1093/nar/gkh676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawase M, Fujimura M, Morita-Fujimura Y, Chan PH. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: implication of the failure of DNA repair in neuronal apoptosis. Stroke. 1999;30:441–448. doi: 10.1161/01.str.30.2.441. discussion 449. [DOI] [PubMed] [Google Scholar]
- 11.Narasimhan P, et al. Overexpression of human copper/zinc-superoxide dismutase in transgenic animals attenuates the reduction of apurinic/apyrimidinic endonuclease expression in neurons after in vitro ischemia and after transient global cerebral ischemia. J Neurochem. 2005;93:351–358. doi: 10.1111/j.1471-4159.2005.03039.x. [DOI] [PubMed] [Google Scholar]
- 12.Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair (Amst) 2005;4:367–379. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Miyata A, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 14.Ohtaki H, Nakamachi T, Dohi K, Shioda S. Role of PACAP in ischemic neural death. J Mol Neurosci. 2008;36:16–25. doi: 10.1007/s12031-008-9077-3. [DOI] [PubMed] [Google Scholar]
- 15.Uchida D, Arimura A, Somogyvári-Vigh A, Shioda S, Banks WA. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996;736:280–286. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- 16.Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- 17.Bhave SV, Hoffman PL. Phosphatidylinositol 3′-OH kinase and protein kinase A pathways mediate the anti-apoptotic effect of pituitary adenylyl cyclase-activating polypeptide in cultured cerebellar granule neurons: modulation by ethanol. J Neurochem. 2004;88:359–369. doi: 10.1046/j.1471-4159.2003.02167.x. [DOI] [PubMed] [Google Scholar]
- 18.Fung H, Liu P, Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol. 2007;27:8834–8847. doi: 10.1128/MCB.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 20.McNeill DR, Wilson DM., 3rd A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 2007;5:61–70. doi: 10.1158/1541-7786.MCR-06-0329. [DOI] [PubMed] [Google Scholar]
- 21.Ono Y, Furuta T, Ohmoto T, Akiyama K, Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994;315:55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Early decrease of apurinic/apyrimidinic endonuclease expression after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:495–501. doi: 10.1097/00004647-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Lewén A, Sugawara T, Gasche Y, Fujimura M, Chan PH. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol Dis. 2001;8:380–390. doi: 10.1006/nbdi.2001.0396. [DOI] [PubMed] [Google Scholar]
- 24.Fujimura M, et al. Copper-zinc superoxide dismutase prevents the early decrease of apurinic/apyrimidinic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30:2408–2415. doi: 10.1161/01.str.30.11.2408. [DOI] [PubMed] [Google Scholar]
- 25.Kopp M, Meissl H, Korf HW. The pituitary adenylate cyclase-activating polypeptide-induced phosphorylation of the transcription factor CREB (cAMP response element binding protein) in the rat suprachiasmatic nucleus is inhibited by melatonin. Neurosci Lett. 1997;227:145–148. doi: 10.1016/s0304-3940(97)00312-1. [DOI] [PubMed] [Google Scholar]
- 26.Sumner AD, Margiotta JF. Pituitary adenylate cyclase-activating polypeptide (PACAP) alters parasympathetic neuron gene expression in a time-dependent fashion. J Mol Neurosci. 2008;36:141–156. doi: 10.1007/s12031-008-9103-5. [DOI] [PubMed] [Google Scholar]
- 27.Formisano L, et al. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci USA. 2007;104:4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao G, et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park YG, Nesterova M, Agrawal S, Cho-Chung YS. Dual blockade of cyclic AMP response element- (CRE) and AP-1-directed transcription by CRE-transcription factor decoy oligonucleotide, gene-specific inhibition of tumor growth. J Biol Chem. 1999;274:1573–1580. doi: 10.1074/jbc.274.3.1573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.