Abstract
Prostate cancer (PCa) and benign prostatic hyperplasia (BPH) are androgen-dependent diseases commonly treated by inhibiting androgen action. However, androgen ablation or castration fail to target androgen-independent cells implicated in disease etiology and recurrence. Mechanistically different to castration, this study shows beneficial proapoptotic actions of estrogen receptor–β (ERβ) in BPH and PCa. ERβ agonist induces apoptosis in prostatic stromal, luminal and castrate-resistant basal epithelial cells of estrogen-deficient aromatase knock-out mice. This occurs via extrinsic (caspase-8) pathways, without reducing serum hormones, and perturbs the regenerative capacity of the epithelium. TNFα knock-out mice fail to respond to ERβ agonist, demonstrating the requirement for TNFα signaling. In human tissues, ERβ agonist induces apoptosis in stroma and epithelium of xenografted BPH specimens, including in the CD133+ enriched putative stem/progenitor cells isolated from BPH-1 cells in vitro. In PCa, ERβ causes apoptosis in Gleason Grade 7 xenografted tissues and androgen-independent cells lines (PC3 and DU145) via caspase-8. These data provide evidence of the beneficial effects of ERβ agonist on epithelium and stroma of BPH, as well as androgen-independent tumor cells implicated in recurrent disease. Our data are indicative of the therapeutic potential of ERβ agonist for treatment of PCa and/or BPH with or without androgen withdrawal.
Keywords: castration, steroid receptors, selective estrogen receptor modulators
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) are the most common benign and malignant diseases in aging men (1, 2). BPH arises in the transition zone or peri-urethral glands where stromal and epithelial nodules develop, whereas PCa arises in the peripheral zone of the prostate gland where epithelial cells undergo malignant transformation. These androgen-dependent diseases are treated by inhibiting androgens or their action. In PCa, androgen ablation fails to target castrate-resistant or androgen-independent cell types, implicated in disease etiology and recurrence. Androgen blockade in men with PCa is effective initially because it causes apoptotic regression in the bulk of the tumor, although significant side effects include hypogonadism, gynecomastia, anemia, and metabolic syndrome, for which further treatments are required. Nevertheless, relapse frequently occurs, as subpopulations of cells are either castrate-resistant or adapt to androgen-deplete conditions, resulting in incurable castrate-resistant PCa (3). For BPH, anti-hormonal treatments are associated with the same side effects and often fail to permanently reduce prostatic volume or to ease lower urinary tract symptoms (4). Thus, new therapies for PCa or BPH are required that are as effective as androgen withdrawal but also target castrate-resistant cells implicated in disease recurrence.
Although estrogens were previously used for PCa therapy, their efficacy was based on indirect suppression of androgen levels; they also resulted in adverse side effects such as cardiovascular and thromboembolic events (5). It is now known that estrogens acting via ERα mediate aberrant epithelial cell proliferation, prostatic inflammation, and malignancy (6 –9), and ERα antagonists such as Toremifine are in clinical trial for PCa prevention/progression (10). In contrast, effects of estrogen mediated by ERβ are beneficial; we and others previously reported anti-proliferative activity of ERβ agonist in the prostate, independent of systemic androgens (and not involving the suppression of serum testosterone) but requiring intraprostatic stromal–epithelial cell signaling (6, 11 –13).
The aim of this study was to investigate the therapeutic potential of an ERβ agonist of proven selectivity (14 –17), specifically investigating its proapoptotic mechanism of action compared with castration. This compound (8β-VE2) has proven selectivity and was previously used to dissect the physiological roles of ERα and ERβ in vivo in bone, cardiovascular, and metabolic studies (14 –18). To circumvent the use of a specific ERβ knock-out mouse model because of reported variation in prostatic phenotypes from different colonies (19), we used aromatase knock-out (ArKO) mice that lack endogenous estrogen ligands but express functional ERs (20), thus obviating any confounding action of ER activation by endogenous ligands. Using these mice, we compared the cellular targets and mechanism of action of ERβ agonist to castration. We further verified our findings by comparing castration and ERβ agonist using human prostatic specimens and cell lines to test the therapeutic potential of ERβ agonists in PCa and BPH. Our results provide independent, unequivocal proof of the concept initially proposed by Gustafsson et al. that ERβ is anti-proliferative and proapoptotic in the prostate (13), and demonstrate a mechanism of ERβ action that is androgen-independent and mediated by TNFα, targeting castrate-resistant epithelial cells.
Results
ERβ Agonist Increases Apoptosis and Reduces Proliferation in Prostatic Stroma and Epithelia.
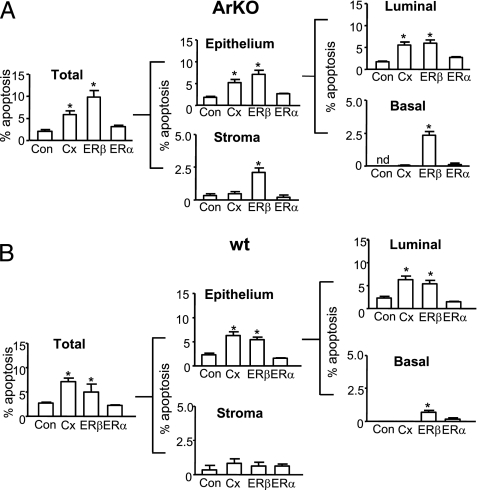
Treatment with ERβ agonist for 6 weeks abrogated prostatic hyperplasia and hypertrophy of ArKO mice (21) because of reduced cellular proliferation; more acutely, a time course study showed that ERβ-induced apoptosis was maximal at 3–7 days, compared with the effect of an ERα agonist that causes inflammation (Fig. S1 A and B). Figure 1 shows proapoptotic effects of ERβ agonist in ArKO or wt mice within 3 days, compared with those in intact vehicle-treated, castrate, or ERα agonist–treated mice. Contemporary stereology and morphometric analyses show that ERβ agonist significantly increased epithelial and/or stromal apoptosis vs. vehicle controls in ArKO (Fig. 1A) and wt mice (Fig. 1B). Castration significantly increased epithelial but not stromal apoptosis, whereas ERα agonist–treated tissues showed levels of apoptosis similar to controls in all cellular compartments (Fig. 1 A and B). Further subdivision into epithelial luminal and basal cells based on location and CKH immunoreactivity (basal cells are CKH-positive) showed that luminal epithelial cell apoptosis was significantly increased by both castration and ERβ agonist, but only ERβ agonist caused apoptosis of basal cells (Fig. 1A and B and Fig. S1C). In ArKO mice, ERβ agonist or castration (but not ERα agonist) significantly reduced epithelial (but not stromal) cell proliferation (quantified by PCNA staining) compared with controls; proliferation was reduced in luminal and basal epithelia (Fig. S2A). Similar results were observed in wild-type (wt) mice in which epithelial (but not stromal) cell proliferation was lowered by castration and ERβ agonist (Fig. S2B). Altogether, these data showed that ERβ agonist uniquely caused apoptosis in the castrate-resistant basal cell layer, reducing cell proliferation and increasing apoptosis in the luminal epithelial and stromal cells of hyperplastic and normal mouse prostate.
Fig. 1.
Effect of selective ERβ agonist on prostatic apoptosis in ArKO (A) and wild-type (wt) (B) mice. (A) Apoptosis (%) in total tissue at 3 days in ArKO Control (Con), Castrate (Cx), ERβ agonist (ERβ), or ERα agonist (ERα)–treated mice. Apoptosis (%) in total tissue was subdivided into epithelial (further subdivided into luminal and basal epithelium) and stromal components. (B) Percentage apoptosis in total tissue at 3 days from wt Control (Con), Castrate (Cx), ERβ agonist (ERβ), or ERα agonist (ERα). Apoptosis (%) in total tissue subdivided into epithelial (luminal or basal) and stromal components. Values are mean ± SEM, n = 5 mice/group. nd, not detectable.*P < 0.05 vs. control.
Epithelial Regeneration After ERβ Agonist Results in Cystic Atrophy and Depletion of p63+ Basal Cells.
Basal cells maintain the structural integrity of the prostatic epithelium (22) and are necessary for tissue regeneration occurring over repeated cycles of androgen deprivation and replacement. Following ERβ agonist-induced apoptosis in basal cells, we examined whether ERβ agonist treatment disrupted epithelial regenerative capacity. Twenty-one days posttreatment, ERβ agonist-treated tissues showed regions of cystic atrophy with expansion of the fluid-filled lumen (Fig. S3A) not seen in control or castrate-recovery tissues as evidence of perturbed glandular secretion. Prostates from castrated animals treated with androgens or intact control animal tissues showed normal morphology. The apparent frequency of p63+ cells within atrophic regions of ERβ–agonist treated tissues was reduced compared with normal control tissue but was unaltered by castration (Fig. S3B). Quantification of atrophy (%) and p63+ cells (p63+/100 epithelial cells) confirmed these observations, showing cystic atrophy in 42.5% of ERβ-recovered tissue within which the frequency of p63+ cells was 4.9 ± 1.4 compared with 9.8 ± 1.2 in normal regions of the same tissue (Fig S3C). Overall, these data show functional and structural difference between ERβ agonist and castrate tissues after recovery, because of loss of p63+ basal cells. In castrate mice treated with ERβ agonist followed by 21 days of T replacement, cystic atrophy was observed in 42.6 ± 17.2% tissue within which the frequency of p63+ cells was significantly reduced (7.9 ± 0.4, compared with 12.2 ± 0.3 in normal regions of the same tissue). Therefore, regardless of androgen supplementation, ERβ agonist perturbs regeneration.
Mechanism of ERβ-Induced Apoptosis Is Androgen Independent and TNFα Mediated.
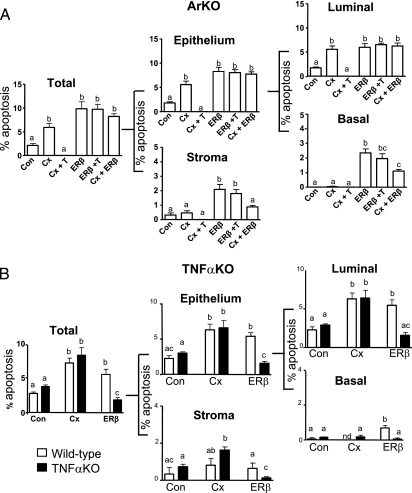
To determine whether the mechanism of ERβ agonist action was androgen independent, we compared the effect of androgen supplementation on agonist-treated (ERβ+T) and castrate (Cx+T) ArKO mice. Morphometric analyses showed that testosterone supplementation did not alter the apoptotic response to ERβ agonist in any cellular compartment of the epithelium or the stroma (Fig. 2A). In contrast, apoptosis induced by castration was completely abrogated by testosterone supplementation (Fig. 2A). To demonstrate the effect of the ERβ agonist in an androgen-deplete environment, we evaluated the effect of ERβ agonist treatment on castrated (ERβ+Cx) ArKO mice. After 3 days of combined treatment, the apoptotic response to ERβ agonist was maintained (as seen in basal cells), except in stroma, where an increase in apoptosis was observed but not significant (Fig. 2A). Finally, although castration significantly reduced serum testosterone levels, ERβ agonist treatment showed no significant alterations in serum androgen levels (Table S1).
Fig. 2.
ERβ agonist–induced apoptosis is androgen independent (A) and involves TNFα signaling (B). (A) Apoptosis (%) in total tissue subdivided into epithelial (luminal or basal) and stromal components at 3 days from control (Con), Castration (Cx), or ERβ agonist (ERβ)–treated ArKO mice. Cx+T, castrated mice receiving T supplementation; ERβ+T, ERβ mice receiving T supplementation; and Cx+ERβ, Cx mice treated with ERβ to maintain serum T levels. (B) Apoptosis (%) in wild-type mice (open bar) and TNFαKO mice (solid bar), after 3 days of vehicle (Con), Castrate (Cx), or ERβ agonist (ERβ). Values are mean ± SEM. Different superscripts indicate groups that are significantly different. P < 0.05; n = 5 mice/group.
To identify ERβ activated gene expression, a pathway-specific DNA microarray for apoptosis was used to compare castrate and ERβ agonist–treated ArKO prostate tissues at 12 h or 3 days posttreatment. Differentially expressed genes included members of the TNF superfamily such as TNFα (Table S2). To confirm a role of TNFα signaling in ERβ agonist–induced apoptosis, we used TNFα knock-out (TNFαKO) mice after establishing normal prostatic phenotype (Fig. S4). Quantitative analysis of prostatic apoptosis (epithelial or stromal) in ERβ agonist–treated TNFαKO and wt mice showed divergent responses; TNFαKO mice failed to show any significant increase, whereas wt mice showed increased apoptosis; in contrast, castration of TNFαKO and wt mice caused the same increase in apoptosis (Fig. 2B).
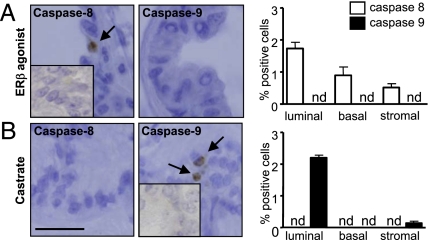
Although extrinsic and intrinsic apoptosis pathways converge with activation of caspase-3, caspase-8 is activated through extrinsic and caspase-9 through intrinsic signaling; these cleaved caspases can differentiate between apoptotic pathways (23). Using wt mice, we showed that ERβ agonist up-regulated caspase-8, but not caspase-9 immunoreactivity, whereas castration up-regulated expression of caspase-9, but not caspase-8 in the prostate (Fig. 3A). Quantification of caspase immunoreactivity showed that ERβ activated apoptosis via caspase-8 in luminal, basal, and stromal cells, whereas castration activated apoptosis via caspase-9 in luminal and stromal cells but not in basal cells (Fig. 3B). Altogether, these data showed ERβ agonist action was mechanistically different from that of castration, independent of androgen levels as well, as activating the extrinsic apoptotic pathway in prostatic luminal and basal epithelial cells and prostatic stroma via TNFα-mediated signaling.
Fig. 3.
Apoptotic pathways activated by ERβ agonist and castration in wt mice. (A) Expression and quantification of cleaved caspase-8 (open bars) and -9 (solid bars) in ERβ tissues. (B) Expression and quantification of cleaved caspase-8 (left) and -9 (right) in castrated tissues. In micrographs, arrows indicate cells positive for cleaved caspase-8 or -9. (Inset) Negative control. Values are mean ± SEM n = 5 mice/group. nd, not detectable. (Scale bar, A, 25 μm).
ERβ Agonist Induces Androgen-Independent Apoptosis in Stroma and Epithelia of Human BPH.
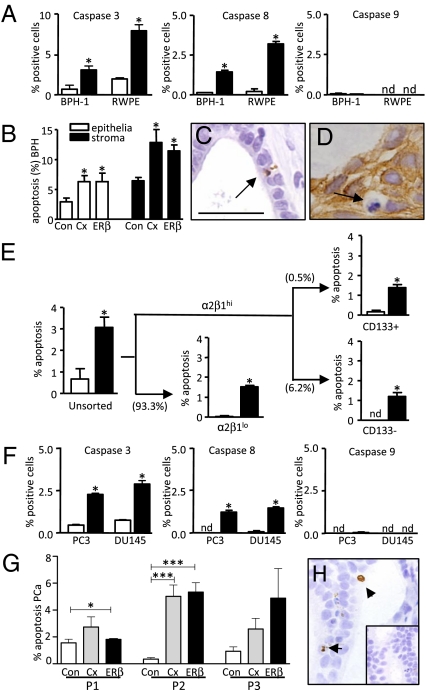
The effect of ERβ agonist in human cells and tissues was determined using benign human epithelial BPH-1 and RWPE-1 cell lines that express ERβ, but not ERα (Fig. S5). Consistent with our animal studies, ERβ agonist activated the extrinsic apoptotic pathway via caspase-8 (Fig. 4A). To examine the in vivo effect of ERβ agonist, we subrenally grafted four human BPH specimens into host male mice. Host mice containing human BPH specimens were then treated for 3 days with ERβ agonist and compared with castrate tissues. Using immunohistochemistry, ERβ expression was confirmed in epithelial and stromal cells, whereas ERα was detectable in stroma and rarely present in epithelia in vehicle-treated xenograft tissues (Fig. S6). Epithelial and stromal apoptosis in human BPH xenografted tissues was significantly increased when the host mice were treated with ERβ agonist or castrated, compared with vehicle-treated tissues (Fig. 4 B and C). Basal cell apoptosis was identified in ERβ-treated xenografts, based on combined CKH staining and morphological markers of apoptosis (chromatin condensation, membrane blebbing, and shrunken cytoplasm) (Fig. 4D).
Fig. 4.
ERβ agonist–induced apoptosis in human xenograft tissues and cells. (A) Quantification of caspase positive cells in BPH-1 and RWPE-1 cells treated with vehicle (open bars) or ERβ agonist (closed bars). (B) Apoptosis (%) in epithelial (open bars) and stromal (solid bars) BPH tissue xenografts (n = 4 patients) after 3 days treatment with control (Con), castration (Cx), or ERβ agonist (ERβ). (C) ApopTag staining of ERβ–treated BPH xenografts. (D) Morphologically identifiable apoptosis in CKH-positive basal cells. (E) Percent apoptosis in unsorted BPH-1 cells treated with ERβ agonist-treated (solid bars) or vehicle-treated (open bars) controls. After further fractionation to enrich for α2β1hi (basal) and α2β1lo (luminal) populations, α2β1hi cells were sub fractionated into CD133+ and CD133− subpopulations (figure representative of two individual experiments; brackets show percent cells per fraction). (F) Quantification of caspase positive cells in PC3 and DU145 cells treated with ERβ agonist (solid bars) or vehicle control (open bars). (G) Quantification of apoptosis (%) in human PCa xenografts from three patients (P1, P2, and P3), treated with vehicle (Con; open bars), castration (Cx; gray bars), or ERβ agonist (ERβ; solid bars). (H) Caspase 8 in ERβ-treated PCa; inset negative control. Values are mean ± SEM; n = 4 replicates per group except in G, where n = 3. nd, not detectable. Analyses by Student’s t test (A, E, and F) or ANOVA (B and G). *P < 0.05 vs. control; ***P < 0.005 vs. control. (Scale bar, C, D, H, and I, 25 μm; F, 100 μm; and G, 200 μm.)
To further identify the cellular targets of ERβ agonist in BPH, we sorted subpopulations of BPH-1 cells by rapid adherence to type-1 collagen (α2β1integrinhi cells, representing <10% of BPH-1 cells (Fig. 4E)) and further fractionated cells using CD133 into α2β1integrinhi/CD133+ cells (<0.5% of BPH-1 cells) and α2β1integrinhi/CD133− (∼6% of BPH-1 cells). These two populations of cells are enriched respectively for stem/progenitor cells (reported to regenerate prostatic acini in vivo) or transit amplifying cells (24 –26). To study the in vitro effects of ERβ agonist on these subpopulations, each fraction was replated and treated for 24 h. In unsorted BPH-1 cells, ERβ agonist increased apoptosis 3-fold compared with vehicle controls (Fig. 4E). Both CD133+ (putative stem/progenitor) and CD133− (transient amplifying) cells showed a significant increase in apoptosis in response to the ERβ agonist, as did the nonadherent α2β1integrinlo subpopulation (luminal cell–enriched), which constituted the bulk of the BPH-1 cell cultures (∼93%) (Fig. 4E). Collectively, these data demonstrated that ERβ agonist induced prostatic apoptosis in xenografted human BPH specimens and in subpopulations of BPH-1 cells, including those enriched for CD133+ that are implicated in regeneration of the prostate and in the transition of benign to malignant disease (26).
ERβ Agonist Induces Androgen-Independent Apoptosis in Human PCa.
The expression of caspase-3, -8 and -9 was examined in androgen-independent (ERα− and ERβ+, Fig. S5) human PCa cell lines, PC3 and DU145. ERβ agonist treatment significantly increased caspase-3 and -8 but not -9 (Fig. 4F), confirming use of the extrinsic pathway of apoptosis. Confirmation of the specific induction of apoptosis by ERβ agonist was obtained by siRNA knockdown of ERβ in DU145 cells. The relative efficacy of siRNA against ERβ, negative control siRNA, and transfection control was determined by RT-PCR and showed a >90% reduction of ERβ transcripts in ERβ siRNA–transfected cells. Subsequent treatment of these transfected cells demonstrated that ERβ agonist–induced apoptosis is abrogated in ERβ siRNA–transfected cells (Fig. S7B).
To demonstrate an ERβ-induced biological outcome, we s.c. grafted human fluorescent PC3 cells into immuno-deficient host male mice. Tumors were monitored using in vivo fluorescent imaging pre- and posttreatment with vehicle or ERβ agonist. Fluorescent intensity was used as a measure of tumor growth. Our data show a ∼2-fold increase in tumor doubling time after ERβ agonist treatment, concurrent with a significant increase in apoptosis and a significant reduction in proliferation, as shown in Table S3.
Finally, we subrenally grafted tissue specimens from three human PCa patients (Gleason Grade 7, epithelial ERβ+, ERα−, Fig S6) into host male mice. After treatment for 3 days, tumor cell apoptosis (detectable by Apoptag staining) was significantly increased after ERβ agonist compared with controls in two of three PCa patient tissues (P1, P2; Fig. 4G), and increased in the third patient (P3), although not significantly. In patient 1 (P1), increased caspase 8 immunoreactivity was detected (Fig. 4H); further semiquantification of the percent sections expressing caspase 8 showed a 2-fold increase compared with castrate and control tissues. Overall, the data showed ERβ agonist-induced apoptosis in primary PCa xenografts and in androgen-independent PCa cell lines, consistent with the androgen-independent mechanism of action identified in mice.
To confirm that TNFα mediates ERβ-agonist induced apoptosis, we used immunohistochemistry to show that TNFα protein expression was up-regulated in human cell lines and tissues after agonist treatment (Fig. S7A). We also used siRNA knockdown of TNFα, reducing TNFα transcripts by ∼30%, and showed abrogation of the ERβ-agonist induced apoptotic response in DU145 cells (Fig. S7C). Therefore, as shown in animal studies, TNFα mediates ERβ-agonist induced apoptosis in human cells and tissues.
Discussion
This study reports beneficial, proapoptotic actions of selective activation of ERβ without the necessity for androgen withdrawal, in both BPH and PCa, diseases that often occur concurrently in different prostatic zones of aging men. ERβ agonist-induced apoptosis was androgen independent and mediated by TNFα signaling, and thus was mechanistically different from castration (or the effects of ERα agonist). Cellular targets of ERβ agonist were luminal, basal, and stromal cells of BPH tissue and cells, as well as androgen-independent PCa cells lines. Therefore our study unequivocally endorses a proposed anti-proliferative/proapoptotic role for ERβ (13), and provides insight into its mechanism of action and cellular targets. As current therapies for benign and malignant prostate disease (androgen blockade) fail to target castrate-resistant cells and are associated with adverse side effects, these findings imply that ERβ agonists may have significant therapeutic potential for treatment of BPH and/or PCa subject to satisfactory pharmacokinetic and toxicity testing (27, 28).
There are several key differences between the mechanism of apoptosis induced by ERβ agonist and castration that may offer some therapeutic advantage. First, ERβ agonist-induced apoptosis via activation of caspase-8 that is not required for castration-induced apoptosis (29) and was absent in TNFαKO mice. The mechanisms of interaction between ERβ and TNFα are unknown; however, we show caspase-8 and -3 activation, and the abrogation of the ERβ-mediated apoptotic response following siRNA knockdown of TNFα in a human prostate cancer cell line. These findings concur with similar data in human hepatocellular carcinoma cell lines where ERβ-activated apoptosis was also mediated by caspase-8 and TNFα (30).
Second, ERβ agonist-induced apoptosis occurs in both the androgen-replete and androgen-deplete milieu. Our conclusion that ERβ agonist action is androgen independent and differs from castration derives from several lines of evidence. In ArKO mice and in human xenografts in which androgen levels are maintained by exogenous testosterone supplementation, or in castrate, androgen-deplete animals, ERβ agonist causes apoptosis, notably in the castrate-resistant androgen-independent basal cell layer. These data concur with our previous report that ERβ is anti-proliferative and occurs in tissue recombinants that exclude a role of systemic hormones (21). In addition, ERβ agonist induced apoptosis in androgen-independent DU145 and PC3 PCa cell lines and BPH-1 cells. Thus ERβ agonist may provide an added therapeutic advantage by obviating the side effects of castration or androgen ablation, including hypogonadism, gynecomastia, anemia, and metabolic syndrome in men.
Another key difference between ERβ-induced apoptosis and castration are the cellular targets, as ERβ agonist causes apoptosis in castrate-resistant epithelial cell subpopulations. The main effect of androgen withdrawal is on the terminally differentiated luminal cells that constitute ∼95% of the epithelium; yet the basal cell layer, which contains stem/progenitor cells, are resistant to androgen blockade, while expressing high levels of ERβ (31, 32). Although the rate of ERβ-induced apoptosis is low, it is comparable to castration over 3 days (proved to be therapeutically effective); but, different from castration, ERβ targets a subpopulation of epithelial cells, including basal cells that are castrate resistant. It could be argued that this difference is more biologically significant because stem cells within the basal layer are required for normal prostatic regeneration that occurs after repeated cycles of androgen withdrawal and replacement. We showed that, unlike castration, ERβ agonist depletes p63+ prostatic basal cells and perturbs epithelial regeneration following recovery. To further address whether ERβ agonist could affect the putative human prostatic stem cells, we studied a subpopulation (∼0.5%) of CD133+ BPH-1 epithelial cells. CD133+ is one of the unique cell surface markers used to enrich for human putative stem cells with demonstrated functional regenerative capacity (25, 26); it is also used to enrich for mouse prostatic stem cells in combination with other markers (33). Here we showed that ERβ induced apoptosis in an enriched CD133+ subpopulation (as well as other cell populations) of BPH-1 cells that are androgen-independent.
In human PCa, castration causes apoptosis in the bulk of tumor cells, but the remaining androgen-independent cells are implicated in disease recurrence. Unlike castration, we show ERβ increased apoptosis in androgen-independent PCa cells (PC3 and DU145), as well as xenografts of primary PCa specimens, expressing ERβ. Whether ERβ agonist targets the castrate-resistant PCa tumor–initiating cells remains to be investigated, and awaits delineation of markers that can isolate and distinguish between normal stem cells and cancer stem cells (25, 26, 34).
Like castration, ERβ targets and induces apoptosis in prostatic stromal cells that play a critical role in the initiation and progression of BPH (and PCa). There are two advantages of ERβ agonist targeting the stroma: first, it has a direct effect on the stromal nodules of BPH themselves; and second, it disrupts stromal–epithelial interactions that are necessary for prostatic epithelial cell proliferation and differentiation (35). Targeting the stroma breaks this cycle of aberrant cell–cell signaling, and therefore it is significant that ERβ agonist targets both stroma as well as epithelial cells, exemplifying its potential therapeutic use. Collectively, the cellular targets of ERβ agonist and castration overlap (including luminal and stromal cells). Uniquely, however, ERβ agonist induces apoptosis in prostatic basal cells, including subpopulations of basal cells enriched for stem/progenitor cells (α2β1hi/CD133+), and androgen-independent PCa cells; this is achieved without altering steroid hormone levels.
Overall, this study demonstrates beneficial effects of ERβ agonist on both BPH and PCa cells and human clinical specimens that are mechanistically different from castration, and targets both castrate-responsive and castrate-resistant cells. These studies support the rationale for the preclinical testing and evaluation of the potential for clinical application of estrogen-based therapies, specifically including ERβ agonists, either alone or in combination with existing androgen blockade, for the treatment of BPH and/or PCa. Future replicate studies using other ER agonists are warranted to determine the full potential of this class of agonist.
Materials and Methods
Animals.
ArKO or homozygous TNFαKO mice generated by targeted disruption of cyp19 or TNFα, respectively (36, 37), and Balb-c/Nude mice housed at Monash Medical Centre were used at 10–14 weeks of age. NOD/SCID mice were housed at British Columbia (BC) Cancer Research Centre.
Specific ER Modulators.
The ERβ-specific agonist (8β-VE2) and ERα-specific agonist (16α-LE2) were gifted by Drs. Karl-Heinrich Fritzemeier and Katja Prelle (Bayer-Schering Pharma AG). Animals were treated for 3 days by s.c. injection (ERβ [300 μg/kg/d] or ERα agonist [3 μg/kg/d], equivalent volume of peanut oil control) or castration as previously described (21). Testosterone replacement (1-cm Silastic implants of testosterone; Sigma) were given either at the time of initial treatment (3 day experiments) or after treatment (recovery experiments) (additional information on siRNA knockdown of ERβ agonist action in SI Text).
Cell Culture Experiments.
Human prostate cell lines DU145, PC3, BPH-1, and RWPE-1 were cultured as previously described (38). Cells were treated as follows; cells were plated (2 × 104 cells/well) in multichambered slides (Nalge Nunc International) in low-serum media (5% FCS) for 12 h before being treated with ERβ (6 μM)or vehicle at doses equivalent to those used in mice. After 12 h of treatment, cells were fixed in 10% neutral buffered formalin for immunostaining. Further information on enrichment for CD133+ stem/progenitor cells is given in SI Text.
Prostate Tissues and Xenografting.
Fresh tissues were processed and implanted in male NOD/SCID mice as previously described (39). Mice received supplementation with testosterone for a period of 2–4 weeks and were divided into three groups: Control (intact mice treated with vehicle); Castrated (castrated mice with no testosterone); and ERβ (intact mice treated for 3 days with ERβ, 300 μg/kg/d). Additionaldetails on patient selection and sample selection are given in SI Text.
Immunohistochemistry.
Immunohistochemical staining was performed as previously described (21). Antibodies used were as follows: high-molecular-weight cytokeratins (CKH), PCNA, ERα (DAKO), androgen receptor (AR); p63 (Santa Cruz Biotechnology) and ERβ (Novocastra Laboratories Ltd) using previously described protocols (21). Apoptosis was detected using ApopTag Plus Peroxidase In situ Apoptosis detection kit (Chemicon) or with antibodies to cleaved caspase-3, -8, -9 according to the instructions of the manufacturer (Cell Signaling Technology). Details on dual immunofluorescence and quantification of immunostaining are provided in SI Text.
RNA Extraction and Oligo Gene Expression Array.
Total RNA was extracted from prostate tissues using TRIzol reagent (Invitrogen Life Technologies) as previously described (38). Gene expression analysis was conducted using GEArray DNA microarray (OMM-012; SuperArray Bioscience Corp.) Details are provided in SI Text.
Statistical Analysis.
Data were analyzed to determine normality, and significant differences were determined by either t test or one-way ANOVA (Prism 5.00, GraphPad Software Inc.) followed by Tukey posthoc analyses. Significance was accepted at P < 0.05. Data are expressed as mean ± SEM unless otherwise noted.
Supplementary Material
Acknowledgments
We thank Prof. M.E. Gleave and Dr. J. Pedersen for specimen collection/pathology, Prof. R. Sutherland and Dr. E. Caldon for assistance with siRNA, and Dr. K. Britt for manuscript review. Grant support was received from National Health and Medical Research Council, US Army, Department of Defense, Cancer Council of Victoria, Prostate Cancer Foundation of Australia, Victorian Cancer Agency, and the Peter and Lyndy White Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905524107/DCSupplemental.
References
- 1.Wei J, Calhoun E, Jacobsen S. Benign prostatic hyperplasia. In: Litwin M, Saigal C, editors. Urologic Diseases in America. Bethesda, MD: National Institutes of Health; 2007. NIH Publication No. 07-5512. [Google Scholar]
- 2.Penson D, Chan J. Prostate cancer. In: Litwin M, Saigal C, editors. Urologic Diseases in America. Bethesda, MD: National Institutes of Health; 2007. NIH Publication No. 07-5512. [Google Scholar]
- 3.Locke JA, et al. Litwin M, Saigal C, editors. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 4.Stone NN, Clejan SJ. Response of prostate volume, prostate-specific antigen, and testosterone to flutamide in men with benign prostatic hyperplasia. J Androl. 1991;12:376–380. [PubMed] [Google Scholar]
- 5.Klotz L, McNeill I, Fleshner N. A phase 1-2 trial of diethylstilbestrol plus low dose warfarin in advanced prostate carcinoma. J Urol. 1999;161:169–172. [PubMed] [Google Scholar]
- 6.Ricke WA, et al. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. 2008;22:1512–1520. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 7.Risbridger G, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–442. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS, et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: Studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 9.Setlur SR, et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price D, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: Results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176:965–970. doi: 10.1016/j.juro.2006.04.011. discussion 970–961. [DOI] [PubMed] [Google Scholar]
- 11.McPherson SJ, Ellem SJ, Risbridger GP. Estrogen-regulated development and differentiation of the prostate. Differentiation. 2008;76:660–670. doi: 10.1111/j.1432-0436.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 12.Imamov O, et al. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci USA. 2004;101:9375–9380. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillisch A, et al. Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol Endocrinol. 2004;18:1599–1609. doi: 10.1210/me.2004-0050. [DOI] [PubMed] [Google Scholar]
- 15.Jazbutyte V, et al. Ligand-dependent activation of ERbeta lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res. 2008;77:774–781. doi: 10.1093/cvr/cvm081. [DOI] [PubMed] [Google Scholar]
- 16.Seidlová-Wuttke D, Prelle K, Fritzemeier KH, Wuttke W. Effects of estrogen receptor alpha- and beta-selective substances in the metaphysis of the tibia and on serum parameters of bone and fat tissue metabolism of ovariectomized rats. Bone. 2008;43:849–855. doi: 10.1016/j.bone.2008.07.237. [DOI] [PubMed] [Google Scholar]
- 17.Escande A, et al. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem Pharmacol. 2006;71:1459–1469. doi: 10.1016/j.bcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Hillisch A, et al. Protein structure-based design, synthesis strategy and in vitro pharmacological characterization of estrogen receptor alpha and beta selective compounds. Ernst Schering Res Found Workshop. 2004;(46):47–62. doi: 10.1007/978-3-662-05386-7_4. [DOI] [PubMed] [Google Scholar]
- 19.Jarred RA, et al. Prostate phenotypes in estrogen-modulated transgenic mice. Trends Endocrinol Metab. 2002;13:163–168. doi: 10.1016/s1043-2760(02)00575-1. [DOI] [PubMed] [Google Scholar]
- 20.McPherson SJ, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 21.McPherson SJ, et al. Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–574. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- 22.Hayward SW, Brody JR, Cunha GR. An edgewise look at basal epithelial cells: Three-dimensional views of the rat prostate, mammary gland and salivary gland. Differentiation. 1996;60:219–227. doi: 10.1046/j.1432-0436.1996.6040219.x. [DOI] [PubMed] [Google Scholar]
- 23.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 24.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 25.Richardson GD, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 26.Vander Griend DJ, et al. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risbridger GP, Frydenberg M, Hayward SW, Clark PE, Appu S. Endocrinology of the Prostate. In: Groot LJD, editor. Endocrinology, Male Reproduction, Endocrinology of the Prostate. 2009. in Press. [Google Scholar]
- 28.Ellem SJ, Risbridger GP. Treating prostate cancer: A rationale for targeting local oestrogens. Nat Rev Cancer. 2007;7:621–627. doi: 10.1038/nrc2174. [DOI] [PubMed] [Google Scholar]
- 29.Rijken AM, et al. Genomic alterations in distal bile duct carcinoma by comparative genomic hybridization and karyotype analysis. Genes Chromosomes Cancer. 1999;26:185–191. [PubMed] [Google Scholar]
- 30.Huang EJ, et al. Opposing action of estrogen receptors alpha and beta on tumor necrosis factor-alpha gene expression and caspase-8-mediated apoptotic effects in HA22T cells. Mol Cell Biochem. 2006;287:137–145. doi: 10.1007/s11010-005-9092-4. [DOI] [PubMed] [Google Scholar]
- 31.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 32.Leav I, et al. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 34.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 35.Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 36.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Körner H, et al. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- 38.Balanathan P, et al. Epigenetic regulation of inhibin alpha-subunit gene in prostate cancer cell lines. J Mol Endocrinol. 2004;32:55–67. doi: 10.1677/jme.0.0320055. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate. 2005;64:149–159. doi: 10.1002/pros.20225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.