Abstract
The mouse mammary gland develops postnatally under the control of female reproductive hormones. Estrogens and progesterone trigger morphogenesis by poorly understood mechanisms acting on a subset of mammary epithelial cells (MECs) that express their cognate receptors, estrogen receptor α (ERα) and progesterone receptor (PR). Here, we show that in the adult female, progesterone drives proliferation of MECs in two waves. The first, small wave, encompasses PR(+) cells and requires cyclin D1, the second, large wave, comprises mostly PR(−) cells and relies on the tumor necrosis factor (TNF) family member, receptor activator of NF-κB-ligand (RANKL). RANKL elicits proliferation by a paracrine mechanism. Ablation of RANKL in the mammary epithelium blocks progesterone-induced morphogenesis, and ectopic expression of RANKL in MECs completely rescues the PR−/− phenotype. Systemic administration of RANKL triggers proliferation in the absence of PR signaling, and injection of a RANK signaling inhibitor interferes with progesterone-induced proliferation. Thus, progesterone elicits proliferation by a cell-intrinsic and a, more important, paracrine mechanism.
Keywords: cell proliferation, cyclin D1, mammary epithelium, progesterone, RANKL
Lifetime exposure to reproductive hormones, in particular estrogens and progesterone, affects the risk of breast cancer, a complex disease that is under hormonal control (1). The same hormones also control development of the mouse mammary gland, most of which occurs after birth. In response to ovarian estrogen secretion at puberty, the rudimentary ductal system extends from the nipple area into the fat pad through dichotomous branching. The complexity of the milk duct system increases through the formation of side branches triggered by cyclic changes in estrogen and progesterone secretion during adulthood. Side branching is enhanced during the first half of pregnancy. Subsequently, saccular outpouchings, alveoli, bud off the ducts and differentiate to become sites of milk production during lactation. Once pups are weaned, the mammary gland returns to a prepregnancy state through involution.
Tissue recombination experiments with mammary epithelium from mice with germ-line deletion of the estrogen receptor (ER) α (2), the progesterone receptor (PR) (3), or the prolactin receptor (PrlR) (4) revealed that epithelial intrinsic ERα is required for ductal elongation (5), PR for ductal side branching (6), and PrlR for alveologenesis and differentiation into milk-producing cells (7). PRs are composed of two proteins that are expressed from a single gene as a result of transcription from two alternative promoters (8), both of which are expressed in the mouse mammary gland (9). Deletion of either PR-A or PR-B from the mouse germ line revealed that PR-B is specifically required for mammary gland development (3, 10, 11).
The mechanisms by which hormones induce proliferation in vivo are poorly understood. The mammary epithelium consists of an inner layer of luminal cells that are surrounded by basal cells juxtaposed to the basal lamina. Some of these are in suprabasal position and thought to comprise progenitor cells others are spindle shaped and called myoepithelial cells because they exhibit features of smooth muscle cells. The receptors for both steroid hormones are expressed in about 30% of the luminal cells and have been shown to colocalize in the human breast (12). Colabeling studies in humans and rodents revealed that most of the proliferating cells are hormone receptor negative (12–14). Chimeric epithelia in which ERα- or PR-deficient cells were mingled with WT cells showed that the mutant cells participated actively in ductal growth in the context of surrounding WT MEC (5, 6).
The tumor necrosis family (TNF) member, receptor activator of NF-κB-ligand (RANKL), is important for osteoclast differentiation and lymph-node organogenesis (15) and was shown to be required for pregnancy-induced lactational mammary gland development (16) and implicated upstream of cyclin D1 in mammary epithelial cells (MECs) acting via the IKKα subunit of IκB kinase (17). Ectopic expression of RANKL in the mammary epithelium was shown to elicit ductal side branching and alveologenesis (18); similarly, overexpression of the cognate receptor RANK resulted in increased proliferation (19). Here, we show that progesterone induces proliferation of a subset of PR-positive (+) MECs by a cell-autonomous, cyclin D1-dependent mechanism and a larger wave of proliferation by a paracrine, RANKL-dependent mechanism.
Results
Two Waves of Progesterone-Induced Proliferation.
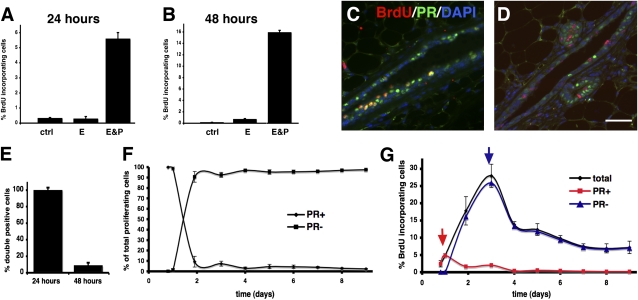
To examine cell proliferation induced by ovarian steroids, we administered 17-β-estradiol and progesterone to adult mice that had been ovariectomized 10 days earlier to deplete their endogenous steroids, and pretreated with 17-β-estradiol for 24 h to restore PR expression (20). Neither vehicle, nor 17-β-estradiol alone (Fig. 1 A and B) elicited proliferation considerably above background levels as assessed by 5′bromo-2′deoxyuridine (BrdU) labeling, but extensive proliferation occurred when progesterone was added (Fig. 1 A and B), highlighting that 17-β-estradiol, a major mitogen in the pubertal gland (21), is merely permissive for the proliferative effects of progesterone in the adult mammary gland.
Fig. 1.
Progesterone induces two waves of proliferation. (A–E) Ten-week-old female mice were ovariectomized, pretreated with 17-β-estradiol 10 days later, and then injected with vehicle, 17-β-estradiol, or 17-β-estradiol and progesterone. BrdU was administered repeatedly for analysis at 24 h (A and C) or as a single bolus at 46 h (B and D). Bars show BrdU incorporating MECs ± SEM in different treatment groups 24 h (A) and 48 h (B) after injection (n = 3). Double immunofluorescence after 24 h (C) and 48 h (D) of stimulation. Green, PR; red, BrdU; blue, DAPI. (Scale bar: 40 μm.) (E) Percentage of PR(+) and PR(−) cells ± SEM among BrdU incorporating MECs 24 h and 48 h after progesterone stimulation. (F and G) Ten-week-old female mice were ovariectomized and treated every 24 h with 17-β-estradiol and progesterone. Percentage of PR(+) and PR(−) BrdU incorporating MECs determined by double immunofluorescence; 40–400 BrdU(+) MECs counted per mouse (n = 3–4) (F). BrdU incorporation indices were determined by counting 3,000 cells per mouse (n = 3–4), and plotted over time. Percentage of PR(+) (red) and PR(−) cells (blue) incorporating BrdU was calculated based on F (G).
Based on previous reports that few proliferating MECs express ERα and PR in humans and rodents (12–14), and genetic evidence that progesterone can act by paracrine mechanisms (6), we hypothesized that progesterone may be directly mitogenic for some PR(+) MECs and elicit proliferation of PR(−) MECs by a paracrine mechanism. As cell divisions elicited by direct mitogenic stimulation should occur earlier than those following paracrine stimulation, we assessed proliferation at distinct time points. To identify all early proliferation events, we administered BrdU continuously for 24 h following hormone stimulation. Anti-BrdU staining revealed that 5% of MECs incorporated the label (Fig. 1A); double immunohistochemistry showed that most of these were PR(+) (Fig. 1 C and E), and found in the luminal compartment (Fig. 1C). Then, we pulsed mice with BrdU 46–48 h after progesterone stimulation. Under these conditions, about 15% of MECs incorporated the label (Fig. 1B); out of these less than 10% were PR(+) (Fig. 1 D and E). A time-course analysis revealed that BrdU incorporation was first above background level at 18 h after stimulation, with 3.2% of the MECs staining positive for the labeled nucleotide. Colabeling for BrdU and PR confirmed that up to 24 h, almost all of the proliferating MECs were PR(+); on day 2, the PR(+) cell fraction represented 9.3%, and by day 5 less than 5% of the proliferating MECs (Fig. 1F). From day 2 on, proliferating cells were found in luminal and subluminal locations as well as in the myoepithelium. Proliferation peaked on day 3 with 27% of MECs incorporating BrdU (Fig. 1G). Analysis of PR(+) and PR(−) MEC populations revealed two distinct peaks of proliferation (Fig. 1G, red and blue line): a small peak of PR(+) cell proliferation at 24 h and a large peak of PR(−) cell proliferation at 72 h. We note that the incorporation index at 48 h is higher under repeated hormone stimulation (Fig. 1 F and G) than following a single bolus (Fig. 1 A–E). Taken together, progesterone stimulation elicits two waves of proliferation in the mammary epithelium, a first small wave of PR(+) cells and a subsequent large wave of PR(−) MECs.
Cyclin D1 and Progesterone-Induced Proliferation.
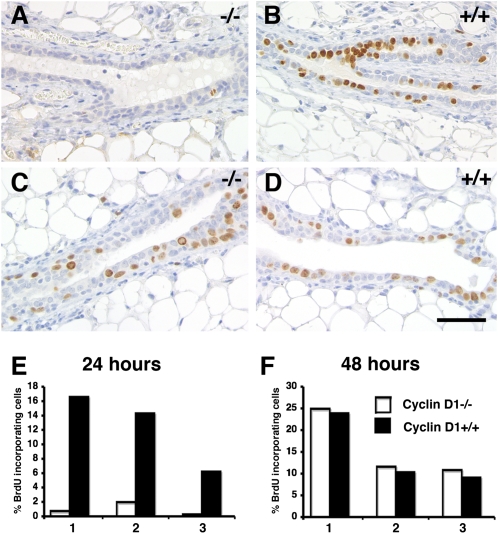
Cyclin D1 is amplified specifically in ERα(+) breast cancers and is a progesterone target gene in the PR(+) breast cancer cell line T47D (22). To assess whether cyclin D1 is required in vivo for progesterone-induced proliferation, we generated mice with cyclin D1−/− and WT mammary epithelia in contralateral glands by tissue recombination. Briefly, in 3-week-old mice, the inguinal glands can be cleared of endogenous epithelium by surgically removing the nipple-near half that contains the rudimentary ductal system. MECs that are introduced into the remaining “cleared” fat pad will give rise to a new ductal system. They can grow out from a piece of mammary tissue that is implanted (23), or from single-cell suspensions injected into the fat pad (24). To distinguish the graft from endogenous epithelium inadvertently left behind, we crossed the mutant cyclin D1 allele into a transgenic strain that ubiquitously expresses green fluorescent protein (GFP) (25) and grafted the GFP(+) donor tissue into GFP(−) hosts. Eight weeks later, recipients were analyzed 24 or 48 h after progesterone stimulation. As expected, within 24 h of progesterone stimulation, 6–16% of WT MECs had incorporated BrdU (Fig. 2 B and E). The contralateral cyclin D1−/− epithelial grafts, however, incorporated only background levels (Fig. 2 A and E). At 48 h, BrdU incorporation indices in both WT (Fig. 2 D and F) and cyclin D1−/− epithelia were in the range of 10–24% (Fig. 2 C and F). Double immunofluorescence revealed that among both WT and mutant MECs more than 90% of the BrdU-incorporating cells were PR(−) (Fig. S1). Thus, cyclin D1 function is required for cell-autonomous division of PR(+) cells induced by progesterone, but the second wave of proliferation is cyclin D1 independent. We note that grafted epithelia in general have higher proliferative indices than endogenous ones.
Fig. 2.
Cyclin D1- and progesterone-induced proliferation. (A and D) Mice engrafted with cyclin D1−/− and WT epithelia were stimulated with progesterone for 24 h (A, B, and E) or 48 h (C, D, and F). Histological sections of contralateral mammary glands engrafted with cyclin D1−/− (A and C) or WT (B and D) epithelia and stained with an anti-BrdU antibody. (Scale bar: 40 μm.) (E and F) Bar graphs showing BrdU incorporation in cyclin D1−/− and WT MECs ± SEM (n = 3), 1,000 cells counted per mouse.
Role of RANKL in the Mammary Epithelium.
Next, we sought to identify the mediator of progesterone’s paracrine mitogenic effects. Several factors had been implicated downstream of progesterone signaling in the mammary gland, including Wnt-4 (26), RANKL (10, 27), Calcitonin (28), and Id4 (29). RANKL had been proposed as a candidate paracrine mediator based on the observation that RANKL protein was shown to be expressed in PR(+) cells (10). Yet, analysis of RANKL-deficient mice indicated that the cytokine is required late in pregnancy for alveologenesis and lactogenic differentiation (16). As analysis of the mammary gland phenotype in RANKL−/− mice may have been confounded by systemic effects of the deletion (15), we performed mammary gland recombination experiments to discern the epithelial intrinsic effects of the deletion.
Fluorescent stereomicroscopy of contralateral glands engrafted with GFP+.RANKL−/− versus GFP+.RANKL+/+ MECs (Fig. S2 A and C) revealed that pubertal ductal outgrowth (9 weeks) was comparable between WT and mutant grafts. At 13 weeks of age, WT grafts gained complexity due to side branching, whereas the RANKL−/− epithelium did not. By day 14.5 of pregnancy, WT grafts had developed side branches and alveolar buds, whereas RANKL−/− grafts had only bifurcated. At the end of pregnancy, WT epithelia showed extensive alveologenesis, whereas RANKL−/− epithelia had few side branches and alveoli (Fig. S2 A–C). Quantification of side branches at 13 weeks confirmed that WT grafts were consistently more complex than the RANKL−/− counterparts (Fig. S2D).
Histological analysis of the engrafted glands revealed normal tissue structure at all stages (Fig. S2B) with immunohistochemistry for the myoepithelial markers p63 (Fig. S2E) and smooth muscle actin (Fig. S2F) showing normal organization of the two epithelial layers. Morphological hallmarks of secretion, such as lipid droplets, characteristic of the WT lactating gland, were sparse in the RANKL−/− epithelium (Fig. S2 B and G). Immunostainings for β-casein revealed the presence of this milk protein (Fig. S2G), suggesting differentiation occurs in the absence of RANKL.
Thus, epithelial RANKL is required for mammary gland side branching before and during pregnancy, consistent with this cytokine mediating progesterone function.
RANKL and Progesterone-Induced Proliferation.
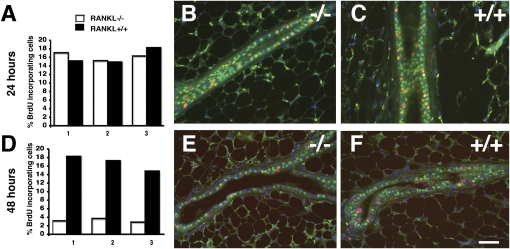
To address whether RANKL is required for progesterone-induced proliferation, mice engrafted with RANKL−/− and WT epithelia were stimulated with progesterone. During the first 24 h, on average 16% of the cells in both epithelia incorporated BrdU (Fig. 3A), most of which expressed PR (Fig. 3 B and C). Forty-eight hours after progesterone injection, an average of 17% of WT but less than 3% of RANKL−/− MECs incorporated BrdU (Fig. 3 D–F), suggesting that RANKL is not required for cell-autonomous proliferation, but rather paracrine proliferation induced by progesterone.
Fig. 3.
Response to progesterone in RANKL−/− MECs. (A–F) Mice engrafted with RANKL−/− or WT MECs stimulated with progesterone for 24 h (A, B, and C) or 48 h (D, E, and F). The percentage of BrdU-incorporating cells ± SEM in contralateral mammary epithelia of three mice after 24 h (A) and 48 h (D) of stimulation. Open bars, RANKL−/−; black bars, WT MECS; total of 1,000 cells counted in three different sections from each mouse. (B, C, E, and F) Double-immunofluorescence at 24 h (B and C) and 48 h (E and F) of stimulation. Green, PR; red, BrdU; blue, DAPI. (Scale bar: 40 μm.) Note: at 24 h, RANKL−/− (B) and WT (C) MECs incorporate BrdU similarly and are PR(+). At 48 h, fewer mutant MECs incorporate BrdU (E) vs. WT (F), and most BrdU-incorporating cells are hormone receptor negative.
Analysis of apoptosis in contralateral glands engrafted with RANKL−/− and WT MECs by caspase 3 staining (Table S1) and TUNEL assays (Fig. S3) revealed no difference.
It was conceivable that RANKL deletion affected PR signaling; however, expression of PR (Fig. S4 A and B) and the PR target genes wnt-4 and calcitonin was comparable (Fig. S4B). Similarly, cyclin D1 protein levels were independent of RANKL status (Fig. S4C), and RANKL expression levels independent of cyclin D1 status. (Fig. S4 D-H). We concluded that the first wave of progesterone-induced proliferation is cyclin D1 dependent and RANKL independent, whereas the second wave is cyclin D1 independent and RANKL dependent.
Ectopic Expression of RANKL in PR−/− Epithelium.
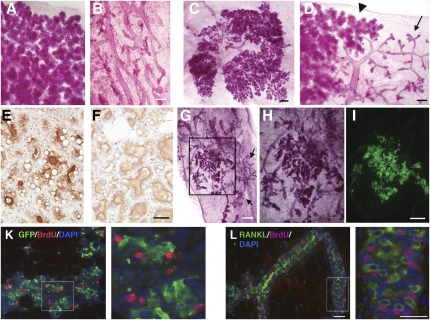
To test whether the paracrine, RANKL-dependent mechanism is sufficient to mediate progesterone function, we ectopically expressed RANKL in PR−/− epithelium using retroviral vectors and used the infected cells to reconstitute cleared fat pads. Two months later, the recipients were mated. At the end of pregnancy, the glands were analyzed by whole-mount microscopy. As expected, endogenous control glands showed full alveolar development (Fig. 4A) and control virus-infected PR−/− cells formed simple ductal systems (Fig. 4B). In glands reconstituted with RANKL-infected cells, some sectors had highly branched ductal systems decorated by alveoli (Fig. 4 C and D), and some of these were undistinguishable from WT control glands (Fig. 4A), indicating that ectopic RANKL expression rescued the PR−/− phenotype. At the cellular level, histological sections containing rescued areas showed fat droplets, a morphological hallmark of milk secretion (Fig. 4 E and F) and expression of the milk protein β-casein (Fig. 4 E and F). Thus, RANKL expression rescued the PR−/− phenotype with regard to morphogenesis and differentiation.
Fig. 4.
RANKL is sufficient to mediate PR function in the mammary epithelium. (A–D) Whole-mount micrographs of mammary glands from WT hosts postpartum. (A) Endogenous gland showing highly branched ductal system with dilated alveoli. (B) Gland engrafted with PR−/− epithelium infected with control virus. Note the simple ductal system. (Scale bar: 300 μm.) (C and D) Two representative mammary glands engrafted with PR−/− MECs infected with a retrovirus expressing RANKL showing extensive alveologenesis (C) or PR−/− phenotype in one area (arrow) next to a fully developed sector (arrowhead) (D). (E and F) Histological sections of postpartum mammary glands stained with an anti-β-casein antibody, gland engrafted with PR−/− MECs infected with a retrovirus expressing RANKL (E), and endogenous control gland (F). Note the presence of fat droplets and β-casein expression in both WT epithelium and PR−/− MECs expressing ectopic RANKL. (Scale bar: 40 μm.) (G–I) Stereomicrographs of a mammary gland reconstituted with PR−/− MECs expressing ectopic RANKL and GFP. The sector highlighted in G is magnified in H; same sector shown with a fluorescent image taken before tissue processing (I). Note: area of rescue coincides with GFP expression. Areas showing the PR−/− phenotype (arrows; G) do not express detectable levels of GFP (G). (Scale bar: 1 mm.) (H and I) 400 μm. (K) Double immunofluorescence for GFP (green) and BrdU (red) on mammary gland reconstituted with PR−/− MECs expressing ectopic RANKL and GFP. Blue, DAPI. Note: BrdU incorporation in MECs adjacent to cells expressing viral GFP. (L) Anti-RANKL (green) and BrdU (red) immunofluorescence of histological sections of mammary glands from WT female at day 12.5 of pregnancy. Note: BrdU incorporation in MECs adjacent to cells expressing RANKL. (K and L Right) Zoom of marked area. (Scale bar: 40 μm.)
In view of the 10–30% efficiency of retroviral infection of primary MECs, which is usually reflected by a similar extent of transgene expression in vivo (27), the large extent of rescue in the grafts with 74% of the grafts showing more than 40% rescue (Table S2) was surprising. To test whether RANKL is diffusible, we generated a retrovirus expressing both RANKL and GFP. In glands reconstituted with PR−/− MECs infected with this virus, GFP expression overlapped with areas of rescue (n = 10) (Fig. 4 G–I), indicating that RANKL confers a growth advantage to the MECs expressing it and their immediate neighbors.
Immunohistochemistry on PR−/− MECs infected with the retrovirus expressing both RANKL and GFP revealed that BrdU-incorporating cells are frequently found next to ectopic RANKL-expressing cells, indicating that RANKL indeed elicits proliferation by a paracrine mechanism (Fig. 4K). Similarly, colabeling of mammary glands from WT females at day 12.5 of pregnancy revealed dissociation of BrdU incorporation and RANKL expression (Fig. 4L), with RANKL expressing cells frequently as direct neighbors of BrdU-incorporating cells (Fig. 4L Right). Thus, RANKL elicits proliferation by a paracrine mechanism.
Systemic Modulation of Proliferation in the Mammary Gland.
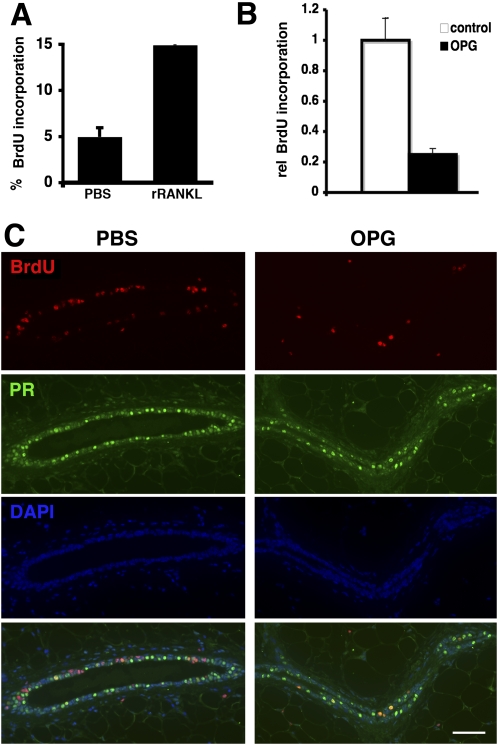
To assess whether proliferation of MECs can be induced by systemic RANKL administration, we injected PR−/− females with recombinant RANKL (rRANKL). In rRANKL-treated mice, 14% of the MECs incorporated BrdU versus 4% in control-injected mice (Fig. 5A). Next, we sought to block the interaction between RANKL and its receptor by administering the decoy receptor OPG. Injection of rOPG resulted in 54% inhibition of the progesterone-induced proliferation (Fig. 5B). Double immunohistochemistry revealed that most of the cells that proliferate in the presence of OPG are PR(+) (Fig. 5C). Thus, systemic interference with RANKL signaling affects proliferation of MECs in vivo.
Fig. 5.
Systemic manipulation of RANKL signaling affects the mammary epithelium. (A) Twelve-week-old PR−/− females were injected i.v. with either 8 μg of Fc-RANKL or PBS. BrdU was administered continuously for 72 h. BrdU-incorporating cells ± SEM; 1,200 cells were counted per mouse (n = 6), representing two independent experiments. (B and C) WT females were stimulated with progesterone and treated either with PBS or OPG. (B) BrdU incorporation in MECs is plotted ± SEM. Open bars, PBS treated (n = 9); filled bars, OPG treated (n = 12); three independent experiments were performed. (C) Double immunofluorescence of histological sections from mammary glands stimulated with progesterone and treated with PBS (Left) or OPG (Right). Green, PR; red, BrdU; blue, DAPI; bottom, overlay. Note: most BrdU(+) MECs in OPG-treated animals are PR(+).
Discussion
The present study shows that progesterone elicits proliferation in the mammary epithelium by two distinct mechanisms. First, a subset of PR(+) cells proliferates by a cyclin D1-dependent mechanism. Second, a large number of PR(−) cells proliferate by a RANKL-dependent mechanism. These findings, with experimental hormone stimulation, are consistent with studies under physiologic conditions in different species showing that most proliferation occurs in hormone receptor-negative cells (12–14).
Approximately 20% of all PR(+) MECs proliferated during the first 24 h of hormone stimulation, prompting the question whether proliferation is a stochastic event or whether distinct populations of PR(+) cells exist, some with a higher proliferation potential and some more differentiated. It is tempting to speculate that the BrdU incorporating PR(+) cells represent hormone receptor-positive stem cells, identified as label retaining epithelial cells (LREC) that incorporate label during pubertal ductal elongation and proliferate again upon hormonal stimulation during adulthood (30, 31), and that these cells may be expressing integrin β3 (CD61), a marker for luminal progenitor cells, some of which express ERα (32).
Our finding that cyclin D1 expression is not affected by deletion of RANKL in the mammary epithelium of virgin mice is in line with observations on pregnant RANKL−/− mice (33). Yet, cross-talk between progesterone and RANKL signaling with cyclin D1 may occur at the level of posttranslational modification. Cyclin D1 function was shown to be controlled by phosphorylation on threonine-286 by GSK3-β (34), and IκB kinase phosphorylates the steroid receptor coactivator (SRC)-3 (35), which is an important coactivator of PR (36).
Cyclin D1 has been shown to be required in MECs for alveolar development (37) downstream of PrlR signaling and IGF-2 (27). Whether the block in alveologenesis is related to the failure of at least some PR(+) cells to divide in response to progesterone or whether it reflects a distinct function of cyclin D1 in MECs remains to be addressed.
Previous overexpression studies of both RANKL (18) and its receptor (19) indicated that RANK signaling is strongly mitogenic in MECs both in vivo and in vitro. Observations in 3D cultures pointed to a synergism between RANKL and hepatocyte growth factor (HGF) in eliciting proliferation of myoepithelial cells (38). Here, we provide genetic evidence that RANKL is required for the proliferation of PR(−) MECs and sufficient to rescue the phenotype of PR−/− MECs, consistent with the finding that ectopic expression of RANKL elicits pregnancy-related morphogenetic changes in virgin mice (18). We reported previously that wnt-4 is required for progesterone-induced side branching (26). How RANKL and wnt-4 interact, and whether they are coexpressed in the same PR-positive cells, remains to be addressed.
RANKL signaling may be directly or indirectly mitogenic. BAFF/BLyS and CD40L/CD144 also belong to the TNF family—the former induces direct proliferation of B cells, possibly through TRAF2-mediated activation of MEKK1, JNK, AP1, and up-regulation of cyclin D2 (39), whereas the latter indirectly promotes mitogenic signals originating from the B-cell receptor by promoting survival (40). RANKL was shown to trigger nuclear translocation of Id2, which in turn resulted in the down-regulation of the cell-cycle inhibitor p21 and proliferation in primary MECs (33).
Breast cancer risk correlates with the number of menstrual cycles a woman experiences, and cell proliferation occurs during the luteal phase of the menstrual cycle when progesterone levels are high, suggesting a link between progesterone exposure and the disease. Similarly, studies on women on hormone replacement therapy showed that concomitant administration of estrogens and progesterone results in increased breast cancer incidence, whereas administration of estrogen only did not have significant effect (41). Our finding that progesterone-induced proliferation can be inhibited by administering OPG may have important implications if RANKL signaling should have a similar role in the human breast. Clinical trials to test the utility of anti-RANKL agents in the therapy of bone erosion diseases such as osteoporosis, rheumatoid arthritis, and multiple myeloma are ongoing (42); might it turn out that these agents interfere with proliferation, in the breast epithelium possibly in a subset of patients, and protect against breast cancer?
Materials and Methods
Mice.
GFP transgenic, RANKL, PR, and cyclin D1 mutant mice (3, 25, 43, 44) were bred in C57Bl6 or 129SV/C57Bl6 genetic background. Transplantation and mammary gland whole mounts were performed as described (5).
Hormone and BrdU Treatments.
Ten-week-old female mice were ovariectomized and injected 10 days later s.c. with 17β-estradiol (Sigma; 4 ng/g body weight) in tocopherol-stripped corn oil (MP Biomedicals). Twenty-four hours later, vehicle, 17β-estradiol, or 17β-estradiol and progesterone (Sigma; 100 μg/g body weight) were injected. For continuous stimulation, both steroids were injected every 24 h. BrdU (Sigma; 30 μg/g body weight) was injected either every 3 h for 24 h, or as bolus 2 h before sacrifice.
Injection of recombinant RANKL and OPG.
Human OPG (amino acids 1–202) was fused at the N terminus of hIgG-Fc, expressed, and purified as described (45). The receptor-binding domain of mRANKL was fused at the C terminus to hIgG-Fc and purified as described (46). Eight micrograms of Fc-RANKL in PBS were administered intravenously, and BrdU was injected every 6 h for 72 h. Twelve-week-old female mice were ovariectomized and injected 10 days later s.c. with 17β-estradiol and 24 h later with progesterone (100 μg/g body weight). Two hours and 24 h later, mice were injected intraperitoneally with OPG (2.4 μg/g of body weight) in PBS. BrdU was injected every 6 h for 48 h.
Histological Examination and Immunohistochemistry.
Glands fixed with 4% paraformaldehyde were paraffin embedded. We stained 4-μm sections with anti-BrdU (1:300) (Oxford Biotechnology; OBT0030), anti-GFP (1:4,000) (Molecular Probes; A6444), anti-PR (1:400) (Neomarkers; SP2), and anti-RANKL (1:200) (R&D Systems) overnight at 4 °C after antigen retrieval in citrate buffer and revealed with Vectastain Elite kit (Vector Laboratories). RANKL was detected using a TSA Signal Amplification System (PerkinElmer). Pictures were acquired with a Leica DM2000 microscope and Pixelink PL-A622C camera, and Zeiss Axioplan 2-imaging fluorescence microscope with Axiocam MRm camera.
Retroviral Production and Cell Infection.
The mRANKL coding region (GenBank accession no. AB022039) was extracted from pCR3-RANKL subcloned via Bluescript into MSCV (47) or PINCO (48). High-titer retrovirus was produced as described (49).
Supplementary Material
Acknowledgments
We thank M. Santos, N. Mueller, (School of Life Sciences, EPFL, Lausanne, Switzerland) and L. Willen (Department of Biochemistry, University of Lausanne, Switzerland) for technical assistance and Drs. P. Scinski (Dana Farber Institute, Boston), J. Lydon (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston), and T. Okabe (Genome Information Research Center, Osaka University, Osaka) for generously providing cyclin D1, PR mutant, and the C47BL/6-Tg(Act-EGFP) strain, respectively. This work was supported by funds from the National Center of Competence in Research Molecular Oncology, SNF3100A0-112090, KFP OCS-01445-12-2003, and US Army Medical Research and Materiel Command Grant DAMD17-03-1-0640 to C.B. M.B. was a Roche Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915148107/DCSupplemental.
References
- 1.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 2.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 3.Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 4.Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisken C, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisken C, et al. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210:96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- 8.Kastner P, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aupperlee MD, Smith KT, Kariagina A, Haslam SZ. Progesterone receptor isoforms A and B: Temporal and spatial differences in expression during murine mammary gland development. Endocrinology. 2005;146:3577–3588. doi: 10.1210/en.2005-0346. [DOI] [PubMed] [Google Scholar]
- 10.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 12.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 13.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 14.Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 15.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 16.Fata JE, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, et al. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Valdivia R, et al. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. 2009;328:127–139. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Suarez E, et al. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol. 2007;27:1442–1454. doi: 10.1128/MCB.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslam SZ, Shyamala G. Effect of oestradiol on progesterone receptors in normal mammary glands and its relationship with lactation. Biochem J. 1979;182:127–131. doi: 10.1042/bj1820127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- 22.Musgrove EA, et al. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deome KB, Faulkin LJ., Jr. Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 24.Daniel CW, Deome KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 25.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 26.Brisken C, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 27.Brisken C, et al. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- 28.Ismail PM, DeMayo FJ, Amato P, Lydon JP. Progesterone induction of calcitonin expression in the murine mammary gland. J Endocrinol. 2004;180:287–295. doi: 10.1677/joe.0.1800287. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Valdivia R, et al. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology. 2008;149:6236–6250. doi: 10.1210/en.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth BW, Boulanger CA, Smith GH. Selective segregation of DNA strands persists in long-label-retaining mammary cells during pregnancy. Breast Cancer Res. 2008;10:R90. doi: 10.1186/bcr2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Booth BW, Smith GH. Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8:R49. doi: 10.1186/bcr1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 33.Kim NS, et al. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol. 2006;26:1002–1013. doi: 10.1128/MCB.26.3.1002-1013.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 35.Wu RC, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–3461. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han SJ, et al. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- 37.Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C. Impaired mammary gland development in Cyl-1(−/−) mice during pregnancy and lactation is epithelial cell autonomous. Dev Biol. 1999;212:1–11. doi: 10.1006/dbio.1999.9329. [DOI] [PubMed] [Google Scholar]
- 38.Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology. 2008;149:2098–2107. doi: 10.1210/en.2007-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher E, et al. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2006;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 40.Smith SH, Cancro MP. Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J Immunol. 2003;170:5820–5823. doi: 10.4049/jimmunol.170.12.5820. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Bartsch R, Steger GG. Role of denosumab in breast cancer. Expert Opin Biol Ther. 2009;9:1225–1233. doi: 10.1517/14712590903146877. [DOI] [PubMed] [Google Scholar]
- 43.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA. 2000;97:10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sicinski P, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 45.Schneider P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 46.Bossen C, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 47.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 48.Grignani F, et al. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- 49.Brisken C, Socolovsky M, Lodish HF, Weinberg R. The signaling domain of the erythropoietin receptor rescues prolactin receptor-mutant mammary epithelium. Proc Natl Acad Sci USA. 2002;99:14241–14245. doi: 10.1073/pnas.222549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.