Abstract
EBV causes infectious mononucleosis and is associated with certain malignancies. EBV nuclear antigen 1 (EBNA1) mediates EBV genome replication, partition, and transcription, and is essential for persistence of the viral genome in host cells. Here we demonstrate that Hsp90 inhibitors decrease EBNA1 expression and translation, and that this effect requires the Gly-Ala repeat domain of EBNA1. Hsp90 inhibitors induce the death of established, EBV-transformed lymphoblastoid cell lines at doses nontoxic to normal cells, and this effect is substantially reversed when lymphoblastoid cell lines are stably infected with a retrovirus expressing a functional EBNA1 mutant lacking the Gly-Ala repeats. Hsp90 inhibitors prevent EBV transformation of primary B cells, and strongly inhibit the growth of EBV-induced lymphoproliferative disease in SCID mice. These results suggest that Hsp90 inhibitors may be particularly effective for treating EBV-induced diseases requiring the continued presence of the viral genome.
Keywords: Epstein–Barr virus, 17-DMAG, 17-AAG, geldanamycin, translation
EBV is a human herpesvirus that causes infectious mononucleosis (IM) and persists in the host for life, but is normally well controlled by the immune system. Nevertheless, EBV is also associated with human malignancies of both epithelial and B-cell origin, including lymphoproliferative disease, Burkitt lymphoma, nasopharyngeal carcinoma (NPC), and gastric cancer (1). In addition, increasing evidence suggests that EBV infection may contribute to certain autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, and lupus (2). Like all herpesviruses, EBV can infect cells in either latent or lytic forms.
EBNA1 is the one viral protein expressed in all three forms of latent viral infection, and is the only viral protein absolutely required for persistence of EBV infection in host cells. EBNA1 mediates replication of the viral episome during latent infection by recruiting host replication initiation factors to the initiation site in the latent origin of replication, oriP (reviewed in ref. 3). EBNA1 also plays essential roles in partitioning of viral episomes during cell division (4, 5), and activates transcription of other essential viral transforming proteins in cells with type III latency (6). In addition, increasing evidence suggests that EBNA1 may directly contribute to tumorigenesis by inhibiting apoptosis (7, 8). Collectively, the fundamental roles of EBNA1 in maintenance of the viral episome, as well as its possible direct contributions to tumorigenesis, make it a particularly desirable target for therapeutic strategies. However, drugs that inhibit expression of EBNA1 or its functions are not currently available.
Here we demonstrate that Hsp90 inhibitors can be used to inhibit expression of EBNA1 in cells with various types of latent EBV infection, and that Hsp90 inhibitors prevent EBV transformation of primary B cells and are highly toxic to EBV-immortalized lymphoblastoid cell lines (LCLs). Heat shock proteins (Hsps) are a class of molecular chaperones that facilitate proper protein folding and stability. Unlike other Hsps, only a small subset of cellular proteins (approximately 100) are thought to be clients of Hsp90 (9). Hsp90 inhibitors such as geldanamycin and its analogues (17-AAG and 17-DMAG) bind to the ATP-binding motif of Hsp90 and inhibit its protein chaperoning activity, consequently resulting in misfolding (and subsequent degradation) of cellular client proteins (10, 11). Hsp90 inhibitors are often more toxic to tumor cells than to normal cells (12), not only because a number of Hsp90 client proteins contribute to tumor cell growth, but also because a particular Hsp90 conformation required for inhibitor binding exists more frequently in tumor cells (13).
EBNA1 is an unusual protein that is translated with extremely poor efficiency, but is highly stable once it is made (14–18). Interestingly, our results suggest that, rather than decreasing the stability of EBNA1, Hsp90 inhibitors further reduce the ability of EBNA1 to be translated. A region in EBNA1 previously shown to inhibit EBNA1 translation (the Gly-Ala repeat domain) (14, 16–18) is required for Hsp90 inhibition of EBNA1 expression. Importantly, the toxic effect of low dose Hsp90 inhibitors in LCLs is substantially reversed following enforced expression of a mutant EBNA1 protein (missing most of the Gly-Ala repeat domain) resistant to the Hsp90 effect. Finally, we also show that EBV-induced lymphoproliferative disease in SCID mice is strongly inhibited using a nontoxic dose of 17-AAG. Our results suggest that Hsp90 inhibitors can be used to decrease EBNA1 expression in a variety of different EBV-infected cell types and thus may prove useful for treating certain EBV-induced diseases.
Results
Hsp90 Inhibitors Decrease EBNA1 Expression in a Variety of Cell Types.
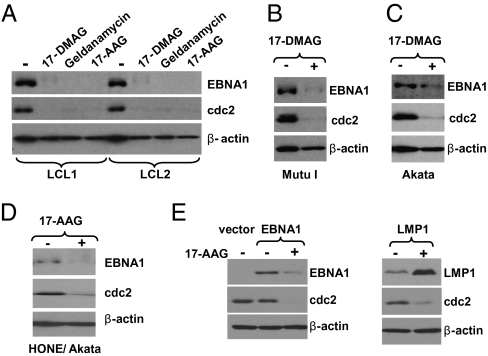
To determine whether Hsp90 inhibitors alter EBNA1 expression, various types of latently infected, EBV-positive cells were treated with vehicle control or Hsp90 inhibitors. Hsp90 inhibitors decreased the expression level of EBNA1 in every EBV-infected cell line examined, including two different LCL lines (Fig. 1A), two different Burkitt lymphoma lines (Fig. 1 B and C and Fig. S1A), two different NPC lines (Fig. 1D and Fig. S1B), and a gastric carcinoma line (Fig. S1C). Treatment with 17-DMAG reduced the EBNA1 expression level to 6% to 8% of its normal expression level in LCL1, LCL2, and Mutu BL lines (Fig. S1D). As expected, expression of the cellular protein, cdc2 (a known substrate of Hsp90) (19), was also decreased, whereas β-actin expression was not affected. The inhibitory effect of Hsp90 inhibitors on EBNA1 expression in B cell lines (in which the EBNA1 half-life is >24 h) required several days of treatment, but was apparent in epithelial cell lines (in which the EBNA1 half-life is shorter) (20) within 48 h.
Fig. 1.
Hsp90 inhibitors decrease expression of EBNA1. (A) LCL cells (line 1 and 2) were treated with no drug, 17-DMAG (0.17 μM), geldanamycin (0.5 μM), or 17-AAG (0.5 μM) for 96 h. (B and C) Mutu I and Akata Burkitt lymphoma cells were treated with no drug or 17-DMAG (0.17 μM) for 96 h. (D) The EBV-infected NPC cell line HONE/Akata was treated with no drug or 17-AAG (0.5 μM) for 48 h. (E) AGS gastric cells (EBV-negative) were transfected with empty vector (pSG5), pSG5-EBNA1, or pSG5-LMP1 as indicated, followed by a 48-h treatment with 17-AAG (0.25 μM) beginning at 4 h after transfection. Whole-cell extracts were prepared and immunoblot analysis was performed to analyze the expression of EBNA1, cdc2 (a known cellular substrate of Hsp90), cellular β-actin, or LMP1 as indicated.
To determine if Hsp90 inhibitors decrease EBNA1 expression outside the context of the EBV genome, EBV-negative AGS gastric carcinoma cells were transfected with an EBNA1 expression vector driven by the SV40 promoter (in the SG5 vector), then treated with or without 17-AAG beginning at 4 h after transfection. As shown in Fig. 1E, 17-AAG treatment significantly decreased expression of transfected SG5-EBNA1, whereas expression of another EBV protein, LMP1, in the same vector was increased. Of note, we found that Hsp90 inhibitors nonspecifically decrease expression of all CMV promoter–driven proteins and thus did not use CMV promoter constructs for these experiments.
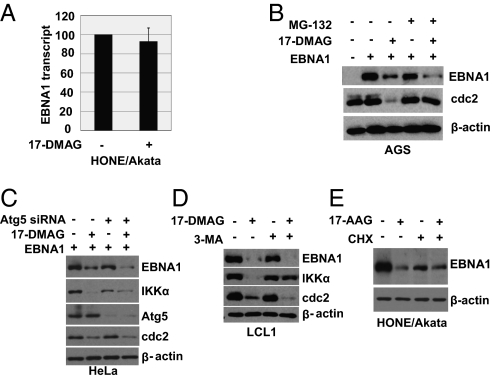
Hsp90 Inhibitors Can Decrease EBNA1 Expression Without Affecting EBNA1 Transcript Level.
The EBNA1 transcript is derived from the Qp viral promoter in EBV+ Burkitt lymphomas, gastric cancers, and NPC tumors, and derived from the Cp promoter in LCLs (1). The level of EBNA1 mRNA in HONE/Akata cells was not significantly affected by 17-DMAG treatment (Fig. 2A), suggesting that Hsp90 inhibitors do not affect EBNA1 transcription or RNA stability in this cell type. In contrast, in cells with type III viral latency (i.e., LCLs), in which EBNA1 activates its own transcription from the viral Cp promoter, 17-DMAG treatment decreased the level of EBNA1 transcripts as expected (Fig. S2), as well other viral proteins derived from Cp such as EBNA2, although LMP1 was increased.
Fig. 2.
Hsp90 inhibitors reduce EBNA1 independent of effects on EBNA1 transcripts or EBNA1 stability. (A) EBV-positive HONE/Akata cells were treated with 17-DMAG (0.17 μM) for 24 h. Expression of the EBNA1 transcript was examined by quantitative RT-PCR. The level of EBNA1 transcript in untreated cells is set as 100. (B) EBV-negative AGS cells were transfected with empty vector (pSG5) or pSG5-EBNA1, followed by a 48-h treatment with no drug or 17-DMAG (0.17 μM) beginning at 4 h after transfection in the presence or absence of the proteasome inhibitor MG-132 (50 μM). (C) HeLa cells were transfected with pSG5-EBNA1 in the presence of Atg5 siRNA or equivalent amounts of a control siRNA, then treated with no drug or 17-DMAG (0.17 μM). (D) LCL1 cells were treated with no drug or 17-DMAG (0.17 μM) in the presence or absence of the autophagy inhibitor 3-MA (10 mM). (E) EBV-positive HONE/Akata cells were treated with no drug or 17-AAG (0.5 μM) for 48 h in the presence or absence of CHX (50 μg/mL) added into medium 12 h before cell harvesting. Whole-cell extracts were prepared and immunoblot analysis was performed to analyze the expression of viral and cellular proteins as indicated (B–E).
Hsp90 Inhibitors Do Not Affect EBNA1 Stability or Half-Life.
Many Hsp90 client proteins are degraded via the proteasome–ubiquitin pathway in the absence of Hsp90, suggesting that proteasomal inhibitors might attenuate the effect of Hsp90 inhibitors on EBNA1 expression. To examine this, AGS cells were transfected with the SG5-EBNA1 vector and treated with 17-DMAG or vehicle control in the presence or absence of the proteosomal inhibitor MG-132. As shown in Fig. 2B, 17-DMAG decreased EBNA1 level to a similar degree in the presence or absence of MG-132, although the effect on cdc2 was attenuated. Similarly, although EBNA1 has been shown to be degraded through autophagy in B cells (21), Administration of 17-DMAG down-regulated EBNA1 levels to a similar degree in HeLa cells even when a key autophagy pathway component, Atg5, was knocked down using siRNA (Fig. 2C). In contrast, the effect of 17-DMAG on IκB kinase–α (IKKα), a cellular protein degraded via the autophagy pathway (22), was reduced by the Atg5 siRNA (Fig. 2C). In addition, treatment of LCL1 cells with the autophagy inhibitor 3-methyladenine (3-MA) attenuated the effect of 17-DMAG on IKKα but not EBNA1 (Fig. 2D). To determine if Hsp90 inhibitors might affect EBNA1 stability through some other mechanism, EBV-positive HONE cells were treated with 17-AAG or vehicle control in the presence or absence of cycloheximide (CHX). As shown in Fig. 2E, the half-life of EBNA1 was not decreased, but increased, in the presence of Hsp90 inhibitors.
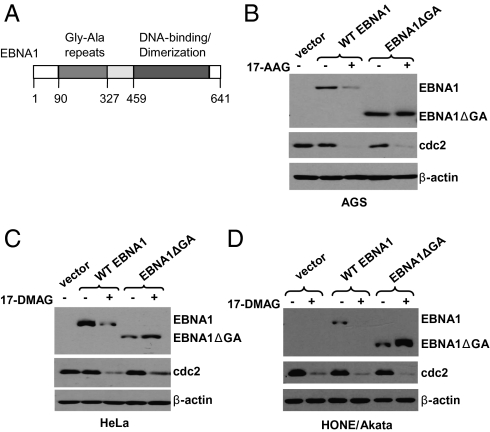
Gly-Ala Repeats Are Required for Inhibition of EBNA1 Expression by Hsp90 Inhibitors.
EBNA1 contains an internal Gly-Ala repeat domain (Fig. 3A) that inhibits both translation of EBNA1 (16–18) and EBNA1 degradation via the proteasomal pathway (15). Therefore, EBNA1 is translated with extremely poor efficiency but is highly stable once it is made. To determine if this region of the protein is required for the effect of Hsp90 inhibitors on EBNA1 expression, we compared the effect of 17-AAG/17-DMAG on the full-length EBNA1 protein or a mutant EBNA1 lacking most of the Gly-Ala repeats (23). In contrast to their effect on full-length EBNA1, neither drug affected expression of the mutant EBNA1 in a variety of different cell types, and in some cell types (HeLa and Hone/Akata) the mutant EBNA1 was consistently increased by the drugs (Fig. 3 B–D). These results suggest that the Gly-Ala repeats domain is required for the Hsp90 inhibitor effect on EBNA1.
Fig. 3.
The Gly-Ala repeats are required for inhibition of EBNA1 expression by Hsp90 inhibitors. (A) Schematic diagram of the EBNA1 protein showing some of its functional elements and amino acid numbers. AGS cells (B), HeLa cells (C), and EBV-positive HONE cells (D) were transfected with empty vector (pSG5), pSG5-EBNA1, or pSG5-EBNA1ΔGA (a mutant protein missing the Gly-Ala repeats), followed by a 48-h treatment with 17-AAG (0.5 μM) or 17-DMAG (0.17 μM) beginning at 4 h after transfection. Immunoblot analysis was performed to analyze the expression of EBNA1, cdc2, and β-actin.
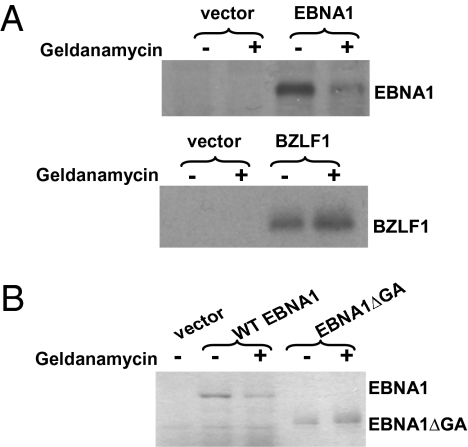
Geldanamycin Inhibits Translation of EBNA1 in Reticulocyte Lysate.
To investigate the effect of Hsp90 inhibitors on EBNA1 translation, we translated EBNA1 in vitro using rabbit reticulocyte lysate in the presence or absence of geldanamycin, using a dose of drug previously shown to inhibit Hsp90 in reticulocyte lysate (24). As shown in Fig. 4A, geldanamycin inhibited the translation of full-length EBNA1 while not affecting translation of the EBV protein, BZLF1, expressed in the same SG5 vector. Furthermore, translation of the mutant EBNA1 protein lacking the Gly-Ala repeats domain was not affected by geldanamycin (Fig. 4B). (Note that 10-fold less of the Gly-Ala deleted EBNA1 mutant versus the full-length EBNA1 protein was loaded on the gel in Fig. 4B to normalize for the greatly enhanced translation of the mutant protein.) These results suggest that Hsp90 inhibitors further reduce the already very poor translation efficiency of EBNA1, and that the Gly-Ala repeat domain is required for this inhibition.
Fig. 4.
Geldanamycin inhibits EBNA1 translation in reticulocyte lysate. (A) pSG5 expression constructs encoding EBNA1 and BZLF1 were transcribed and translated in vitro with T7 RNA polymerase using a coupled transcription–translation reticulocyte lysate system supplemented with [35S]methionine/cysteine in the presence or absence of geldanamycin (0.5 μM). EBNA1 translation was reduced by 74% in the presence of geldanamycin. (B) pcDNA3.1 expression constructs encoding EBNA1 and EBNA1ΔGA were also transcribed and translated in vitro using the same method in the presence or absence of geldanamycin (0.5 μM). Ten fold less EBNA1ΔGA versus EBNA1 protein is loaded on the gel.
Hsp90 Does Not Associate with EBNA1.
To determine if Hsp90 forms a complex with EBNA1, the full-length EBNA1 and the mutant EBNA1 lacking the Gly-Ala repeats were transfected into AGS cells and immunoprecipitated with anti-EBNA1 antibodies. As shown in Fig. S3, no detectable Hsp90 protein was coimmunoprecipitated with either full-length or mutant EBNA1 protein. These results suggest that Hsp90 does not detectably associate with EBNA1.
Hsp90 Inhibitors Reduce Viability of EBV-Immortalized LCLs and Prevent EBV Transformation of Primary B Cells.
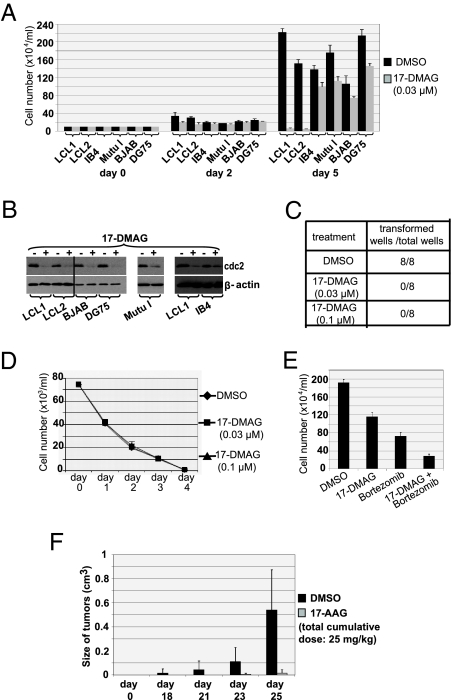
To determine if Hsp90 inhibitors affect the viability of LCLs in vitro, two different LCLs were treated for 5 d with low-dose 17-DMAG (0.03 μM) or vehicle (DMSO) and cell viability was determined by trypan blue exclusion. As shown Fig. 5A, 17-DMAG treatment induced close to 100% cell death of both lines. This drug-induced death in LCLs required several days of treatment, consistent with the long half-life of EBNA1 in B cells. In contrast, the same low dose of 17-DMAG had minimal effect on the growth of two EBV-negative B-cell lymphoma lines, BJAB and DG75; an EBV-positive Burkitt line, Mutu I, which can survive in the absence of EBV (in contrast to most EBV+ Burkitt lymphomas) (25); or an LCL line (IB4) previously shown to be EBNA1-independent as a result of an integrated EBV genome (26) (Fig. 5A). The effect of 17-DMAG on cellular cdc2 level was similar in each line (Fig. 5B), confirming that the drug is active in all cell types. To determine if Hsp90 inhibitors prevent EBV transformation of B cells, primary B cells (15,000 cells per condition) were infected with 100 infectious units of EBV and treated with low-dose 17-DMAG or DMSO beginning 1 h after infection. EBV infection of B cells resulted in the formation of LCLs by 3 to 4 weeks after infection in each of eight conditions treated with the vehicle control (Fig. 5C), whereas none of the 16 conditions treated with 17-DMAG (eight wells given 0.03 μM of 17-DMAG and eight wells given 0.1 μM of 17-DMAG) formed LCLs. Administration of 17-DMAG did not affect the viability of primary B cells (Fig. 5D). These results indicate that Hsp90 inhibitors prevent EBV transformation of primary B cells, and that even established LCLs are highly susceptible to the toxic effect of Hsp90 inhibitors. The combination of very low–dose 17-DMAG (0.005 μM) and low-dose bortezomib (an inhibitor of the 26S proteasome that is highly effective against LPD-like lesions in SCID mice) (27) killed more LCLs than either drug alone (Fig. 5E), suggesting the 17-DMAG/bortezomib combination might be particularly potent.
Fig. 5.
Hsp90 inhibitors reduce viability of EBV-transformed LCLs and prevent EBV transformation of primary B cells. (A) Two different independently derived LCLs (late passage line LCL1 and early passage line LCL2), IB4 (an LCL in which the EBV genome is integrated), two EBV-negative B-cell lymphoma lines (BJAB and DG75), and an EBV+ Burkitt lymphoma line (Mutu I), were treated with either vehicle control (DMSO) or 17-DMAG (0.03 μM). Cell counts in each condition were determined by trypan blue exclusion at the time points indicated. (B) Whole-cell extracts were prepared from cells treated with or without 17-DMAG (0.17 μM) and immunoblot analysis was performed to analyze the expression of cellular proteins as indicated. (C) Primary B cells were infected with EBV and treated with either vehicle control (DMSO) or 17-DMAG (0.03 μM or 0.1 μM) beginning 1 h after infection. Media (and drug) were replaced once per week. Numbers of transformed wells and the total numbers of wells treated with either no drug (DMSO) or 17-DMAG (0.03 μM or 0.1 μM) at 3 weeks after infection are given. (D) Primary B cells were treated with either no drug or 17-DMAG (0.03 μM or 0.1 μM). Cell counts in each condition were determined by trypan blue exclusion at the time points as indicated. (E) LCL1 cells were treated with no drug, 17-DMAG (0.005 μM), bortezomib (0.01 μM), or both, and cell counts determined. (F) EBV-positive lymphoblastoid cells (5 × 106 LCL1 cells) were implanted s.c. into the flanks of SCID mice. Tumors (six mice in each group) were treated with either no drug or three low doses (total cumulative dose, 25 mg/kg) of 17-AAG (given i.p. on d 7, 9, and 11 after injection of LCL1 cells). Columns show mean tumor volumes at different time points; bars show SE.
17-AAG Inhibits Lymphoproliferative Disease in SCID Mice.
To investigate whether Hsp90 inhibitors can inhibit the growth of EBV-induced lymphoproliferative disease at a nontoxic dose in SCID mice, mice were injected with 5 × 106 LCL1 cells in the flank at d 0, and then given three low doses of 17-AAG (total cumulative dose, 25 mg/kg) or DMSO on d 7, 9, and 11 following injection of the cells. As shown in Fig. 5F, 17-AAG dramatically inhibited the growth of EBV-transformed lymphoblastoid cells in SCID mice. These results suggest that 17-AAG may be particularly useful for treating EBV-positive lymphoproliferative disease in humans.
Expression of an EBNA1 Mutant Missing the Gly-Ala Repeat Domain Reduces the Toxic Effect of Hsp90 Inhibitors in LCLs.
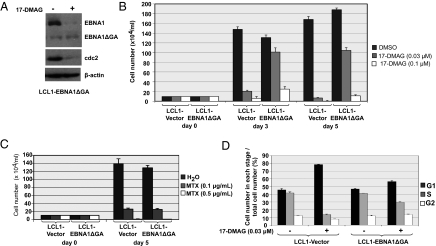
To determine if reduced EBNA1 expression contributes to Hsp90 inhibitor killing of LCLs, LCL1 cells were stably infected with a pBABE-puro retrovirus vector expressing the EBNA1 mutant missing the Gly-Ala repeat domain, or the empty retrovirus vector. The Gly-Ala repeat domain of EBNA1 is not required for any of the essential functions of EBNA1 in vitro. As expected, the EBNA1 mutant protein (driven by the retrovirus LTR promoter) was less susceptible than the full-length endogenous EBNA1 protein to Hsp90 inhibitors in the stably infected LCL line (Fig. 6A). LCLs expressing the mutant EBNA1 were much more resistant than vector control LCLs to the toxic effect of very low–dose 17-DMAG (0.03 μM; Fig. 6B). A higher dose of 17-DMAG (0.1 μM) prevented cellular replication in cells infected with the EBNA1 mutant retrovirus (consistent with the loss of cdc2 expression; Fig. 6A) but did not induce cell killing, whereas the vector control cells were killed by d 5. In contrast, the EBNA1 mutant did not protect LCLs from the toxic effect of methotrexate (MTX; Fig. 6C). In addition, LCLs expressing the mutant EBNA1 were more resistant than vector control LCLs to G1 arrest (Fig. 6D) and apoptotic events (Fig. S4) induced by low-dose 17-DMAG (0.03 μM). These results indicate that decreased EBNA1 expression substantially contributes to the unusual susceptibility of LCLs to Hsp90 inhibitors.
Fig. 6.
Expression of an EBNA1 mutant missing the Gly-Ala repeat domain decreases the toxic effect of Hsp90 inhibitors in LCLs. (A) LCL1 cells were infected with a retrovirus vector expressing EBNA1ΔGA or the control retrovirus vector. Immunoblot analysis was performed to analyze the expression of EBNA1, EBNA1ΔGA, cdc2, and β-actin in the presence and absence of 17-DMAG. (B) LCL1-vector and LCL1-EBNA1ΔGA cells were treated with either no drug or 17-DMAG (0.03 μM or 0.1 μM). Cell counts in each condition were determined by trypan blue exclusion at the time points indicated. (C) LCL1-vector and LCL1-EBNA1ΔGA cells were treated with either no drug or MTX (0.1 μg/mL or 0.5 μg/mL). Cell counts in each condition were determined by trypan blue exclusion at the time points indicated. (D) LCL1-vector and LCL1-EBNA1ΔGA cells were treated with either no drug or 17-DMAG (0.03 μM) for 48 h. The cell cycle distribution is given.
Discussion
The essential roles of EBNA1 in EBV genome maintenance, as well as its consistent expression in all proliferating EBV-positive cells, provide an attractive target for developing antiviral and antitumor strategies. Hsp90 inhibitors have recently been shown to inhibit the expression of some cellular, oncogenic Hsp90 clients at doses safe for humans. Here we show that Hsp90 inhibitors also effectively decrease expression EBNA1, and that this effect requires the EBNA1 Gly-Ala repeat domain. Furthermore, we show that Hsp90 inhibitors kill EBV-transformed B cells at nontoxic doses, and that this effect is at least partially caused by the loss of EBNA1 expression. Thus, Hsp90 inhibitors have been shown to inhibit EBNA1.
Although the exact mechanism for the Hsp90 inhibitor effect on EBNA1 remains unclear, the finding that Hsp90 inhibitors decrease translation of EBNA1 in vitro while not decreasing EBNA1 stability or half-life strongly suggests that their primary effect is to attenuate EBNA1 translation. Decreased translation of EBNA1 then leads to decreased transcription of EBNA1 in cells with type III latency, in which EBNA1 activates its own transcription. As EBNA1 and Hsp90 were not found to directly interact, we speculate that a cellular protein required to translate EBNA1 efficiently is an Hsp90 client protein. At least two ribosomal proteins, S3 and S6, are known to be Hsp90 client proteins (28). Our results suggest that the effect of Hsp90 inhibitors on translation is protein-specific. Interestingly, inhibition of EBNA1 translation by the Gly-Ala repeats is mediated at the nucleotide rather than protein sequence level (17).
Consistent with the ability of Hsp90 inhibitors to decrease EBNA1 expression, we found that these drugs prevent EBV transformation of primary B cells at nontoxic doses, and are highly toxic to established EBV-transformed LCLs. Our finding that Hsp90 inhibitors do not affect EBNA1 stability once the protein has been successfully translated, along with the very long half-life of EBNA1 in B cells, helps to explain why killing of LCLs by Hsp90 inhibitors requires a number of days. Thus, a previous study suggesting that Hsp90 inhibitors are not particularly toxic to LCLs (29) likely underestimated the toxicity of these drugs because cells were treated for only 1 d.
As the toxicity of low-dose Hsp90 inhibitors in LCLs is substantially reversed by expression of an EBNA1 mutant resistant to the Hsp90 inhibitor effect, the toxicity of these drugs in LCLs is at least partially mediated through loss of EBNA1 expression. Nevertheless, the ability of Hsp90 inhibitors to decrease expression and/or function of certain cellular proteins, particularly NF-κB, no doubt collaborates with the loss of EBNA1 to induce killing of EBV-transformed LCLs. Interestingly, as we also found that expression of the EBV protein LMP1 is rather dramatically increased (at least in LCLs) by Hsp90 inhibitors, and high level LMP1 expression is toxic (30), LMP1 overexpression may also contribute to the death of LCLs. The antiapoptotic effect (7, 8) of EBNA1 may normally attenuate the toxicity of LMP1. Finally, we also demonstrated that a nontoxic dose of 17-AAG effectively inhibits the growth of EBV-induced lymphoproliferative disease in SCID mice.
In addition to EBNA1, recent evidence suggests that some other important viral proteins also require Hsp90 for proper folding and/or stability. For example, poliovirus capsid protein P1 is expressed at only low levels in the presence of Hsp90 inhibitors, and geldanamycin treatment prevents the death of poliovirus-infected mice (31). Geldanamycin and 17-AAG delay growth of influenza A virus in cell culture and reduce half-life of the PB1 and PB2 subunits of the viral RNA polymerase complex (32). Hsp90 is also required for lytic replication of HSV-1 and human cytomegalovirus (33, 34).
Our results suggest that Hsp90 inhibitors might be useful for treating a variety of different EBV-induced diseases, provided that the continued presence of the viral genome is required for these EBV-associated illnesses. Given our finding that Hsp90 inhibitors prevent EBV transformation of B cells in vitro and inhibit the growth of EBV-induced lymphoproliferative disease in SCID mice, the most obvious target for Hsp90 inhibitor therapy in humans would be EBV-induced lymphoproliferative disease. In this disease, each of the known EBV-encoded transforming proteins is expressed, and there is little doubt that the continued presence of EBV is required for growth of these lesions. Another often fatal illness that appears to be highly dependent upon the presence of EBV, and might thus respond to Hsp90 inhibitors, is chronic active EBV disease. This rare disease, which most commonly occurs in Asia, is caused by persistent latent EBV infection of T cells and/or natural killer cells, and frequently culminates in EBV-positive T cell/natural killer cell malignancies (1).
Whether the loss of EBNA1 expression induced by Hsp90 inhibitors in EBV-positive tumors such as Hodgkin lymphoma, NPC, gastric carcinoma, and Burkitt lymphoma, which have additional genetic abnormalities and express only a subset of the EBV transforming proteins, would result in EBV-dependent killing is less clear. However, given that inhibition of EBNA1 induces apoptosis in most (but not all) EBV-positive Burkitt lymphoma cells in vitro (7, 25) and reduces the growth and survival of some EBV-positive epithelial tumors (35), these malignancies may indeed continue to require EBNA1 expression for their growth in vivo, similar to the recently described “oncogene addiction” theory for cellular oncogenes (36).
Finally, it is interesting to speculate whether Hsp90 inhibitors could be used to treat nonmalignant illnesses associated with EBV infection. In the case of EBV-induced IM, Hsp90 inhibitors would be predicted to not only reduce the number of cells infected with EBV, but would also likely attenuate the host immune response through their effect on cellular proteins such as NF-κB. As the host immune response to EBV-infected B cells is largely responsible for the clinical symptoms of this illness, short-term treatment of patients with low-dose Hsp90 inhibitors might alleviate the clinical symptoms of IM without increasing the risk of EBV-induced lymphoproliferative disease. In addition to IM, an increasing number of autoimmune illnesses have also been linked to EBV infection (including multiple sclerosis, lupus, and rheumatoid arthritis) (2), and continued expression of EBV-encoded antigens may contribute to these diseases. Hence, reducing the total number of EBV-infected cells in such patients might be useful. Nevertheless, as humans may be infected by different strains of EBV (37), long-term suppression of EBV infection using Hsp90 inhibitors would likely require lifelong therapy, and the long-term toxicities of these drugs are not known. In addition, EBV can persist in nonreplicating memory B cells without any EBNA1 expression. Thus, clinical trials will be required to assess the potential of these drugs for different types of EBV-induced illnesses.
Materials and Methods
In Vitro Cell Killing Studies with Hsp90 Inhibitors or MTX.
LCL cells (1 × 105 cells/mL) were treated with vehicle control (DMSO; 0.016%, vol/vol) or 17-DMAG (0.005, 0.03, or 0.1 μM) or bortezomib (0.01 μM). Human primary B cells infected or uninfected with EBV (the B95-8 strain) were treated with no drug (DMSO; 0.006%, vol/vol) or 17-DMAG (0.03 μM or 0.1 μM). LCL1 vector and LCL1 EBNA1ΔGA cells were treated with no drug (DMSO; 0.006%, vol/vol), 17-DMAG (0.03 μM, 0.1 μM, or 0.17 μM), or MTX (0.1 μg/mL or 0.5 μg/mL). Media containing DMSO or 17-DMAG were exchanged every 7 d. Cell killing was determined by trypan blue (Sigma-Aldrich) exclusion.
In Vitro Translation Assays.
The pSG5-EBNA1, pSG5-BZLF1, pcDNA3-EBNA1, and pcDNA3-EBNA1ΔGA constructs were transcribed and translated in vitro with T7 RNA polymerase using a coupled transcription/translation reticulocyte lysate system (Promega), supplemented with 20 μCi of 35[S]methionine/cysteine (GE Healthcare), in the absence or presence of geldanamycin (0.5 μM). Lysates were incubated for 5 min at 95 °C with protein loading sample buffer and subjected to SDS/PAGE. Gels were fixed, incubated with Amplify (GE Healthcare), dried, and then subjected to autoradiography.
EBV Transformation Assays.
Human primary B cells were incubated with WT EBV (B95.8) for 1 h at room temperature and divided into aliquots into wells of a 96-well plate (15,000 cells/well; 100 infectious units of virus/well). RPMI 1640 supplemented with 20% FBS and 10% penicillin–streptomycin was added to bring the medium volume up to 200 μL/well. 17-DMAG (or DMSO) was then added to achieve a final concentration of 0.03 μM or 0.1 μM. Media (and drug) were replaced once per week until transformation into LCLs was apparent in the vehicle treated cells (at approximately 3–4 weeks after infection).
In Vivo Tumor Studies.
All animal experiments were performed in accordance with the guidelines of the University of Wisconsin–Madison Animal Care Committee. LCL1 cells (5 × 106) were implanted s.c. into the flanks of 6-week-old SCID mice. The mice (six mice per group) were then given either three low doses of 17-AAG i.p. (total cumulative dose, 25 mg/kg) or vehicle control (DMSO) on d 7, 9, and 11 after injection of tumor cells. The mice were examined and tumor size was measured in three dimensions every 2 to 3 d. Mice were euthanized when the tumor size exceeded 1 cm3 in size. Statistical analysis was done using the t test.
Additional Methods.
Detailed methodology is described in SI Methods.
Supplementary Material
Acknowledgments
We thank Bill Sugden for helpful discussion, reviewing the manuscript, and multiple EBNA1 plasmid reagents; David Vereide for help with the cell cycle analysis; and Sarah Dickerson for help preparing the manuscript. This work was supported by National Institutes of Health grant P01 CA022443.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910717107/DCSupplemental.
References
- 1.Kieff E, Richinson AB. 2007. Epstein-Barr virus and its replication. Fields’ virology. (Lippincott Williams & Wilkins, Philadelphia) 5th ed. eds Knipe D, etal., pp 2603–2654. [Google Scholar]
- 2.Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- 3.Lindner SE, Sugden B. The plasmid replicon of Epstein-Barr virus: mechanistic insights into efficient, licensed, extrachromosomal replication in human cells. Plasmid. 2007;58:1–12. doi: 10.1016/j.plasmid.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor P, Lavoie BD, Frappier L. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by aurora family kinases. Mol Cell Biol. 2005;25:4934–4945. doi: 10.1128/MCB.25.12.4934-4945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Ceccarelli DF, Frappier L. The DNA segregation mechanism of Epstein-Barr virus nuclear antigen 1. EMBO Rep. 2000;1:140–144. doi: 10.1093/embo-reports/kvd026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altmann M, et al. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV’s transforming genes. Proc Natl Acad Sci USA. 2006;103:14188–14193. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saridakis V, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol Cell. 2005;18:25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs. 2002;7:277–288. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- 10.Grenert JP, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 11.Prodromou C, et al. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 12.Dai C, Whitesell L. HSP90: a rising star on the horizon of anticancer targets. Future Oncol. 2005;1:529–540. doi: 10.2217/14796694.1.4.529. [DOI] [PubMed] [Google Scholar]
- 13.Kamal A, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 14.Daskalogianni C, et al. Gly-Ala repeats induce position- and substrate-specific regulation of 26 S proteasome-dependent partial processing. J Biol Chem. 2008;283:30090–30100. doi: 10.1074/jbc.M803290200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellam J, et al. Influence of translation efficiency of homologous viral proteins on the endogenous presentation of CD8+ T cell epitopes. J Exp Med. 2007;204:525–532. doi: 10.1084/jem.20062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellam J, et al. Regulation of protein translation through mRNA structure influences MHC class I loading and T cell recognition. Proc Natl Acad Sci USA. 2008;105:9319–9324. doi: 10.1073/pnas.0801968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Y, Manoury B, Fåhraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301:1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 19.Nomura N, Nomura M, Newcomb EW, Zagzag D. Geldanamycin induces G2 arrest in U87MG glioblastoma cells through downregulation of Cdc2 and cyclin B1. Biochem Pharmacol. 2007;73:1528–1536. doi: 10.1016/j.bcp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Tellam J, et al. Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus-encoded nuclear antigen 1. J Exp Med. 2004;199:1421–1431. doi: 10.1084/jem.20040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 22.Qing G, Yan P, Xiao G. Hsp90 inhibition results in autophagy-mediated proteasome-independent degradation of IkappaB kinase (IKK) Cell Res. 2006;16:895–901. doi: 10.1038/sj.cr.7310109. [DOI] [PubMed] [Google Scholar]
- 23.Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 24.Fuller W, Cuthbert AW. Post-translational disruption of the delta F508 cystic fibrosis transmembrane conductance regulator (CFTR)-molecular chaperone complex with geldanamycin stabilizes delta F508 CFTR in the rabbit reticulocyte lysate. J Biol Chem. 2000;275:37462–37468. doi: 10.1074/jbc.M006278200. [DOI] [PubMed] [Google Scholar]
- 25.Cameron JE, et al. Epstein-Barr virus growth/latency III program alters cellular microRNA expression. Virology. 2008;382:257–266. doi: 10.1016/j.virol.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang MS, Hung SC, Kieff E. Epstein-Barr virus nuclear antigen 1 activates transcription from episomal but not integrated DNA and does not alter lymphocyte growth. Proc Natl Acad Sci USA. 2001;98:15233–15238. doi: 10.1073/pnas.211556598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou P, Kawada J, Pesnicak L, Cohen JI. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J Virol. 2007;81:10029–10036. doi: 10.1128/JVI.02241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TS, et al. Interaction of Hsp90 with ribosomal proteins protects from ubiquitination and proteasome-dependent degradation. Mol Biol Cell. 2006;17:824–833. doi: 10.1091/mbc.E05-08-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robles AI, et al. Schedule-dependent synergy between the heat shock protein 90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin and doxorubicin restores apoptosis to p53-mutant lymphoma cell lines. Clin Cancer Res. 2006;12:6547–6556. doi: 10.1158/1078-0432.CCR-06-1178. [DOI] [PubMed] [Google Scholar]
- 30.Lam N, Sandberg ML, Sugden B. High physiological levels of LMP1 result in phosphorylation of eIF2 alpha in Epstein-Barr virus-infected cells. J Virol. 2004;78:1657–1664. doi: 10.1128/JVI.78.4.1657-1664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 2007;21:195–205. doi: 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chase G, et al. Hsp90 inhibitors reduce influenza virus replication in cell culture. Virology. 2008;377:431–439. doi: 10.1016/j.virol.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Basha W, et al. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibits gene expression and replication of human cytomegalovirus. Antivir Chem Chemother. 2005;16:135–146. doi: 10.1177/095632020501600206. [DOI] [PubMed] [Google Scholar]
- 34.Burch AD, Weller SK. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J Virol. 2005;79:10740–10749. doi: 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Q, Flemington EK. siRNAs against the Epstein Barr virus latency replication factor, EBNA1, inhibit its function and growth of EBV-dependent tumor cells. Virology. 2006;346:385–393. doi: 10.1016/j.virol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitki-Green D, Covington M, Raab-Traub N. Compartmentalization and transmission of multiple epstein-barr virus strains in asymptomatic carriers. J Virol. 2003;77:1840–1847. doi: 10.1128/JVI.77.3.1840-1847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.