Abstract
The human pathogens enteropathogenic (EPEC) and enterohemorrhagic Escherichia coli and the related mouse pathogen Citrobacter rodentium subvert a variety of host cell signaling pathways via their plethora of type III secreted effectors, including triggering of an early apoptotic response. EPEC-infected cells do not develop late apoptotic symptoms, however. In this study we demonstrate that the NleH family effectors, homologs of the Shigella effector kinase OspG, blocks apoptosis. During EPEC infection, NleH effectors inhibit elevation of cytosolic Ca2+ concentrations, nuclear condensation, caspase-3 activation, and membrane blebbing and promote cell survival. NleH1 alone is sufficient to prevent procaspase-3 cleavage induced by the proapoptotic compounds staurosporine, brefeldin A, and tunicamycin. Using C. rodentium, we found that NleH inhibits procaspase-3 cleavage at the bacterial attachment sites in vivo. A yeast two-hybrid screen identified the endoplasmic reticulum six-transmembrane protein Bax inhibitor-1 (BI-1) as an NleH-interacting partner. We mapped the NleH-binding site to the N-terminal 40 amino acids of BI-1. Knockdown of BI-1 resulted in the loss of NleH’s antiapoptotic activity. These results indicate that NleH effectors are inhibitors of apoptosis that may act through BI-1 to carry out their cytoprotective function.
Keywords: Citrobacter rodentium, type 3 secretion system, enterohemorrhagic E. coli, OspG, Shigella
Enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli (EHEC), and the mouse pathogen Citrobacter rodentium (reviewed in refs. 1 and 2) are closely related diarrheagenic pathogens that intimately adhere to gut enterocytes and instigate localized effacement of brush border microvilli (3), collectively known as attaching and effacing (A/E) lesions. Injection of bacterial effector proteins via a type III secretion system is an integral part of the EPEC, EHEC, and C. rodentium infection strategy (4, 5). These pathogens encode a plethora of effector proteins (6, 7) that target an intricate array of host cell signaling processes to facilitate colonization, multiplication, dissemination, and infection (5). Importantly, 21 effectors (known as core effectors) are conserved among EPEC, EHEC, and C. rodentium (6), whereas other effectors are strain-specific.
NleH is one of these core effectors (8). EPEC and EHEC contain two nleH genes (nleh1 and nleH2), and C. rodentium harbors a single copy of nleH. NleH effectors are homologous to the Shigella effector OspG, a protein kinase that prevents ubiquitination and subsequent degradation of phospho-IκBα and downstream activation of the transcriptional factor NF-κB (9). Using C. rodentium, we have shown that NleH increases NF-κB activity and TNF-α expression in the mouse colonic mucosa and confers a competitive advantage in mixed infections (10).
Among the other core effectors, EspF disrupts the mitochondria membrane potential (11), opens the tight junctions (12), and induces degradation of the antiapoptotic protein AbcF2 (13). But despite the potent proapoptotic effect of EspF, EPEC-infected cells exhibit early features of apoptosis, including expression of phosphatidylserine on the cell surface and cleavage of cellular DNA (14, 15), but do not undergo cell shrinkage, membrane blebbing, or nuclear condensation and fragmentation, all of which are key features of late-stage apoptosis (14–16). In fact, the proportion of apoptotic cells in monolayers infected with EPEC has been shown to be significantly lower than that of cells infected with Salmonella (14).
Apoptosis can occur via two major pathways, intrinsic (mitochondria- and ER-mediated pathways) and extrinsic (receptor-mediated pathway) (17). Induction of apoptosis via the intrinsic pathway involves activation of the Bcl-2 homology 3–only proteins and oligomerization of the proapoptotic proteins Bak and Bax (18), leading to permeabilization of the mitochondrial outer membrane and release of cytochrome c (17). Cytosolic cytochrome c interacts with the apoptosis activating factor 1 and procaspase-9 in the presence of dATP, forming an apoptosome that cleaves and activates the executioner caspases procaspase-3, -6, and -7 (19, 20), which in turn cleave numerous protein substrates, leading to apoptosis (21). Because apoptosis relies on a fine balance between proapoptotic and antiapoptotic factors, we hypothesized that A/E pathogens encode effector(s) with antiapoptotic activity that neutralize the EspF effects and promote cell survival. In this study, we demonstrated that NleH plays a role in modulating apoptotic responses during EPEC and C. rodentium infections by inhibiting caspase activation.
Results
Cells Infected with EPEC ΔnleH Undergo Apoptosis.
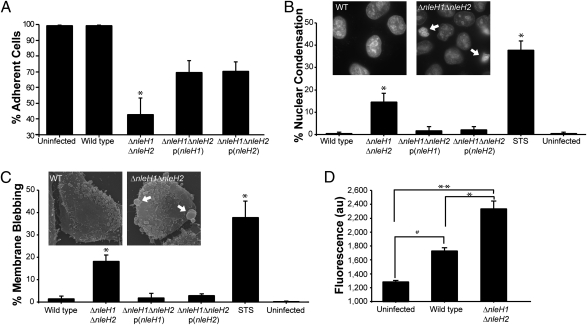
To investigate the role of NleH effectors, we generated a double-nleH EPEC mutant, and used it to infect HeLa cells. Quantification of the number of adherent living cells after 5 h of infection showed that <50% of cells infected with the EPEC ΔnleH1ΔnleH2 mutant remained attached, whereas no significant cell loss was observed in wild-type (WT) EPEC-infected cells compared with uninfected cells. Complementation of the EPEC mutant with either nleH1 or nleH2 significantly restored cell survival (Fig. 1A).
Fig. 1.
Cells infected with EPEC ΔnleH undergo apoptosis. Quantification of live adherent HeLa cells (A), nuclear condensation (B), membrane blebbing (C), and cytoplasmic Ca2+ (D) following infection of HeLa cells with WT EPEC, ΔnleH1ΔnleH2 mutant, and p(nleH1) or p(nleH2) complemented strains. The number of adherent cells was significantly reduced in cells infected with EPEC ΔnleH1ΔnleH2 (*) compared with uninfected cells and cells infected with WT EPEC. Complementing the mutant with either p(nleH1) or p(nleH2) partially restored the WT phenotype (A). The number of cells displaying nuclear condensation or fragmentation (B) or membrane blebbing (C) was significantly higher in HeLa cells infected with ΔnleH1ΔnleH2 or after treatment with STS as a control (*) compared with cells infected with EPEC WT or the complemented mutant strains. Although significant Ca2+ release was observed in WT EPEC-infected cells compared with uninfected cells (#), HeLa cells infected with the ΔnleH1ΔnleH2 had significantly elevated cytosolic Ca2+ levels compared with cells infected with WT EPEC (*) or uninfected cells (**) (D). In A, B, and C, the nonparametric Mann-Whitney test was used to determine significance, because the data did not follow Gaussian distribution. *P < 0.05. In D, significance was tested using the one-way ANOVA Bonferroni multiple-comparisons test. *,#,**P < 0.05.
We next explored whether the cells infected with the WT, ΔnleH1ΔnleH2, and nleH1 or nleH2 complemented ΔnleH1ΔnleH2 strains exhibited apoptotic phenotypes by assessing nuclear condensation (through Hoechst staining) and membrane blebbing (through phase-contrast and scanning electron microscopy [SEM]). We used staurosporine (STS), a potent inducer of apoptosis (22), as a control. Quantification of the number of cells with condensed nuclei revealed that cells infected with the EPECΔnleH1ΔnleH2 mutant (15%) and STS-treated cells (38%) contained significantly more condensed nuclei compared with uninfected cells and cells infected with WT EPEC or the nleH1- or nleH2-complemented mutants (all ≤2%) (Fig. 1B and Fig. S1A). Similar results were observed for membrane blebbing (Fig. 1C and Fig. S1B).
Free cytoplasmic Ca2+ is an important second messenger of apoptosis (reviewed in ref. 23). Thus, we measured cytosolic Ca2+ levels after 3.5 h of infection with the different EPEC strains using the Ca2+-sensitive fluorescent indicator Fluo-4 Direct (Invitrogen) in a 96-well fluorometer. Although an elevation of cytosolic Ca2+ concentration was observed during infection with WT EPEC compared with uninfected cells, significantly higher cytosolic Ca2+ levels were observed during infection with the ΔnleH1ΔnleH2 mutant (Fig. 1D). Taken together, these results suggest that NleH effectors have antiapoptotic activity.
NleH Inhibits Cleavage of Procaspase-3 Independently of Its Kinase Activity.
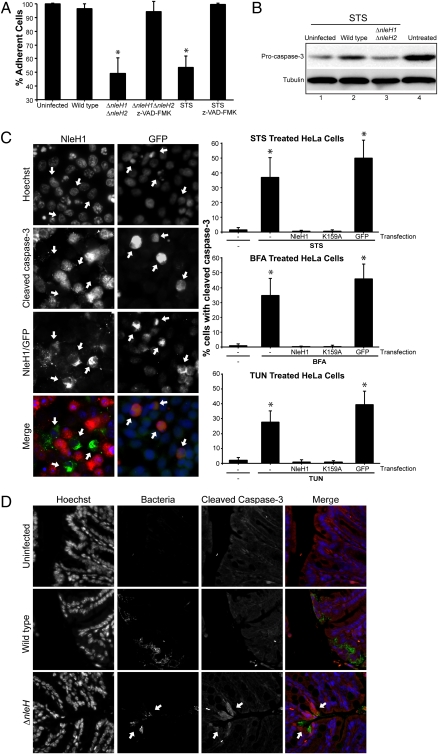
We used the global caspase inhibitor Z-VAD-fmk to explore whether the observed cell loss in the absence of nleH1 and nleH2 was due to caspase-dependent apoptosis. The addition of Z-VAD-fmk restored survival of cells infected with the ΔnleH1ΔnleH2 mutant, as well as control cells treated with STS (Fig. 2A). These results suggest that NleH1 and NleH2 inhibit caspase-dependent apoptosis during EPEC infection.
Fig. 2.
NleH inhibits caspase-3 activation. (A) Z-VAD-fmk inhibits EPEC-induced cell detachment. HeLa cells treated with the caspases inhibitor Z-VAD-fmk were infected with WT EPEC, ΔnleH1ΔnleH2 mutant, and p(nleH1)- or p(nleH2)-complemented strains, and the number of live adherent cells was counted. Z-VAD-fmk inhibited loss of STS-treated control and EPEC ΔnleH1ΔnleH2-infected cells. *P <.05. The nonparametric Mann-Whitney test was used to determine significance, because the data did not follow a Gaussian distribution. *P <.05. (B) Analysis of procaspase-3 cleavage. HeLa cell lysates, infected for 1 h with WT EPEC or ΔnleH1ΔnleH2 mutant and then treated for 3 h with STS, were analyzed by Western blot with procaspase-3 (inactive) and tubulin-control antibodies. Untreated uninfected lysates served as controls (lane 4). STS treatment induced capase-3 activation (lane 1). Infection with WT EPEC inhibited STS-induced procaspase-3 cleavage (lane 2), whereas infection with ΔnleH1ΔnleH2 mutant did not (lane 3). (C) Cleaved caspase-3 (active) was quantified in cells transfected with nleH1, nleHK159A, or pEGFP and treated with STS, BFA, or TUN. Anti-HA (green) and anti–cleaved caspase-3 (red) were used to detect HA-NleH1 and NleH1K159A or active caspase-3 by immunofluorescence. Ectopic expression of NleH1 or NleH1K159A prevented cleavage of procaspase-3 by treatment with STS (B), BFA (C), and TUN (D) compared with mock-transfected cells or cells transfected with pEGFP. The nonparametric Mann-Whitney test was used to determine significance. *P <.05. (D) NleH inhibits caspase-3 activation in vivo. Colonic sections extracted from mice infected for 9 days with WT or ΔnleH C. rodentium were immunostained with anti–cleaved caspase-3 antibody, anti-intimin antibody (to label bacteria), and Hoechst. Specific caspase-3 activation at bacterial attachment sites was observed in mice infected with ΔnleH mutant, but not in those infected with WT C. rodentium.
Active (cleaved) caspase-3 is one of the main apoptosis executioner. To investigate whether NleH effectors can prevent apoptosis, we infected HeLa cells with WT EPEC, the ΔnleH1ΔnleH2 mutant, or the complemented mutant strain. Whereas 40% of HeLa cells infected with the double-nleH mutant exhibited cleaved caspase-3 staining, only 3% of cells infected with WT EPEC (Fig. S1C) and 20% of cells infected with the complemented strain (Table S4) demonstrated caspase-3 activation. To examine whether NleH can protect HeLa cells from STS-induced apoptosis, we analyzed the level of procaspase-3 (inactive) by Western blot analysis. Infection of cells with WT EPEC inhibited caspase-3 cleavage induced by STS (Fig. 2B, lane 2) compared with uninfected cells and cells infected with the ΔnleH1ΔnleH2 mutant (Fig. 2B, lanes 1 and 3). These data show that NleH inhibits STS-induced procaspase-3 cleavage.
To further investigate whether NleH1 alone can prevent apoptosis, we transfected cells with nleH1 and treated them with STS or with inducers of ER stress-related apoptosis, brefeldin A (BFA), or tunicamycin (TUN) (24). The number of transfected cells with cleaved caspase-3 was quantified by immunofluorescence. Mock-transfected cells or cells transfected with the gfp control displayed high levels of cleaved caspase-3 (40–50%), whereas no cleaved caspase-3 was observed in cells transfected with nleH1 or in untreated cells (Fig. 2C). These results indicate that NleH1 can prevent caspase-3 activation in cells treated with proapoptotic compounds.
NleH1 contains the highly conserved kinase subdomains I, II, and III (9) and has a conserved Lys (K159) in the subdomain II associated with the ATP-binding site. Before investigating whether the putative kinase activity plays a role in antiapoptotic function, we determined whether NleH is indeed a protein kinase. Toward this end, purified His-NleH1WT and His-NleH1K159A were incubated with γ-33P-ATP in the presence or absence of the general kinase substrate myelin basic protein (MBP); Abl kinase was used as a positive control. Autoradiography revealed autophosphorylation of NleH1WT and strong MBP phosphorylation by Abl and NleH1WT. NleH1K159A phosphorylated neither itself nor MBP (Fig. S2A). These results indicate that NleH1 is an autophosphorylated protein kinase. We then investigated whether the kinase activity of NleH1 plays a role in its antiapoptotic activity by incubating NleH1K159A-transfected cells with the different proapoptotic compounds. As shown in Fig. 2C, cells transfected with both nleH1K159A and nleH1WT equally inhibited caspase-3 activation after treatment with STS, BFA, or TUN. However, infection of HeLa cells with the double-nleH mutant complemented with nleH1K159A resulted in intermediate cell detachment phenotype (Fig. S2C). Quantitative analysis revealed that complementing the double-nleH mutant with a plasmid encoding NleH1K159A significantly increased the number of adherent cells, although not to the level in WT (Fig. S2C). Together, the transfection and infection data suggest that NleH’s kinase activity does not have a major role in its cytoprotection function.
To explore whether NleH also can exert its antiapoptotic activity in the context of an in vivo infection, we infected C57BL/6 mice with WT and ΔnleH C. rodentium. Both strains colonized the colon at similar levels (10). Immunofluorescence of thin colonic sections revealed increased cleaved caspase-3 levels only in mice infected with the ΔnleH mutant strain (Fig. 2D). These results demonstrate that NleH also can inhibit apoptosis in vivo.
NleH Effectors Interact with BI-1.
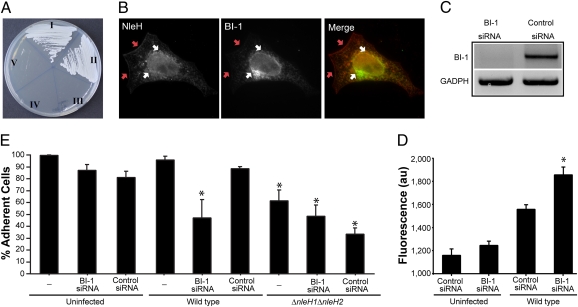
To identify the signaling pathways by which the NleH effectors are able to inhibit apoptosis, we performed a yeast two-hybrid (Y2H) screen using NleH1 as bait and a commercial HeLa cDNA library as prey. A binding partner identified in the screen was Bax inhibitor-1 (BI-1), an evolutionarily conserved apoptosis inhibitor (25, 26). The interaction of BI-1 with NleH1 and NleH2 was confirmed by a direct Y2H assay (Fig. 3A and Fig. S3C).
Fig. 3.
NleH1 and NleH2 target the BI-1 pathway. (A) NleH–BI-1 protein interaction. Yeast cells cotransformed with pGBT9-NleH1/pGAD-BI-1 [I] and pGBT9-NleH1/pGAD-BI-11–40 [II] grew on selective medium, whereas yeast cotransformed with pGBT9/pGAD-BI-1 [III], pGBT9-NleH1/pGAD [IV], and pGBT9/pGAD-BI-11–40 [V] did not, demonstrating specific interaction of NleH1 with BI-1 and BI-11–40. (B) NleH1 and BI-1 are colocalized. HeLa cells cotransfected with HA-NleH1 and Myc-BI-1 immunostained with anti-HA (red) and anti-Myc (green) antibodies. NleH1 colocalized with BI-1 at a reticular perinuclear region (white arrows) and also localized to the plasma membrane (red arrows). (C) BI-1 knockdown was verified by semiquantitative RT-PCR. GADPH RT-PCR was used as a total mRNA concentration control. (D) Quantification of cytoplasmic Ca2+ level in HeLa cells depleted of BI-1 or treated with control siRNA. Whereas no significant Ca2+ release was observed in uninfected cells transfected with BI-1 or control siRNA, a significant increase of Ca2+ release was observed in BI-1–depleted cells infected with WT EPEC compared with infected cells transfected with control siRNA. Significance was tested using the one-way ANOVA Bonferroni multiple- comparisons test. *P < 0.05. (E) Quantification after infection of live adherent HeLa cells depleted of BI-1 or treated with control siRNA. The number of adherent cells treated with BI-1 siRNA and infected with WT EPEC was similar to that of ΔnleH1ΔnleH2-infected cells but was significantly lower than cells treated with control siRNA and infected with WT EPEC. Significance was tested using the one-way ANOVA Bonferroni multiple-comparisons test. *P < 0.05.
Sequence alignment of NleH and its Shigella homolog OspG revealed that NleH effectors contain an N-terminal 100 amino acids fragment that is missing from OspG (Fig. S3A). To investigate whether this region is involved in BI-1 binding, we deleted the first 300 bp of nleH1 and tested whether the truncated NleH1 still binds BI-1 using a Y2H assay. The cotransformants grew on selective medium (Fig. S3C), indicating that the N terminus of NleH1 is not involved in binding BI-1. But despite the fact that binding of NleH to BI-1 is mediated by a region homologous to OspG, OspG neither interacted with BI-1 in the Y2H screen (Fig. S3C) nor protected cells from STS-induced caspase-3 activation (Fig. S3B). Taken together, these results demonstrate that NleH and OspG have distinct intracellular functions.
BI-1 is a six-transmembrane ER protein containing a predicted cytoplasmic N-terminal 40–amino acid domain (BI-11–40). To determine whether NleH1 binds this domain, we performed a Y2H assay. Yeast cotransformed with BI-11–40 and NleH1 grew on selective medium (Fig. 3A), indicating that the N-terminal domain of BI-1 includes the NleH-binding site.
BI-1 Colocalizes with NleH and Is Essential for Cytoprotection During EPEC Infection.
To investigate colocalization of NleH and BI-1, we cotransfected HeLa cells with vectors encoding myc-tagged BI-1 and HA-tagged NleH1. As shown in Fig. 3B, NleH1 and BI-1 were colocalized in reticular perinuclear structures, while NleH was found at the plasma membrane as well.
To explore whether BI-1 is directly involved in the antiapoptotic phenotype, we treated HeLa cells with BI-1 or control (scrambled) siRNA. Knockdown of BI-1 expression was confirmed by semiquantitative RT-PCR using GADPH as a total mRNA control (Fig. 3C). The cells were then infected with WT EPEC, and cytosolic Ca2+ levels were measured 3.5 h later. As shown in Fig. 3D, the level of cytosolic Ca2+ was 20% higher in infected BI-1 knockdown cells compared with infected cells treated with control siRNA. No significant difference was observed between uninfected cells treated with BI-1 and those treated with control siRNA. These results suggest that BI-1 modulates cytosolic Ca2+ levels during EPEC infection.
Quantification of live adherent cells revealed that infection of BI-1–depleted cells with WT EPEC for 5 h resulted in significant cell loss (50%) compared with control (scrambled) siRNA-treated cells, demonstrating that BI-1 plays an important role in cell survival during EPEC infection. In addition, no significant difference in the levels of remaining adherent cells was observed between control and BI-1 siRNA-treated cells infected with the EPEC ΔnleH1ΔnleH2 mutant and BI-1–depleted cells infected with WT EPEC (Fig. 3E). The absence of an additive effect between the deletion of nleH and depletion of BI-1 suggests that BI-1 is directly involved in the antiapoptotic NleH-signaling pathway.
Discussion
Whereas induction of cell death is a defense strategy used by the host to remove infected cells, bacterial pathogens use diverse strategies to inhibit apoptotic pathways. For example, Chlamydia trachomatis secrets the CPAF protease, which inhibits apoptosis by cleaving the proapoptotic BH3-only proteins (27); Neisseria gonorrhea injects PorB (28), which blocks caspase activation by preventing mitochondrial depolarization and release of cytochrome c (29); Salmonella enterica translocates the type III secretion system effector SopB, which inhibits apoptosis by activating Akt (30); and Legionella pneumophila inhibits apoptosis by translocating the type IV secretion system effector SdhA (31).
Several reports have demonstrated that different EPEC proteins induce early-stage apoptosis. For example, EspF has been reported to bind the antiapoptotic protein AbcF2, leading to its degradation and thereby facilitating cell death (13). In addition, targeting of Map and EspF to the mitochondria (11, 32) also might trigger apoptotic signals. Moreover, several studies have demonstrated that purified outer membrane EPEC proteins are able to induce expression of TNF-α and activate caspase-3, leading to apoptosis (33, 34). It is notable, however, that despite the presence of proapoptotic factors and the stress signals induced by the EPEC effectors, the proportion of apoptotic cells in monolayers infected with WT EPEC is lower than that of cells infected by Salmonella (14). The first indication that EPEC has an antiapoptotic activity was provided by Roxas et al. (35), who reported that EPEC induces phosphorylation of EGFR. Interestingly, similar activity was recently found in H. pylori, which activates the prosurvival phosphoinositide 3-kinase/Akt pathway via the EGFR, increasing the expression of the antiapoptotic factor Bcl-2 and decreasing expression of the proapoptotic factor Bax (36).
In this work, we have demonstrated that EPEC counteracts the proapoptotic signals by translocating the T3SS effectors NleH1 and NleH2, which block caspase-3 activation, cytosolic Ca2+ accumulation, nuclear condensation and fragmentation, membrane blebbing and cell death and detachment, whereas OspG (9) does not have the same antiapoptotic effect. Moreover, whereas inhibition of NF-κB activation by OspG is dependent on its kinase activity, we have demonstrated that the kinase activity of NleH1 is not essential for its antiapoptotic function, although it appears to increase the cytoprotective activity.
Our results demonstrate that NleH effectors inhibit apoptosis through a mechanism involving BI-1. BI-1 is a six-transmembrane protein localized at the ER membrane, from which it exerts its cytoprotective function (26, 37). We found that the cytosolic N-terminal 40 amino acids of BI-1, which face the cytosol, consist of the NleH-binding site, whereas the first 100 amino acids of NleH, which are missing from OspG, are not required for NleH–BI-1 interaction; OspG does not bind BI-1.
BI-1 was originally identified in a cDNA library screen for human proteins that inhibit cell death in yeast expressing Bax, a proapoptotic member of the Bcl-2 family (26, 37). Overexpression of BI-1 in mammalian cells suppresses apoptosis induced by various stimuli, including Bax, etoposide, STS, and growth factor deprivation, suggesting its ability to prevent more than one form of apoptosis (26). Indeed, overexpression of BI-1 reduces Bax activation, procaspase cleavage, mitochondrial membrane depolarization, and ultrastructural changes induced by proapoptotic ER stress agents such as TUN, BFA, and thapsigargin (25). These data correlate with our observation that ectopic expression of NleH1 inhibits cleavage of procaspase-3 in the presence of STS, TUN, and BFA. The mechanism through which BI-1 inhibits apoptosis is not currently known, but the identification of a bacterial binding partner may help decipher its physiological function.
Our data indicate that NleH effectors are multifunctional. They are protein kinases that trigger NF-κB and production of TNF-α during C. rodentium infections (10), but have a cytoprotective function as well. Importantly and uniquely, we have shown that NleH exerts its antiapoptotic activity in vivo, which provides a plausible explanation for why the nleH mutant is outcompeted by the WT strain during mixed infections (10).
The gastrointestinal epithelium has a high turnover rate and renews every 3–5 days in a process involving proliferation, differentiation, and apoptosis (38). A high rate of apoptosis would result in detachment and shedding of enterocytes, along with any associated bacteria. A recent report has shown that Shigella uses the effector protein OspE, which modulates integrin-linked kinase to inhibit cell detachment and exfoliation of mucosal epithelial cells (39). Similarly, expression of NleH may facilitate pathogenesis by slowing enterocyte loss and sustain colonization of the attached, extracellular, EPEC, EHEC, and C. rodentium bacteria.
Materials and Methods
The bacterial strains (Table S1), plasmids (Table S2), primers (Table S3), growth conditions, molecular biology, protein purification, kinase assay, Y2H assay, and experiments with cultured cells and mice used in this study are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Siouxsie Wiles and Francis Girard for assisting with the in vivo work and Claude Parsot for providing the ospG transfection vector. This work was supported by grants from the Medical Research Council and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911609106/DCSupplemental.
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 3.Knutton S, Lloyd DR, McNeish AS. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frankel G, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: More subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 5.Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: Translocation, translocation, translocation. Infect Immun. 2005;73:2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iguchi A, et al. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol. 2009;191:347–354. doi: 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobe T, et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci USA. 2006;103:14941–14946. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W, et al. Dissecting virulence: Systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci USA. 2004;101:3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DW, et al. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc Natl Acad Sci USA. 2005;102:14046–14051. doi: 10.1073/pnas.0504466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemrajani C, et al. Role of NleH, a type III secreted effector from attaching and effacing pathogens, in colonization of the bovine, ovine, and murine gut. Infect Immun. 2008;76:4804–4813. doi: 10.1128/IAI.00742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nougayrède JP, Donnenberg MS. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 2004;6:1097–1111. doi: 10.1111/j.1462-5822.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 12.McNamara BP, et al. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nougayrède JP, Foster GH, Donnenberg MS. Enteropathogenic Escherichia coli effector EspF interacts with host protein Abcf2. Cell Microbiol. 2007;9:680–693. doi: 10.1111/j.1462-5822.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Crane JK, Majumdar S, Pickhardt DF., 3rd Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane JK, McNamara BP, Donnenberg MS. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell Microbiol. 2001;3:197–211. doi: 10.1046/j.1462-5822.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 16.Allen RT, Hunter WJ, 3rd, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 17.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 20.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium–apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 21.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonsson A, Persson JL. Induction of apoptosis by staurosporine involves the inhibition of expression of the major cell cycle proteins at the G(2)/m checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer Res. 2009;29:2893–2898. [PubMed] [Google Scholar]
- 23.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: The unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 25.Chae HJ, et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Xu Q, Reed JC. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1:337–346. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- 27.Pirbhai M, Dong F, Zhong Y, Pan KZ, Zhong G. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis–infected cells. J Biol Chem. 2006;281:31495–31501. doi: 10.1074/jbc.M602796200. [DOI] [PubMed] [Google Scholar]
- 28.Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun. 2004;72:6408–6417. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 2003;5:99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 30.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 31.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila–translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papatheodorou P, et al. The enteropathogenic Escherichia coli (EPEC) Map effector is imported into the mitochondrial matrix by the TOM/Hsp70 system and alters organelle morphology. Cell Microbiol. 2006;8:677–689. doi: 10.1111/j.1462-5822.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 33.Shankar B, Krishnan S, Malladi V, Balakrishnan A, Williams PH. Outer membrane proteins of wild-type and intimin-deficient enteropathogenic Escherichia coli induce Hep-2 cell death through intrinsic and extrinsic pathways of apoptosis. Int J Med Microbiol. 2009;299:121–132. doi: 10.1016/j.ijmm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Abul-Milh M, Wu Y, Lau B, Lingwood CA, Barnett Foster D. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli–expressing bundle-forming pili. Infect Immun. 2001;69:7356–7364. doi: 10.1128/IAI.69.12.7356-7364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roxas JL, Koutsouris A, Viswanathan VK. Enteropathogenic Escherichia coli–induced epidermal growth factor receptor activation contributes to physiological alterations in intestinal epithelial cells. Infect Immun. 2007;75:2316–2324. doi: 10.1128/IAI.01690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan F, et al. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori–induced apoptosis. Gastroenterology. 2009;136:1297–1307. doi: 10.1053/j.gastro.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chae HJ, et al. Evolutionarily conserved cytoprotection provided by Bax inhibitor-1 homologs from animals, plants, and yeast. Gene. 2003;323:101–113. doi: 10.1016/j.gene.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 39.Tegtmeyer N, Backert S. Bacterial type III effectors inhibit cell lifting by targeting integrin-linked kinase. Cell Host Microbe. 2009;5:514–516. doi: 10.1016/j.chom.2009.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.