Abstract
Epidermal growth factor (EGF), a mitogen, also stimulates neurite extension during development, but the underlying mechanism is elusive. This study reveals a functional role for kappa opioid receptor (KOR) in EGF-stimulated neurite extension, and the underlying mechanism. EGF and activated EGF receptor (EGFR) levels are elevated in embryonic spinal cords during late gestation stages, with concurrent rise in protein levels of KOR and axon extension markers, growth-associated protein 43 (GAP43), and transient axonal glycoprotein-1 (TAG-1). Both GAP43 and TAG-1 levels are significantly lower in KOR-null (KOR−/−) spinal cords, and EGFR inhibitors effectively reduce the levels of KOR, GAP43, and TAG-1 in wild-type embryonic spinal cords. For KOR−/− or KOR-knockdown dorsal root ganglion (DRG) neurons, EGF can no longer effectively stimulate axon extension, which can be rescued by introducing a constitutive KOR expressing vector but not by a regulated KOR vector carrying its 5′ untranslated region, which can be bound and repressed by growth factor receptor-bound protein 7 (Grb7). Furthermore, blocking KOR activation by application of anti-dynorphin, KOR antagonist, or EGFR inhibitor effectively reduces axon extension of DRG neurons. Thus, EGF-stimulated axon extension during development is mediated, at least partially, by specific elevation of KOR protein production at posttranscriptional level, as well as activation of KOR signaling. The result also reveals an action of EGF to augment posttranscriptional regulation of certain mRNAs during developmental stages.
Keywords: 5′ untranslated region, dorsal root ganglion, epidermal growth factor, growth factor receptor-bound protein 7, posttranscriptional regulation
Opioid receptors (ORs) bind opioid drugs to modulate pain sensation, cognition functions, and animal behaviors, as demonstrated in many OR gene knockout studies (1, 2). Three ORs, μ, δ, and κ, all are detected in the developing nervous systems (3, 4). However, it is unclear how ORs may participate in developmental processes. Among the three OR genes, the kappa OR (KOR) gene is the first to exhibit its gene activity (mRNA expression) in development, detectable as early as E9.5 (3, 5). KOR is then elevated in the nervous system throughout later stages of development (3) when active neuritogenesis occurs (6). Although this early expression pattern of KOR mRNA would suggest a physiological function for KOR in development, it has been unclear as to the specific cellular processes associated with KOR activity. We have also observed that KOR mRNA distribution in adult spinal cords is altered after nerve injury (7). Given the KOR mRNA distribution pattern, it is tempting to speculate a functional role for KOR in the developmental and/or regenerating process of the nervous systems.
At the molecular level, the expression of KOR protein is heavily dependent on the efficiency of posttranscriptional events (8, 9), specifically its mRNA transport to neurites and its locally controlled translation (10–13). Previously, we have shown that these events can be stimulated by neuron activity and growth factors such as axon guidance cue, Netrin 1 (11–14). After transcription, KOR mRNA is mostly silenced by its specific RNA-binding protein, Grb7, which binds to its 5′ untranslated region (5′-UTR) and impedes cap recognition for translational initiation (14). An important switch to activate the silenced KOR mRNA for translation is the phosphorylation of Grb7 by Focal Adhesion Kinase (FAK), which is a downstream signal mediator for various growth factors such as Netrin and EGF (13–15). To determine the physiological function of KOR in the developing nervous system, we conducted preliminary experiments using primary DRG neurons dissected from the wild-type (WT) and KOR-null animals (16) to examine several developmental parameters and the sensitivity of the dissected DRG cultures to stimulation by potential growth factors. Preliminary experiments suggested that, in developing embryos, KOR could play a functional role in neurite extension, particularly in response to EGF stimulation.
EGF is a mitogen that is best known to regulate multiple cellular processes including proliferation, survival, and differentiation. Although it has not been as widely emphasized, EGF is also known to exert neurotrophic and/or neuromodulatory effects, thereby regulating neurite extension and neuronal activity as shown in studies of primary neurons (17, 18) and PC12 cells (19, 20). Indirectly, EGF also modulates neurite extension stimulated by hormones/factors such as thyroid hormones (21), suppressor of cytokine signaling-2 (18), and Versican G3 domain (22). EGF signal is primarily mediated by its binding to the EGF receptor (EGFR), which activates two signal transduction pathways. The first signal transduction pathway involves multiple cytoplasmic signaling molecules such as PKC, MAPK, PI3K, STATs, etc., which regulate cellular growth, survival, and differentiation (23, 24). The second pathway is through the nuclear translocation of EGFR to coregulate gene transcription (25, 26). However, the direct molecular mediator that transmits EGF signal to regulate neurite extension in development has remained elusive.
This study reports EGF as a physiological stimulus to elevate KOR protein level during development. Elevation in KOR protein level, as well as KOR activation by its agonist, plays a role in neurite, including axon, extension of the developing nervous system. Furthermore, the target of EGF action resides at posttranscriptional regulation of KOR, which involves a specific KOR 5′-UTR-binding protein, Grb7.
Results
KOR Ablation Lowers the Levels of Axon Extension Markers in the Developing Spinal Cord Without Affecting EGF Production or EGFR Activation.
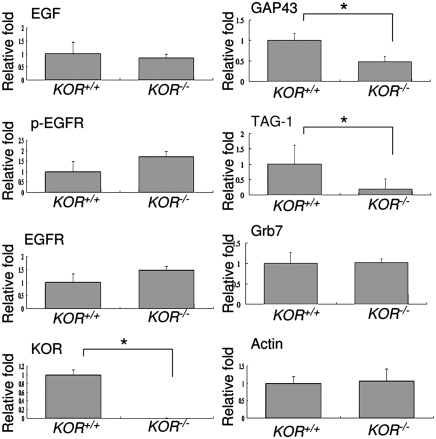
Our preliminary tests suggested that EGF-stimulated neurite extension was less effective in KOR-null primary neurons as compared to the WT neurons. To determine the causal relationship of KOR and EGF-stimulated neurite extension in development, we first examined the endogenous components relevant to EGF signal activation and neurite extension in the WT and KOR-null animals. This was achieved by determining protein levels of endogenous EGF, activated (phosphorylated) EGFR (p-EGFR), and two axon extension markers, growth associated protein 43 (GAP43) and transient axonal glycoprotein-1 (TAG-1), in the spinal cords of WT (KOR+/+) and KOR-null (KOR−/−) P0 newborn mice. As shown in Fig. 1 (corresponding Western blots shown in Fig. S1), despite retaining constant levels of EGF and p-EGFR, the KOR-null spinal cord had significantly lower GAP43 and TAG-1 protein levels as compared to the WT spinal cord. Neither EGFR nor Actin level was significantly different between the KOR-null and WT spinal cords. To rule out the possibility that this phenomenon might be due to nonspecific compensatory effects resulted from KOR knockout in the animals, the cerebellums, where KOR is known to be absent (3), from WT and KOR-null newborn mice were analyzed. As shown in Fig. S2, the expression levels of GAP43 and TAG-1 were not significantly different between WT and KOR-null animals. Therefore, the possible compensatory effect in spinal cord, due to KOR knockout, is largely ruled out. All together, our data reveal that KOR is involved in the expression of both GAP43 and TAG-1, indicators of axon extension, in the developing spinal cord. Furthermore, ablating KOR does not interfere with the production of EGF or EGFR, neither does it affect the activation of EGFR in developmental stages.
Fig. 1.
The axon extension marker proteins, GAP43 and TAG-1, were down-regulated in KOR-null (KOR−/−) spinal cord. Quantifications of Western blot of WT and KOR-null (KOR−/−) newborn spinal cords are presented. The data represent the mean ± SEM of quadruple samples (*P < 0.05). Corresponding Western blots are shown in Fig. S1.
Endogenous EGF Modulates KOR Protein Level and Axon Extension Markers in Animals.
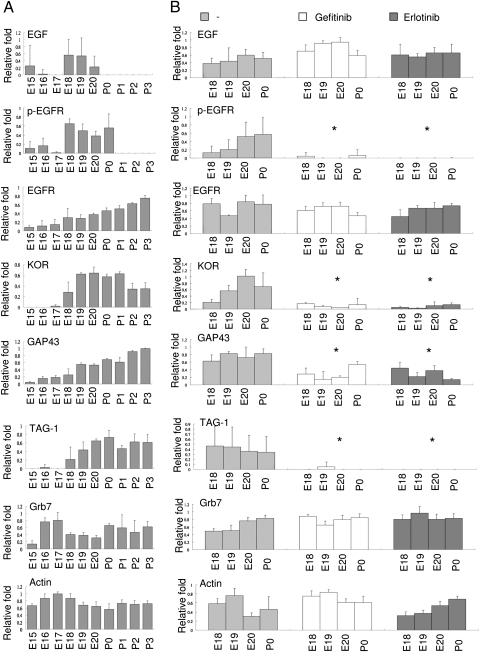
The fact that ablating KOR has no effect on EGF production or its receptor activation but does affect the levels of axon extension markers would suggest that KOR could be a downstream mediator of EGF to stimulate neurite/axon extension. We then monitored changes in the endogenous levels of these components in the WT mouse spinal cord along development. As shown in Fig. 2A (corresponding Western blots shown in Fig. S1), both endogenous EGF and activated EGFR (p-EGFR) were clearly detected from E18 to E20. The levels of KOR protein and axon extension markers, GAP43 and TAG-1, were significantly elevated on E18. The levels of Actin and the KOR mRNA-binding protein, Grb7 (see later, Fig. 5), stayed relatively constant throughout the time period monitored. Therefore, it seems that KOR expression parallels the production of endogenous EGF and EGFR activation in the spinal cord, which is closely correlated with the significant elevation of axon extension markers during the developmental period when active neuritogenesis occurs (20).
Fig. 2.
Endogenous EGF up-regulates KOR and axonal marker protein expression in mouse spinal cord. (A) Quantifications of Western blot (densitometry) of EGF, EGFR, pEGFR, KOR, GAP43, TAG-1, Grb7, and Actin from mouse embryos or newborn spinal cords are presented. The data represent the mean ± SEM of quadruple samples. (B) Quantifications of Western blot from control, Gefitinib-treated, or Erlotinib-treated mouse embryo or newborn spinal cords. The data represent the mean ± SEM of quadruple samples, analyzed by two-way ANOVA comparing drug-treated and control (*, P < 0.05). Corresponding Western blots are shown in Fig. S1.
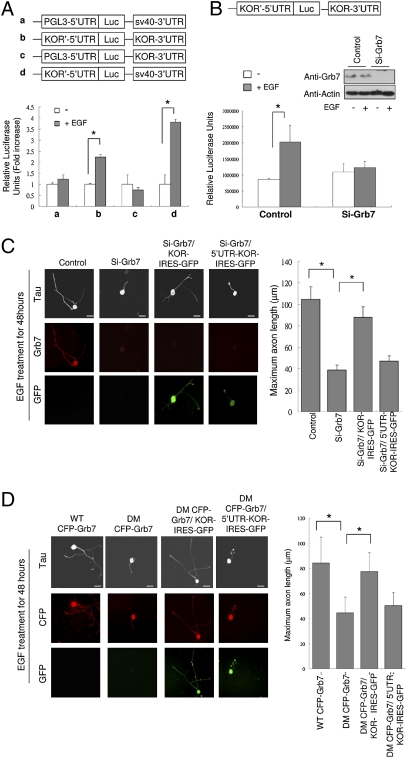
Fig. 5.
Grb7 mediates EGF-induced, KOR-dependent axon extension in primary DRG neurons. (A) Luciferase reporter activity in P19 cells transfected with different KOR UTR reporters shown on the top. Control or EGF treatment are shown with white or gray bars (*, P < 0.05). (B) Luciferase reporter activity in P19 cells transfected with KOR 5′ and 3′-UTR reporter along with either control siRNA (Left) or Grb7-specific siRNA (Right) (*, P < 0.05). Efficient silencing was confirmed by Western blots shown on the top right. (C) Immunohistochemistry (anti-Tau) of rat DRG neurons transfected with control siRNA (first column), Grb7 siRNA (second column), Grb7 siRNA plus KOR-IRES-GFP (third column), or Grb7 siRNA plus 5′-UTR-KOR-IRES-GFP (fourth column) upon EGF treatment. Quantitative analysis of axon length by scoring 50 neurons in each experiment is shown on the right (*, P < 0.05). (Scale bars: 25 μm.) (D) Immunohistochemistry (anti-Tau) of rat DRG neurons transfected with WT or DM Flag-Grb7 or KOR-IRES-GFP constructs as indicated. Quantitative analysis of axon length by scoring 50 neurons of each experiment is shown on the right (*, P < 0.05). (Scale bars: 25 μm.)
To determine whether the elevation of KOR, GAP43, and TAG-1 in spinal cords was indeed modulated by EGF, we employed two EGFR inhibitors, Gefitinib and Erlotinib, which could cross placenta (27) to block the action of endogenous EGF in embryos. We administered these EGFR inhibitors into pregnant mice on E17 and examined the spinal cords of E18 to P0 animals. As shown in Fig. 2B, the levels of pEGFR were significantly lower in the spinal cords of Gefitinib or Erlotinib-treated animals (E18 to P0), confirming the efficacy of these EGFR inhibitors in these experiments. Blockage of the endogenous EGF signals lowered the levels of endogenous KOR, GAP43 and TAG-1, but exerted no effects on the control, Actin, or the KOR RNA-binding protein Grb7. These data show that EGFR activation is required for the elevation of KOR and axon extension markers during late gestation and early postnatal stages.
KOR and Its Ligands Are Required for EGF-Stimulated Axon Extension of Primary DRG Neurons.
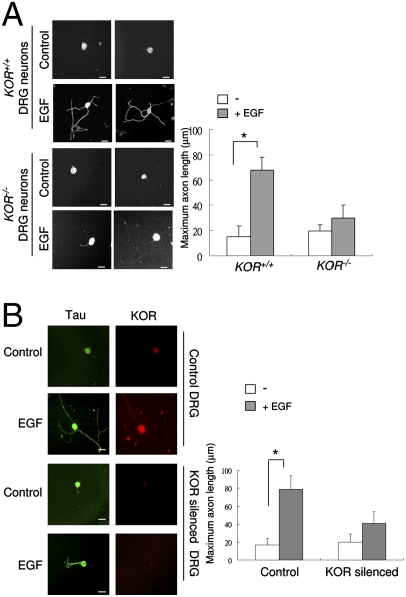
The observation that two axon extension markers were down-regulated in KOR-null spinal cord would suggest a functional role for KOR in EGF-stimulated axon extension. This was tested by using primary DRG neurons obtained from normal and KOR-null mutant mice (16), as well as normal and KOR-silenced primary rat DRG neurons. As shown in Fig. 3A, EGF effectively stimulated axon outgrowth from the WT (KOR+/+) mouse DRG neurons, but it failed to efficiently exert such effects for KOR-null (KOR−/−) mouse DRG neurons. This observation was further confirmed using rat primary DRG neurons transfected with either the control- or KOR-specific siRNA before EGF treatment (Fig. 3B) The efficiency of siRNA knockdown of KOR in rat DRG neurons was accessed by immunohistochemistry (Fig. 3B) and by Western blot (Fig. S3), with anti-KOR antibody. Therefore, the functional role for KOR in mediating the effect of EGF-stimulated axon outgrowth appears to be common to both the mouse and the rat.
Fig. 3.
KOR is required for EGF-induced axonal extension of primary DRG neurons. (A) Two immunohistochemistry images of KOR+/+ and KOR−/− primary mouse DRG neurons treated with, or without, EGF for 48 h and detected with anti-Tau antibody. Quantitative analysis of axon length by scoring 50 neurons is shown on the right (*P < 0.05). (Scale bars: 25 μm.) (B) Immunohistochemistry of rat DRG neurons transfected with control siRNA (first and second panels from the top) or KOR-specific siRNA (third and fourth panels from the top) on control (first and third panels from the top) or EGF treatment (second and fourth panels from the top), stained with anti-Tau or anti-KOR antibodies. Quantitative measurement of axon length from 50 neurons is shown on the right (*, P < 0.05). (Scale bars: 25 μm.)
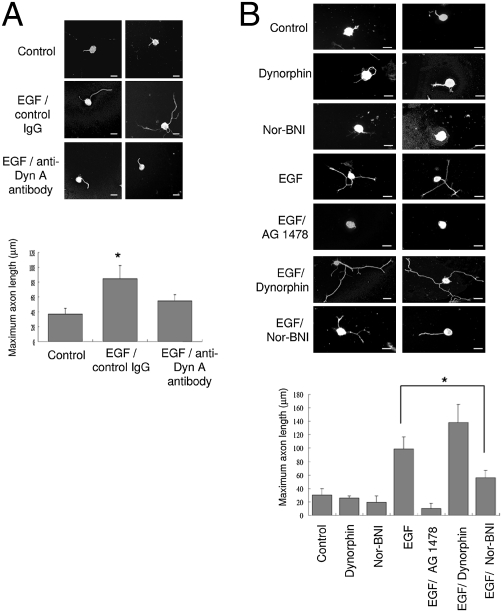
To determine whether EGF-stimulated axon extension required both KOR and its ligand, we examined the effects of the endogenous KOR ligand, dynorphin, on EGF-stimulated axon extension by using anti-dynorphin antibody to block endogenous dynorphin in rat DRG cultures (28). As shown in Fig. 4A, anti-dynorphin indeed reduced EGF-stimulated rat DRG neuron's axon extension, as compared to the IgG control. The reduction in axon extension by anti-dynorphin antibody was abolished when the antibody had been preincubated with the dynorphin peptide (Fig. S4), supporting the specificity and efficiency of this antibody in blocking endogenous dynorphin in the DRG neuron cultures. Together, these results suggests that both KOR and its ligand are required for EGF's action in stimulating axon outgrowth.
Fig. 4.
KOR ligand is required for EGF-induced axon extension in primary DRG neurons. (A) Two immunohistochemical images from primary rat DRG neurons treated with EGF and anti-dynorphin A antibody (Bottom). Untreated neurons (Top) or neurons treated with IgG and EGF (Middle) serve as controls. Quantitative analysis of axon length from 50 neurons of each experiment is shown on the bottom (*, P < 0.05). (B) Two immunohistochemical images from primary rat DRG neurons treated with dynorphin, nor-BNI, EGF, EGF plus AG 1478, EGF plus dynorphin, and EGF plus nor-BNI. Quantitative analysis of axon length from 50 neurons of each experiment is shown below (*, P < 0.05). (Scale bars: 25 μm.)
To support this viewpoint, we employed several KOR ligands in rat DRG cultures. Interestingly, without EGF treatment, neither KOR agonist (dynorphin) nor antagonist (nor-BNI) exerted a significant effect to stimulate axon outgrowth (Fig. 4B). Additional dynorphin slightly enhanced the effect of EGF, whereas the KOR antagonist nor-BNI significantly reduced the effect of EGF (Fig. 4B). The EGFR inhibitor, AG 1478, blocked the effect of EGF on axon extension as predicted. These data confirm that both the elevation of KOR protein level and KOR agonist binding are required for efficient EGF-stimulated axon extension.
Signal of EGF to Stimulate KOR/Dynorphin-Dependent Axon Extension Depends on Grb7-Regulated KOR Protein Expression.
To determine the target of EGF action on the regulation of KOR protein level, i.e., through transcriptional or posttranscriptional events, we first performed a real-time RT-PCR experiment, which ruled out the effect of EGF on the steady state level of KOR mRNA (Fig. S5). Therefore, the target would reside at the posttranscriptional level that involved, primarily, the UTRs of KOR mRNA. We conducted a series of reporter assays in P19 embryonal carcinoma cells to identify the target region (within the KOR UTRs) of EGF action. As shown in Fig. 5A, the full-length (containing KOR 5′- and 3′-UTRs) and 5′-UTR KOR reporters, but not the 3′-UTR reporter, showed increased activities after EGF treatment, suggesting the 5′-UTR of KOR mRNA as the target of EGF action. One crucial regulator for this 5′-UTR is its specific binding protein, Grb7 (14). We then examined the activity of Grb7 in mediating the effect of EGF by silencing the endogenous Grb7. As shown in Fig. 5B, in the Grb7-silenced cellular background, this 5′-UTR reporter basal activity (without EGF stimulation) was not changed. Importantly, EGF could no longer enhance this KOR reporter activity under the Grb7-silencing condition, suggesting that EGF acted to augment KOR mRNA regulation by Grb7.
Based on the results from the reporter assays, we predicted that Grb7 should also be involved in EGF-stimulated axon extension. We tested this possibility by silencing the endogenous Grb7 in rat DRG neurons and monitored the effect of EGF on their axon extension. As shown in Fig. 5C, Grb7 knockdown rendered these DRG neurons resistant to EGF stimulation, supporting that EGF-stimulated axon outgrowth depended on KOR mRNA regulation by Grb7, which could modulate the level of KOR protein. The efficiency of siRNA knockdown of Grb7 in rat DRG neuron cultures was determined by immunohistochemistry (Fig. 5C) and Western blot (Fig. S3), with anti-Grb7 antibody. This was further confirmed by effective rescue of Grb7 knockdown of these DRG neurons by introducing a KOR constitutive expression vector [an expression vector deleted in the Grb7-binding site (KOR-IRES-GFP)]. This KOR expression vector does not require the action of Grb7, and therefore would constitutively produce KOR protein. Intriguingly, reduced axon outgrowth seen in Grb7 knockdown DRG neurons could not be effectively rescued by the Grb7-regulated KOR expression vector 5′-UTR-KOR-IRES-GFP that carried the Grb7-binding sequence. This confirmed that Grb7 was required for elevating KOR level. However, this outcome seemed to contradict its previously established role as a KOR translational repressor (14). A potential explanation was that Grb7 could also regulate other posttranscriptional regulatory events through its binding to KOR mRNA, such as mobilizing KOR mRNA, in addition to its repressing KOR translation. This was supported by studying these two KOR expression vectors (Fig. S6A) in P19 cells that expressed endogenous Grb7. KOR protein level and ligand binding could be better quantified in this system. As shown in Fig. S6B, the Grb7-regulated 5′-UTR-KOR-IRES-GFP vector indeed expressed a higher level of KOR protein than the KOR vector lacking its 5′-UTR (KOR-IRES-GFP). Fig. S6C showed that KOR ligand binding activity indeed was also higher in cells receiving the 5′-UTR-containing KOR expression vector (5′-U TR-KOR-IRES-GFP). These results revealed additional posttranscriptional regulation of KOR protein production by Grb7 binding to its 5′-UTR (see Discussion). The possibility that silencing Grb7 might have affected KOR mRNA stability was ruled out, because KOR mRNA half-life was unaffected by silencing Grb7, as monitored by real-time RT-PCR at different time points (Fig. S7).
One key feature of Grb7 binding to the 5′-UTR of KOR mRNA resides at its phosphorylation, which abolishes its KOR RNA-binding ability. We then exploited a previously generated Grb7 dominant negative mutant that could not be phosphorylated (14) to confirm the functionality of Grb7 in mediating the stimulating effect of EGF on axon extension. We compared the effects of WT Grb7 (WT CFP-Grb7) and this dominant negative mutant Grb7 (DM CFP-Grb7) in rat DRG neuron axon outgrowth experiments (Fig. 5D). The dominant negative mutant Grb7 would remain tightly binding to KOR mRNA, thereby ultimately arresting KOR mRNA translation. In the presence of this dominant negative mutant Grb7 that tightly bound KOR 5′-UTR and could not release KOR mRNA for translation (14), the 5-UTR-carrying KOR vector would be resistant to EGF stimulation. Indeed, as shown in Fig. 5D, forced expression of DM CFP-Grb7, as compared to the WT CFP-Grb7, effectively inhibited EGF-stimulated axon outgrowth in DRG neurons. Furthermore, blockage by this mutant Grb7 could be rescued by coexpressing the constitutive KOR expression vector KOR-IRES-GFP, but not by the Grb7-regulated KOR expression vector 5′-UTR-KOR-IRES-GFP. Together, these data confirm that Grb7-regulated elevation in KOR protein level indeed contributes to EGF-stimulated axon outgrowth.
Discussion
EGF has been shown to both stimulate and inhibit neuritogenesis (29, 30), mediated by its direct or indirect effects in the system. However, during developmental stages, EGF is known to stimulate neurite extension as shown in most studies (18, 31–33); but the mechanism has been elusive. This current study reveals a functional role for KOR in mediating EGF-stimulated axon extension in development. Interestingly, this effect requires the elevation of KOR protein level that is regulated primarily at the posttranscriptional level and involves a KOR mRNA 5′-UTR binding protein Grb7.
The correlation of EGF level with the levels of KOR protein and axon extension markers in spinal cords is very obvious in late embryonic to early postnatal stages, at least for the mouse. In dissociated primary DRG neuron cultures, EGF acts to stimulate axon extension by elevating KOR protein levels through posttranscriptional regulation of KOR mRNA, which involves the KOR 5′-UTR binding protein Grb7. Whereas EGF has been shown to induce neurite outgrowth in several studies (17–20), this report reveals one physiologically relevant mediator for the action of EGF in neurite extension. More importantly, this study provides evidence for the functional role of endogenous KOR and its ligand in neurite/axon extension, and establishing the crosstalk between EGF and the endogenous opioid/opioid receptor system. The data also show that agonist binding to KOR is important for EGF-stimulated neurite/axon extension. Activation of opioid receptor, including KOR, by agonists is known to modulate multiple down stream signaling effectors that are potential axon outgrowth regulators, such as extracellular signal-regulated kinase 1/2 (ERK1/2) (34), p38 mitogen-activated protein kinase (MAPK) (35), and c-Jun N-terminal kinase (JNK) (36). Whether and how these effectors contribute specifically to EGF-stimulated neurite outgrowth needs to be evaluated in the future. Opioid receptors have been shown to be involved in neuronal differentiation (37–39). This study adds a functional role for KOR in the development of the nervous system, specifically in neurite/axon extension. Whether other opioid receptors also participate in neurite outgrowth is an interesting topic to examine in the future.
An important key regulatory target of EGF action resides at Grb7-binding to the 5′-UTR of KOR mRNA. During embryonic and postnatal development, Grb7 level stays relatively constant, whereas the levels of EGF, activated EGFR (pEGFR) and KOR peak in a specific time window when active neuritogenesis occurs [E18 to early postnatal periods (Fig. 2)]. This would suggest that although KOR mRNA can be expressed much earlier during embryonic stages, KOR protein production is probably not very active until later when growth factors, such as EGF, are available to regulate its posttranscriptional events. Thus, posttranscriptional regulatory events are even more critical than transcriptional events in modulating KOR protein levels at specific time and in selected tissues, i.e., the temporal and spatial control for KOR protein is likely to depend heavily on the availability of signals/factors available locally and in a timely fashion. Because we only profiled EGF signaling pathway in the spinal cords of mouse embryos and early postnatal animals, it remains to be determined whether other factors/stimuli for KOR protein production during development would be as efficient as EGF in stimulating neurite extension.
An intriguing finding pertinent to the functional role of Grb7, a specific KOR 5′-UTR binding protein and a known KOR translational repressor, is the surprising outcome of studies using the 5′-UTR-carrying KOR vector under Grb7-silencing (Fig. 5B and Fig. S6). Given the established function of Grb7 in repressing KOR translation, silencing Grb7 would be sufficient to remove the barrier for KOR protein production, and therefore this alone would elevate the basal level of the KOR reporter (Fig. 5B). However, this experiment shows similar basal levels of KOR reporter activity, with or without Grb7 silencing in P19 (Fig. 5B), which suggests that removing Grb7 alone is unable to elevate KOR production, and indicates the requirement for Grb7 in other posttranscriptional events to modulate KOR production, such as mobilizing KOR mRNA. This is partially supported by the results of expressing the 5′-UTR-containing KOR vector (Fig. S6) in the Grb7-containing P19 cellular background where deleting this Grb7-binding 5′-UTR reduces the efficiency of KOR protein production (as shown by the lower KOR level expressed by KOR-IRES-GFP that lacks the 5′-UTR). However, the complicated functional role of Grb7 in regulating KOR posttranscriptional regulation remains to be further investigated. Currently, we have ruled out the effect of Grb7 on KOR mRNA stability (Fig. S7).
Grb7 belongs to the Grb7 super family of adaptors (40). Studies have shown that other Grb7 family members can also be expressed in the nervous system (41–43). This current study provides evidence for a specific functional role of Grb7 in regulating neurite extension through controlling the level of KOR protein at posttranscriptional levels. Whether this is specific to EGF signals or may also be triggered by other growth factors such as Netrin remains to be validated.
Materials and Methods
Antibody and Reagent.
The antibodies were from Santa Cruz Biotechnology (anti-Grb7, anti-Actin, anti-EGF, anti-EGFR, and anti-p-EGFR). Abcam (anti-KOR and anti-TAG-1) and Sigma (anti-Tau and anti-GAP43). EGF, U69,593, nor-binaltorphimine (nor-BNI), and dynorphin were from Sigma. Gefitinib and Erlotinib were from LC Laboratories. AG 1478 was from Calbiochem. 3H-U69,593 was from PerkinElmer. Dynorphin peptide was from AnaSpec.
Plasmid Construction, siRNAs, and Transfection.
WT Flag-Grb7 and DM Flag-Grb7 were as described in refs. 14 and 44. KOR-IRES-GFP or 5′-UTR-KOR-IRES-GFP was made by inserting KOR or 5′-UTR-KOR into NotI/SpeI site of pShuttle-IRES-hrGFP-1 vector (Stratagene). siRNA against rat KOR were from Qiagen with targeting sequences TCC GGG TAG AGA AGA GTT CAA and AAG AGA TAT ATA TGT GGA CAA. The plasmid transfection was performed with Lipofectamine 2000 (Invitrogen), and the siRNA transfection was performed with Hyperfect reagent (Qiagen).
Cell Culture and Ligand Binding Assay.
P19 cells and DRG neurons were cultured as described in ref. 13, with slight modification. To exclude extrinsic effects on KOR expression and axon extension, NGF and N2 supplements were excluded from the regular culture medium. Experiments were all done within 5 days after initial plating to prevent neuronal death due to lack of NGF. EGF treatment was at final concentration 100 μg/mL added at the second day after plating. The ligand binding assay was performed as described in ref. 13.
Immunohistochemistry and Western Blotting.
The immunohistochemistry and Western blotting was performed as described in ref. 45.
Animal Experiments.
The KOR-null animals are reported in ref. 16. To collect spinal cords at different developmental stages, pregnant mice (C57/B6) were killed. Four spinal cords from each timed-embryos or newborn mice were surgically collected and total protein was extract for Western blotting. To block EGFR, pregnant mice were orally administered with Gefitinib (150 mg/kg/day) or Erlotinib (100 mg/kg/day) at E17. The spinal cords were collected from E18 to P0 and total protein was obtained for Western blotting. The Western blot results were quantified by using ImageJ software. The band with highest intensity among the same gene was set as 1.
Measuring Axon Length and Statistical Analysis.
The maximum axon length of 50 DRG neurons under same EGF treatment (100 μg/mL) was measured under microscope with Image ProPlus software (Media Cybernetics). Vectors of CFP-Grb7 fusion protein and those carrying IRES-GFP were used in transfection studies to identify specific cells acquiring these expression vectors. Only fluorescence-positive cells were scored and analyzed. The data in this study is presented as means ± SDs and analyzed with Student’s t test or two-way ANOVA where P < 0.05 was considered as significant.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants DA11190, DA11806, DK54733, DK60521, and K02-DA13926.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912367107/DCSupplemental.
References
- 1.Gavériaux-Ruff C, Kieffer BL. Opioid receptor genes inactivated in mice: the highlights. Neuropeptides. 2002;36:62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- 2.Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Land BB, et al. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, Cao S, Loh HH, Wei LN. Promoter activity of mouse kappa opioid receptor gene in transgenic mouse. Brain Res Mol Brain Res. 1999;69:35–43. doi: 10.1016/s0169-328x(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 6.Mahalik TJ, Carrier A, Owens GP, Clayton G. The expression of GAP43 mRNA during the late embryonic and early postnatal development of the CNS of the rat: an in situ hybridization study. Brain Res Dev Brain Res. 1992;67:75–83. doi: 10.1016/0165-3806(92)90027-t. [DOI] [PubMed] [Google Scholar]
- 7.Sung B, Loh HH, Wei L. Association of kappa opioid receptor mRNA upregulation in dorsal root ganglia with mechanical allodynia in mice following nerve injury. Neurosci Lett. 2000;291:163–166. doi: 10.1016/s0304-3940(00)01394-x. [DOI] [PubMed] [Google Scholar]
- 8.Wei LN, Law PY, Loh HH. Post-transcriptional regulation of opioid receptors in the nervous system. Front Biosci. 2004;9:1665–1679. doi: 10.2741/1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei LN, Loh HH. Regulation of opioid receptor expression. Curr Opin Pharmacol. 2002;2:69–75. doi: 10.1016/s1471-4892(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 10.Bi J, Hu X, Loh HH, Wei LN. Mouse kappa-opioid receptor mRNA differential transport in neurons. Mol Pharmacol. 2003;64:594–599. doi: 10.1124/mol.64.3.594. [DOI] [PubMed] [Google Scholar]
- 11.Bi J, Tsai NP, Lin YP, Loh HH, Wei LN. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc Natl Acad Sci USA. 2006;103:19919–19924. doi: 10.1073/pnas.0607394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi J, Tsai NP, Lu HY, Loh HH, Wei LN. Copb1-facilitated axonal transport and translation of kappa opioid-receptor mRNA. Proc Natl Acad Sci USA. 2007;104:13810–13815. doi: 10.1073/pnas.0703805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai NP, Bi J, Loh HH, Wei LN. Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA. J Neurosci. 2006;26:9743–9749. doi: 10.1523/JNEUROSCI.3014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai NP, Bi J, Wei LN. The adaptor Grb7 links netrin-1 signaling to regulation of mRNA translation. EMBO J. 2007;26:1522–1531. doi: 10.1038/sj.emboj.7601598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, et al. A novel variant of human Grb7 is associated with invasive esophageal carcinoma. J Clin Invest. 1998;102:821–827. doi: 10.1172/JCI2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansonoff MA, et al. Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther. 2006;318:641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg A, Noble EP. EGF-induced neuritogenesis and correlated synthesis of plasma membrane gangliosides in cultured embryonic chick CNS neurons. J Neurosci Res. 1989;24:531–536. doi: 10.1002/jnr.490240411. [DOI] [PubMed] [Google Scholar]
- 18.Goldshmit Y, Greenhalgh CJ, Turnley AM. Suppressor of cytokine signalling-2 and epidermal growth factor regulate neurite outgrowth of cortical neurons. Eur J Neurosci. 2004;20:2260–2266. doi: 10.1111/j.1460-9568.2004.03698.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasai A, Shima T, Okada M. Role of Src family tyrosine kinases in the down-regulation of epidermal growth factor signaling in PC12 cells. Genes Cells. 2005;10:1175–1187. doi: 10.1111/j.1365-2443.2005.00909.x. [DOI] [PubMed] [Google Scholar]
- 20.Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse. 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- 21.Martinez R, Gomes FC. Neuritogenesis induced by thyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem. 2002;277:49311–49318. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- 22.Xiang YY, et al. Versican G3 domain regulates neurite growth and synaptic transmission of hippocampal neurons by activation of epidermal growth factor receptor. J Biol Chem. 2006;281:19358–19368. doi: 10.1074/jbc.M512980200. [DOI] [PubMed] [Google Scholar]
- 23.Doroquez DB, Rebay I. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit Rev Biochem Mol Biol. 2006;41:339–385. doi: 10.1080/10409230600914344. [DOI] [PubMed] [Google Scholar]
- 24.Sibilia M, et al. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation. 2007;75:770–787. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 25.Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19:124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat. 2006;95:211–218. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 27.Perry MC. The Chemotherapy Source Book. Hagerstown, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 28.Sweetnam PM, Wrathall JR, Neale JH. Localization of dynorphin gene product-immunoreactivity in neurons from spinal cord and dorsal root ganglia. Neuroscience. 1986;18:947–955. doi: 10.1016/0306-4522(86)90110-7. [DOI] [PubMed] [Google Scholar]
- 29.Povlsen GK, Berezin V, Bock E. Neural cell adhesion molecule-180-mediated homophilic binding induces epidermal growth factor receptor (EGFR) down-regulation and uncouples the inhibitory function of EGFR in neurite outgrowth. J Neurochem. 2008;104:624–639. doi: 10.1111/j.1471-4159.2007.05033.x. [DOI] [PubMed] [Google Scholar]
- 30.Koprivica V, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 31.Kornblum HI, et al. Epidermal growth factor and basic fibroblast growth factor: effects on an overlapping population of neocortical neurons in vitro. Brain Res. 1990;535:255–263. doi: 10.1016/0006-8993(90)91608-j. [DOI] [PubMed] [Google Scholar]
- 32.Morrison RS, Kornblum HI, Leslie FM, Bradshaw RA. Trophic stimulation of cultured neurons from neonatal rat brain by epidermal growth factor. Science. 1987;238:72–75. doi: 10.1126/science.3498986. [DOI] [PubMed] [Google Scholar]
- 33.Morrison RS, Keating RF, Moskal JR. Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J Neurosci Res. 1988;21:71–79. doi: 10.1002/jnr.490210111. [DOI] [PubMed] [Google Scholar]
- 34.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruchas MR, et al. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SW, He Y, Ha SG, Loh HH, Wei LN. Epigenetic regulation of kappa opioid receptor gene in neuronal differentiation. Neuroscience. 2008;151:1034–1041. doi: 10.1016/j.neuroscience.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim E, et al. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–33760. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narita M, et al. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J Neurochem. 2006;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- 40.Daly RJ. The Grb7 family of signalling proteins. Cell Signal. 1998;10:613–618. doi: 10.1016/s0898-6568(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 41.Sanz LA, et al. A mono-allelic bivalent chromatin domain controls tissue-specific imprinting at Grb10. EMBO J. 2008;27:2523–2532. doi: 10.1038/emboj.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monk D, et al. Reciprocal imprinting of human GRB10 in placental trophoblast and brain: evolutionary conservation of reversed allelic expression. Hum Mol Genet. 2009;18:3066–3074. doi: 10.1093/hmg/ddp248. [DOI] [PubMed] [Google Scholar]
- 43.Colley BS, Cavallin MA, Biju K, Marks DR, Fadool DA. Brain-derived neurotrophic factor modulation of Kv1.3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neurosci. 2009;10:8. doi: 10.1186/1471-2202-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai NP, Ho PC, Wei LN. Regulation of stress granule dynamics by Grb7 and FAK signalling pathway. EMBO J. 2008;27:715–726. doi: 10.1038/emboj.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai NP, Tsui YC, Wei LN. Dynein motor contributes to stress granule dynamics in primary neurons. Neuroscience. 2009;159:647–656. doi: 10.1016/j.neuroscience.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.