Abstract
We provide unprecedented genetic and biochemical evidence that the antiapoptotic transcription factor STAT3 serves as a substrate for SYK tyrosine kinase both in vitro and in vivo. Induction of SYK in an ecdysone-inducible mammalian expression system results in STAT3 activation, as documented by tyrosine phosphorylation and nuclear translocation of STAT3, as well as amplified expression of several STAT3 target genes. STAT3 activation after oxidative stress (OS) is strongly diminished in DT40 chicken B-lineage lymphoma cells rendered SYK-deficient by targeted disruption of the syk gene. Introduction of a wild-type, C-terminal or N-terminal SH2 domain-mutated, but not a kinase domain-mutated, syk gene into SYK-deficient DT40 cells restores OS-induced enhancement of STAT-3 activity. Thus, SYK plays an important and indispensable role in OS-induced STAT3 activation and its catalytic SH1 domain is critical for this previously unknown regulatory function. These results provide evidence for the existence of a novel mode of cytokine-independent cross-talk that operates between SYK and STAT3 pathways and regulates apoptosis during OS. We further provide experimental evidence that SYK is capable of associating with and phosphorylating STAT3 in human B-lineage leukemia/lymphoma cells challenged with OS. In agreement with a prerequisite role of SYK in OS-induced STAT3 activation, OS does not induce tyrosine phosphorylation of STAT3 in SYK-deficient human proB leukemia cells. Notably, inhibition of SYK with a small molecule drug candidate prevents OS-induced activation of STAT3 and overcomes the resistance of human B-lineage leukemia/lymphoma cells to OS-induced apoptosis.
Keywords: apoptosis, cancer, radiation
B-lineage acute lymphoblastic leukemia (ALL) is the most common form of cancer in children and adolescents (1). Resistance of B-lineage ALL cells to the proapoptotic effects of radiation-induced oxidative stress (OS) hampers the attempts to improve the survival outcome of relapsed ALL patients undergoing total body irradiation (TBI) and stem cell transplantation (SCT) and only < 20% of patients become long-term leukemia-free survivors after SCT (1–3). In recent years, intense research efforts have therefore concentrated on elucidating the components of the cellular signal transduction pathways controlling the apoptotic response vs. resistance to OS (4–9).
The signal transducer and activator of transcription (STAT)3 protein, has recently been identified as an important regulator of cell survival after exposure to apoptotic signals, including OS (10–14). The protective effect of STAT3 against apoptosis has been explained partly by upregulation of antiapoptotic proteins such as Bcl-XL and survivin as well as inactivation of caspases (12–14). It has been well established that OS induces the activation of various protein tyrosine kinases (PTK) but the PTK responsible for OS-mediated activation of STAT3 has not yet been identified (7, 9, 15–17). SYK is a cytoplasmic PTK with multiple important regulatory functions in B-lineage lymphoid cells (18). SYK has been demonstrated to play a crucial role in OS signaling in B-cells (9, 17). Here we provide unprecedented genetic and biochemical evidence that SYK plays a mandatory role in OS-induced activation of STAT3 in B-lineage leukemia/lymphoma (BLL) cells. Notably, inhibition of SYK with a small molecule drug candidate prevents OS-induced activation of STAT3 and overcomes the resistance of human BLL cells to OS-induced apoptosis.
Results and Discussion
STAT3 Is a PTK Substrate for SYK.
Far Western blot analyses (WBA) showed that purified recombinant SYK and STAT3 bind each other in vitro in a concentration-dependent manner (Fig. S1A and B). Recombinant SYK phosphorylates recombinant STAT3 during in vitro kinase assays (Fig. S1C and D). Phosphoamino acid analysis of the SYK-phosphorylated STAT3 confirmed that SYK phosphorylates STAT3 exclusively on tyrosine (Y) residues (Fig. S1E). Evaluation of STAT3/SYK interactions in a heterologous baculovirus expression system documented that ectopically expressed recombinant STAT3 physically associates with (Fig. S2A and B) and serves as an in vivo PTK substrate for coexpressed recombinant SYK (Fig. S2C and D). SYK-mediated Y-phosphorylation of ectopically expressed STAT3 enhances its DNA binding activity (Fig. S2E). We next sought to determine if native STAT3 in human B-lineage lymphoid cells is also capable of physically interacting with native SYK. We found that STAT3 associates with SYK in BCL-1 cells challenged with pervanadate (PV)-induced (15) OS (Fig. S2F and G).
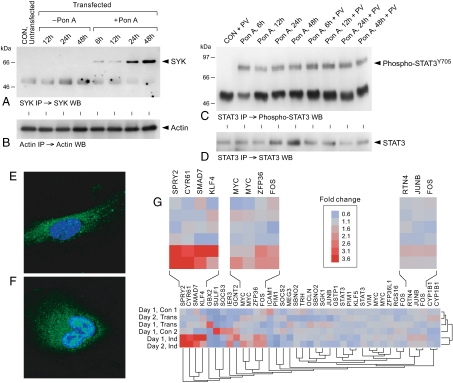
These results prompted us to further examine the regulatory role of SYK in STAT3 activation using an ecdysone-inducible mammalian expression system (19). Exposure of SYK-deficient U373 cells stably transfected with wild-type syk gene to Pon-A induced expression of SYK in a time-dependent fashion (Fig. 1A and B). Induction of SYK resulted in Y-phosphorylation (Fig. 1C and D) and nuclear translocation (Fig. 1E and F) of STAT3. Exposure of transfected but uninduced control U373 cells to PV did not result in STAT3 activation, showing that, in the absence of SYK, exposure to oxidative stress is unable to cause STAT3 activation via stimulation of another PTK. SYK induction without any exposure to PV was sufficient for activating Y-phosphorylation of STAT3 and exposure of SYK-expressing U373 cells to PV did not substantially alter the Y-phosphorylation level of STAT3 (Fig. 1C). Treatment of syk-transfected U373 cells with the pan-JAK inhibitors AG-490 (100 μM) and Pyridone 6 (5 nM) plus the JAK3 inhibitor JANEX-1/WHI-P131 (100 μM) during the Pon-A exposure resulted in abrogation of JAK activity, as documented by the absence of autophosphorylation in the anti-phosphotyrosine (APT) Western blots of JAK kinases immunoprecipitated with a cocktail of antibodies to JAK1, JAK2, and JAK3 (Fig. S3A and B). However, JAK inhibition did not prevent the Y-phosphorylation of STAT3 after Pon-A induced SYK expression (Fig. S3C and D), which excludes any direct or indirect involvement of JAK kinases in SYK-mediated STAT3 activation. We next used the U95Av2 GeneChip microarrays from Affymetrix to interrogate the expression levels of validated STAT3 target genes (20) before and after induction of SYK expression in the U373 expression platform (Fig. 1G, SI Text). Six of the 29 interrogated STAT3 targets belonging to 3 subclusters of genes showed marked upregulation following SYK induction (Fig. 1G). In addition to these 6 genes, 3 additional genes belonging to the same 3 subclusters also showed a SYK induction-associated increase in expression levels that did not reach statistical significance (Fig. 1G, SI Text). None of the remaining 20 STAT3 target genes were down-regulated after SYK induction (Fig. 1G). Thus, SYK is capable of causing Y-phosphorylation, nuclear translocation, and activation of transcription factor function of STAT3 in mammalian cells.
Fig. 1.
Activation of STAT3 in an ecdysone-inducible mammalian expression system for SYK. (A) AntiSYK WBA of SYK immune complexes from whole cell lysates of untransfected vs. transfected U373 cells before and after exposure to the ecdysone-analogue Pon-A (10 μM). (B) Antiactin WBA of actin IC from the same lysates used in (A). (C) Anti-phospho-STAT3Y705 WBA of STAT3 immune complexes from whole cell lysates of transfected uninduced vs. induced U373 cells. Lysates from syk-transfected U373 cells were prepared before (CON) and at various time points after addition of Pon-A (10 μM) as indicated. Some samples were exposed to PV as well where indicated. (D) Anti-STAT3 WBA of the STAT3 immune complexes shown in (C). (E) Confocal image depicting the cytoplasmic localization of native STAT3 in a representative syk-transfected U373 cell before exposure to Pon-A (10 μM). (F) Confocal image showing substantial nuclear (in addition to cytoplasmic) localization of native STAT3 in a representative syk-transfected U373 cell 6-h after exposure to Pon-A (10 μM). (G) Pon-A (10 μM)-induced SYK induction leads to upregulation of STAT3-responsive genes. The heat map represents the color-coded expression value reported as fold change relative to the average expression levels in the control samples (key shows from 0.6- to 3.6-fold change, Blue to Red, respectively). The two-way dendrogram depicts the similarity of expression pattern for all probes (39 probesets for 29 genes) across the 6 treatments and the 6 treatments across the 39 probesets arranged in rows and columns respectively.
SYK Plays a Pivotal Role in OS-Induced Activation of STAT3 in B-lineage Lymphoid Cells.
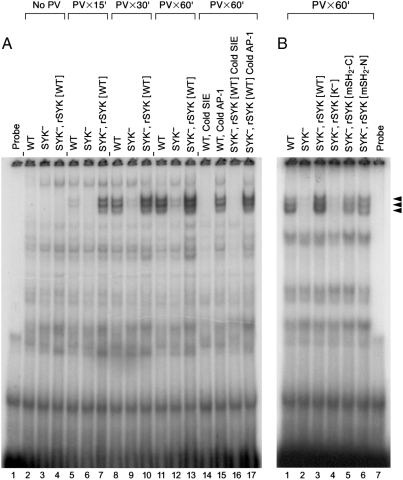
We next examined the role of SYK in OS-induced STAT3 activation in B-lineage lymphoid cells using SYK-deficient DT40 clones that were established by homologous recombination knockout (Fig. S4A–C). In these experiments we used PV, a strong oxidizing agent that triply oxidizes the catalytic cysteine of protein tyrosine phosphatases (21). EMSAs of nuclear proteins from wild-type DT40 cells treated with PV revealed significant mobility shifts in complexes binding the SIE probe especially after 30–60 min of treatment (Fig. 2). This binding was specific to the SIE probe, as indicated by homologous versus nonhomologous competition with 100x excess cold probe. The shifted bands, previously shown to be STAT3 homo- and heterodimers (8), were not detectable in nuclear proteins from SYK-deficient (SYK-) DT40 cells (Fig. 2). The diminished STAT3 signal in the SYK-deficient DT40 cells was due to the lack of SYK because SYK- DT40 cells reconstituted with wild-type SYK showed a rapid and strong STAT3 response to PV. By contrast, SYK- DT40 cells reconstituted with the kinase domain-mutant of SYK failed to show any restoration of the PV-induced STAT3 signal (Fig. 2). Thus, SYK plays an important and indispensable role in OS-induced STAT3 activation and its catalytic domain is critical for this previously unknown regulatory function. Both the C-terminal SH2 mutant and N-terminal SH2 mutant were effective in mediating SYK function in triggering STAT3 activation (Fig. 2), demonstrating that the two tandem SH2 domains of SYK are not necessary for optimal STAT3 signaling during OS. In agreement with the EMSA data, comparison of H2O2-treated SYK- DT40 cells vs. SYK- DT40 cells reconstituted with wild-type SYK by confocal microscopy showed that only the nuclei of the SYK-reconstituted DT40 cells showed the presence of translocated active phospho-STAT3 protein (Fig. S4D).
Fig. 2.
Role of SYK in oxidative stress-induced activation of STAT3 in DT40 chicken lymphoma B-cells (7, 9). EMSAs in (A) and (B) were performed with end-labeled SIE probe and nuclear extracts prepared from wild-type DT40 cells, SYK-deficient DT40 cells (SYK-), SYK-deficient DT40 cells reconstituted with wild-type SYK (SYK-, rSYK [WT]), catalytic kinase domain-mutant of SYK (SYK-, rSYK [K-], and SH2 domain mutants of SYK (SYK-, rSYK [mSH2-C] and SYK-, rSYK [mSH2-N]). Cells were left untreated (no PV) or treated with 400 μM PV for 15 min, 30 min, or 60 min, as indicated. The nuclear extracts were preincubated and then the labeled probe was added (A, Lanes 2–13). In unlabeled competition reactions, 100-fold excess unlabeled homologous SIE (A, Lanes 14 and 16) or nonhomologous AP-1 probe (A, Lanes 15 and 17) was added prior to the preincubation. Controls included samples containing only the SIE probe without any nuclear extract (A, Lane 1; B, Lane 7). Mobility shifts were determined by electrophoresis as described in Materials and Methods. Following electrophoresis, gels were dried and subjected to autoradiography on film. Shifted bands are indicated by arrows.
Inhibition or Deficiency of SYK Prevents OS-Induced, SYK-Mediated Y-Phosphorylation of STAT3 in Human B-lineage Lymphoid Cells.
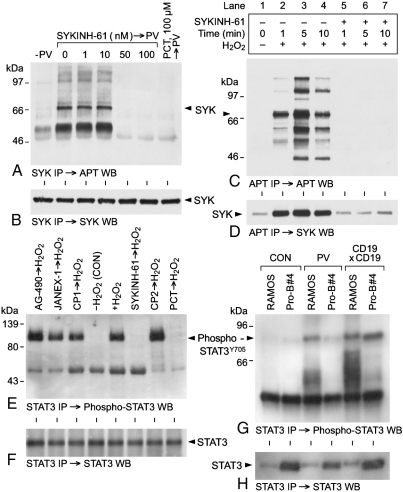
We next used SYKINH-61 as a potent and selective SYK inhibitor to examine the role of SYK in OS-induced Y-phosphorylation of STAT3 in human B-lineage lymphoid cells. SYKINH-61 is a pentapeptide mimic targeting the substrate-binding site of SYK (Fig. S5), that inhibits SYK at nanomolar concentrations, but does not inhibit EGF-R, IRβ, BTK, HCK, JAK1, JAK2, or JAK3 kinases (Fig. S6A–I). Incubation of NALM-6 B-lineage ALL cells with SYKINH-61 (but not WHI-P131, the multifunctional ATP site inhibitor of JAK3/EGF-R) results in inhibition of constitutively active native SYK without any change in SYK protein expression levels (Fig. S6J and K). SYKINH-61 prevented PV-induced as well as H2O2-induced Y-phosphorylation of SYK in RAMOS (Fig. 3A and B) and BCL-1 cells (Fig. 3C and D), respectively. As shown in Fig. 3E and F, the SYK inhibitors SYKINH-61 and Piceattanol (PCT) inhibited H2O2-induced Y-phosphorylation of STAT3 in RAMOS cells. In contrast to SYKINH-61 and PCT, tyrphostin AG-490 inhibiting JAK1, JAK2, and JAK3 (22, 23), JANEX-1 inhibiting JAK3 (24) or BTK inhibitors CP-1/HI-12 and CP-2/HI-86 (25) did not prevent PV-induced Y-phosphorylation of STAT3.
Fig. 3.
Role of SYK in oxidative stress-induced tyrosine phosphorylation of STAT3 in human B-lineage lymphoid cells. (A and B) RAMOS Burkitt’s leukemia/lymphoma cells were either left untreated or treated with the oxidative agent PV at a 400 μM concentration in the presence or absence of SYKINH-61 (1 nM, 10 nM, 50 nM, 100 nM) or 100 μM PCT. Cells were lysed using Nonidet-P40 buffer after 30 min exposure to PV, PV + SYKINH-61, or PV + PCT and lysates were immunoprecipitated with antiSYK antibodies, as indicated. The SYK immune complexes were resolved by SDS-PAGE and examined by APT (A) or antiSYK (B) Western blot analysis. (C and D) BCL-1 cells were either left untreated or treated with 100 mM H2O2 as the oxidative agent in the presence or absence of 100 nM SYKINH-61. Cells were lysed using Nonidet-P40 buffer after 1 min, 5 min, or 10 min exposure to H2O2 or H2O2 + SYKINH-61 and lysates were immunoprecipitated with APT antibodies. The immune complexes were resolved by SDS-PAGE and examined by APT (C) or antiSYK (D) WBA. (E and F) RAMOS cells were left untreated (CON, -H2O2) (Lane 4) or treated with 100 mM H2O2 for 10 min in the absence (Lane 5) or presence of SYK kinase inhibitors SYKINH-61 (100 nM) (Lane 6) and PCT (100 μM) (Lane 8), JAK1,2,3 inhibitor AG-490 (100 μM) (Lane 1), JAK3 kinase inhibitor JANEX-1 (100 μM), or BTK inhibitors compound 1 (CP-1)/HI-12 (100 μM) (Lane 3), and compound 2 (CP-2/HI-86) (100 μM) (Lane 7). STAT3 immune complexes from whole cell lysates of these cells were subjected to WBA with antiphospho STAT3Y705 (E) or anti-STAT3 (F) antibodies. (G and H) RAMOS Burkitt’s leukemia/lymphoma cell and ProB#4 cells (27) were left untreated (CON) (Lanes 1 and 2) or treated with 400 μM PV for 30 min (PV) (Lanes 3 and 4). Controls were treated with an anti-CD19 antibody homoconjugate (1 μg/mL) (Lanes 5 and 6) to stimulate the CD19-linked signaling pathway. STAT3 immune complexes from whole cell lysates were subjected to WBA with antiphospho STAT3Y705 (G) or anti-STAT3 (H) antibodies.
We next compared the PV-responsiveness of SYK+ RAMOS (26) cells vs. SYK-deficient cells from a proB ALL patient (ProB#4) with a missplicing at the 5′ end of syk exon 5 leading to a 4 bp deletion (Δ[G865-G868]) in the syk coding sequence that causes a frameshift starting after Trp239 with absent SYK protein expression and SYK kinase activity (27). Notably, PV [but not CD19 receptor engagement with an anti-CD19 homoconjugate (26)] failed to induce Y-phosphorylation of the STAT3(Y705) phosphoepitope in SYK-deficient ProB#4 cells (Fig. 3G and H). Thus, STAT3 in SYK-deficient ProB#4 cells is capable of undergoing Y-phosphorylation and the inability of PV to induce STAT3 phosphorylation in ProB#4 cells is not due to a defective STAT3 protein. We next examined the effects of SYKINH-61 on DNA binding activity of STAT3. In accord with our previous studies (8), PV-induced OS triggered STAT3 activation in all human B-lineage lymphoid cell lines tested, and this activation was abrogated by SYK kinase inhibitors PCT and SYKINH-61, but not by inhibitors of JAK3, (24), BTK (25), or tubulin polymerization (28) (Fig. S7).
Inhibition of SYK Promotes OS-Induced Apoptosis in Radiation-Resistant Human B-lineage Leukemia/Lymphoma Cells.
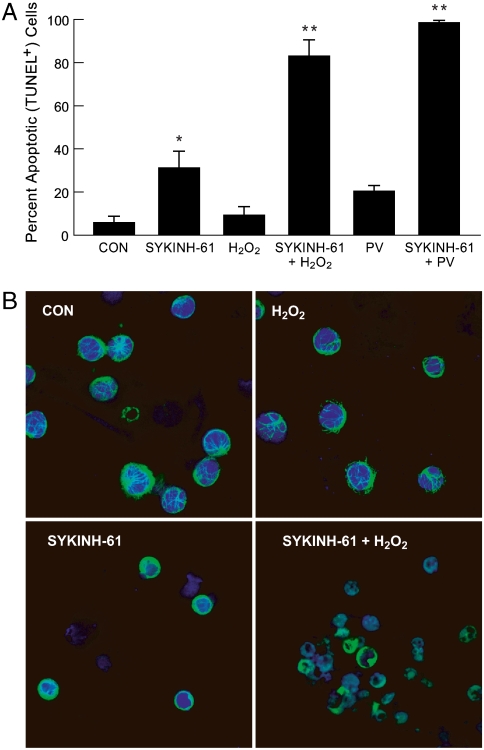
In agreement with the antiapoptotic function of the SYK-regulated STAT3 response to OS, the sensitivity of DT40 cells to PV was remarkably enhanced when they were rendered SYK-deficient (Fig. S8). As shown in Fig. 4A, SYKINH-61 markedly enhanced both H2O2-induced (83.3% vs. 9.5%, P < 0.001) and PV-induced apoptosis (98.8% vs. 20.6%, P < 0.001) in human BLL cells, as measured by in vitro TUNEL assays, including primary leukemic cells from two B-lineage ALL patients, who had relapsed after TBI and allogeneic SCT. Examination of each treatment versus control mean values showed significant effects of SYKINH-61 (Dunnett’s post hoc, p = 0.004) (“*”), SYKINH-61 + H2O2 (p < 0.0001) (“**”), and SYKINH-61 + PV (p < 0.0001) (“**”) (Fig. 4A). Fig. 4B depicts the confocal images of leukemic cells from a radiation-resistant relapsed ALL patient after treatment with H2O2, SYKINH-61, and H2O2 + SYKINH-61. Whereas H2O2-treated leukemic cells maintained their viability, virtually all of the cells treated with H2O2 + SYKINH-61 showed morphologic signs of advanced apoptosis, including shrinkage, nuclear fragmentation, loss of cytoplasmic, and nuclear integrity (Fig. 4B). Thus, inhibition of SYK with SYKINH-61 promotes OS-induced apoptosis against human BLL cells.
Fig. 4.
SYKINH-61 promotes oxidative stress-induced apoptosis in primary B-lineage ALL cells. (A) Cells from two EBV-transformed lymphoblastoid cell lines BCL-1 and BCL-2, Burkitt’s leukemia/lymphoma cell line RAMOS, as well as primary leukemic cells from two B-lineage ALL patients were either left untreated or treated with 100 mM H2O2, 50 nM SYKINH-61, or 100 mM H2O2 + 50 nM SYKINH-61 for 30 min at 37 °C. TUNEL assays were used after 24 hr to determine the percentage of apoptotic cells after treatment. (B) Cells from a B-lineage ALL patient in postSCT relapse were either left untreated or treated with 100 mM H2O2, 50 nM SYKINH-61, or 100 mM H2O2 + 50 nM SYKINH-61 for 30 min at 37 °C. After 24 hr of culture, cells were costained with a rabbit polyclonal antitubulin antibody (Green Fluorescence) and the DNA-specific dye Toto-3 (Blue Fluorescence), and examined by laser scanning confocal microscopy (7, 8, 48).
SYK has recently emerged as a potential new molecular target for the treatment of B-lineage leukemias and lymphomas (29–34). Compelling evidence from gene profiling studies indicates that abundant STAT3 expression in ALL is associated with chemotherapy and steroid resistance (35–37). Our experimental findings presented herein provide unprecedented genetic and biochemical evidence that SYK plays an important and indispensable role in OS-induced activation of STAT3 and its catalytic domain is critical for this previously unknown survival-promoting function in B-lineage leukemia/lymphoma cells. SYK is the first cytoplasmic nonJAK PTK to be identified as a positive regulator of STAT3 in B-lineage lymphoid cells exposed to OS. Several studies have independently demonstrated that cytokine-mediated (38) as well as B-cell antigen receptor (BCR) mediated (39) activation of STAT3 bypasses JAKs in B-lineage lymphoid cells. Several nonJanus family PTK, including TEC family kinase ETK/BMX (40, 41), FES (42), SRC (43), SRC family kinases LCK (44) and LYN (39), and breast tumor kinase BRK (45) have been shown to be capable of activating STAT3 by phosphorylating STAT3(Y705) phosphoepitope independent of JAKs and without inducing activation of endogenous JAKs. Thus, it appears that STAT3 licenses different cytoplasmic PTK in distinct activation pathways.
In the present study, we documented that OS induces rapid stimulation of Y-phosphorylation and nuclear translocation of STAT3 in B-lineage lymphoid cells, which differs from the published reports of STAT3 responses that follow BCR engagement (39, 46, 47). BCR stimulation of murine B-cells has been shown to result in a delayed accumulation of Y-phosphorylated STAT3 via activation of a lymphokine pathway mediated by interleukins 6 and 10 produced by the stimulated B-cells (46). Although BCR stimulation in DT40 cells has been shown to rapidly activate STAT3 Y-phosphorylation through LYN in a JAK independent pathway (39), others have reported that BCR stimulation of murine B-cells induces rapid serine phosphorylation of STAT3 without any Y-phosphorylation (47).
SYK has been reported to participate in both upregulation as well as downregulation of apoptosis in B-lineage lymphoid cells (17, 18). SYK is required for BCR-independent calcium induced apoptosis as well as BCR-mediated apoptosis (48, 49). SYK is not required for radiation or OS-induced apoptosis (7, 50). To the contrary, SYK has been reported to have an antiapoptotic function in the context of OS (17). However, SYK may also play a proapoptotic role via activation of PLCγ2 when oxidative stress is caused by low doses of H2O2 (17, 51). Han et al. (51) reported that the presence of the B-cell linker protein (BLNK) (also known as SLP-65) is required for this apoptosis-accelerating function of SYK under suboptimal OS. Notably, BLNK deficiency plays an important role in the leukemogenesis of B-lineage ALL (52) and a significant portion of B-lineage ALL cases are BLNK-negative (53). The remarkable radiation resistance of relapsed B-lineage ALL patients (pre-pre-B immunophenotype) (6) with abundant expression of SYK (27) indicates that the balance of SYK-linked proapoptotic vs. antiapoptotic signals triggered by OS is an antiapoptotic signal, which may in part be related to their underlying BLNK deficiency. It is noteworthy that SYK also has been reported to have an antiapoptotic function in DT40 cells in the context of ceramide responses that is independent of its enzymatic activity (54). However, in the context of STAT3 activation after OS, the kinase activity of SYK is clearly required for tyrosine phosphorylation of STAT3, as PV did not activate STAT3 in SYK- DT40 cells reconstituted with a kinase domain-mutant SYK and inhibition of the kinase activity of SYK with small molecule inhibitors SYKINH-61 or PCT prevented OS-induced STAT3 activation in the absence of any alteration in SYK protein levels. Likewise, the kinase activity of SYK has been shown to be essential for its reported antiapoptotic activity in the context of OS (9), tonic or ligand-mediated BCR signaling (31, 33, 34), and cytokine signaling (55).
The identification of SYK as a regulator of the antiapoptotic STAT-3 response to OS prompts the hypothesis that PTK inhibitors targeting SYK may overcome the resistance to OS-induced apoptosis and thereby provide the foundation for more effective multimodality treatment regimens for poor prognosis B-lineage ALL patients. This hypothesis is strongly supported in this study by the documented ability of SYK inhibitor SYKINH-61 to markedly enhance OS-induced apoptosis in primary leukemic cells from radiation-resistant ALL patients. In a recent study, constitutive STAT3 phosphorylation in CLL cells appeared to correlate with their SYK expression levels and was reduced by SYK inhibitors (34). Therefore, the antiapoptotic transcription factor STAT3 may also serve as a PTK substrate for SYK in multiple survival-promoting signaling pathways, including the tonic BCR signaling in mature B-cell neoplasms.
Materials and Methods
Recombinant Baculovirus Construction and Protein Expression.
The gene encoding wild-type SYK was ligated into pFastBac1 (PFB) (Gibco-BRL) (8, 56). The resulting vector, PFB-syk, was then used to generate the recombinant baculovirus by site-specific transposition in E. coli DH10Bac cells (Gibco-BRL), which harbor a baculovirus shuttle vector (bacmid), bMON14272. The resulting recombinant bacmid DNA was introduced into Sf21 insect cells (Invitrogen) by transfection as reported (8, 56). Recombinant baculovirus containing the gene encoding murine STAT3 was a kind gift from J. Feng and J.N. Ihle (St. Jude Children’s Hospital). Coexpression experiments were carried out to analyze potential interactions between SYK and STAT3 using previously published procedures (8, 56).
Biochemical Assays.
Immunoprecipitations, immune complex kinase assays (KA), cell-free KA, phosphoamino acid analysis, and WBA were performed as previously reported (7, 8, 15, 16, 26, 27, 56–58). Antibodies against phospho-STAT3 (anti-phospho-STAT3[pY705) were obtained from Sigma–Aldrich.
Electrophoretic Mobility Shift Assays (EMSAs).
EMSAs were performed with a double-stranded m67-SIE probe (top strand: ttgcATTTCCCGTAAATcttgtctaga), end-labeled with 32P using T4 polynucleotide kinase and γ32P-ATP, as previously described (8).
Molecular Modeling.
Fixed docking in the Affinity program within Insight II (Molecular Simulation Inc.) was used for docking SYKINH-61 to the ATP vs. substrate-binding sites of SYK, which was built, based on the crystal structure of IR kinase ternary complex with an ATP analog, a peptide, and Mg ions using the homology module of InsightII. The final docked position of the molecule was chosen based on both the lowest energy estimation, as we previously reported for other PTK inhibitors (24, 56, 59–61).
For more information, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
DT40 and its subclones were obtained from T. Kurosaki (Yale University School of Medicine). The authors thank the members of the Uckun Lab from Parker Hughes Institute for providing the PTK inhibitors, technical assistance, and immunoprecipitations/immunoblotting, confocal microscopy, and molecular modeling. This work was funded by Parker Hughes Trust and Hughes Chair in Molecular Oncology at Parker Hughes Institute (F.M.U.).
Footnotes
The authors declare no conflict of interest.
This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909086107/DCSupplemental.
References
- 1.Trigg ME, Gaynon P, Uckun FM. In: Cancer Medicine. 4th Ed. Holland JF, et al., editors. London: B.C. Decker, Inc.; 1996. pp. 2945–2960. [Google Scholar]
- 2.Gaynon PS, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children’s Oncology Group Study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 3.Bailey LC, Lange BJ, Rheinhold SR, Bunin NJ. Bone marrow relapse in paediatric acute lymphoblastic leukemia. Lancet Oncol. 2008;9(9):873–83. doi: 10.1016/S1470-2045(08)70229-8. [DOI] [PubMed] [Google Scholar]
- 4.Weston VJ, et al. Apoptotic resistance to ionizing radiation in pediatric B-precursor acute lymphoblastic leukemia frequently involves increased NF kappa B survival pathway signaling. Blood. 2004;104:1465–1473. doi: 10.1182/blood-2003-11-4039. [DOI] [PubMed] [Google Scholar]
- 5.Marston E, et al. Stratification of pediatric ALL by in vitro cellular responses to DNA double-strand breaks provides insight into the molecular mechanisms underlying clinical response. Blood. 2009;113:117–126. doi: 10.1182/blood-2008-03-142950. [DOI] [PubMed] [Google Scholar]
- 6.Uckun FM, et al. Intrinsic radiation resistance of primary clonogenic blasts from children with newly diagnosed B-cell precursor acute lymphoblastic leukemia. J Clin Invest. 1993;91(3):1044–51. doi: 10.1172/JCI116261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uckun FM, et al. BTK as a mediator of radiation-induced apoptosis in DT40 lymphoma B-cells. Science. 1996;273:1096–1100. doi: 10.1126/science.273.5278.1096. [DOI] [PubMed] [Google Scholar]
- 8.Uckun FM, Ozer Z, Vassilev A. Bruton’s protein tyrosine kinase prevents activation of the anti-apoptotic transcription factor STAT3 in B-lineage lymphoma cells exposed to OS. Brit J Haematol. 2007;136(4):574–589. doi: 10.1111/j.1365-2141.2006.06468.x. [DOI] [PubMed] [Google Scholar]
- 9.Ding J, et al. Syk is required for the activation of Akt survival pathway in B cells exposed to OS. J Biol Chem. 2000;275:30873–877. doi: 10.1074/jbc.M004813200. [DOI] [PubMed] [Google Scholar]
- 10.Otero DC, Poli V, David M, Rickert RC. Cutting edge: Inherent and acquired resistance to radiation-induced apoptosis in B-cells: A pivotal role for STAT3. J Immunol. 2006;177:6593–6597. doi: 10.4049/jimmunol.177.10.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haga S, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and independent mechanisms. J Clin Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandis JR, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda K, et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: Generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- 14.Terui K, et al. Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J Hepatol. 2004;41(6):957–65. doi: 10.1016/j.jhep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Schieven GL, Kirihara JM, Myers DE, Ledbetter JA, Uckun FM. Reactive oxygen intermediates activate NF-kappa B in a PTK-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn PTKs in human lymphocytes. Blood. 1993;82:1212–1220. [PubMed] [Google Scholar]
- 16.Uckun FM, et al. Physical and functional interactions between Lyn and p34cdc2 kinases in irradiated human B-cell precursors. J Biol Chem. 1996;271:6396–6397. doi: 10.1074/jbc.271.11.6389. [DOI] [PubMed] [Google Scholar]
- 17.Tohyama Y, Takano T, Yamamura H. B cell responses to oxidative stress. Curr Pharm Design. 2004;10:835–9. doi: 10.2174/1381612043452947. [DOI] [PubMed] [Google Scholar]
- 18.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 19.No D, Yao T-P, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourillot P-Y, et al. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells. 2009;27:1760–1771. doi: 10.1002/stem.110. [DOI] [PubMed] [Google Scholar]
- 21.Wijk TV, Overvoorde J, Hertog JD. H2O2-induced intermolecular disulfide bond formation between receptor protein-tyrosine phosphatases. J Biol Chem. 2004;279:44355–44361. doi: 10.1074/jbc.M407483200. [DOI] [PubMed] [Google Scholar]
- 22.Kirken RA, et al. Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T-cells. J Leukocyte Biol. 1999;65:891–899. doi: 10.1002/jlb.65.6.891. [DOI] [PubMed] [Google Scholar]
- 23.Xiong H, et al. Inhibition of JAK1,2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudbeck EA, et al. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin Cancer Res. 1999;5(6):1569–1582. [PubMed] [Google Scholar]
- 25.Uckun FM, Zheng Y, Ghosh S. BTK Inhibitors and methods for their identification and use. 1999. WO/1999/054286. International Application No. PCT/US1999/008556.
- 26.Uckun FM, et al. Signal transduction through the CD19 receptor during discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1993;268:21172–21184. [PubMed] [Google Scholar]
- 27.Goodman PA, Wood CM, Vassilev A, Mao C, Uckun FM. Spleen tyrosine kinase (Syk) deficiency in childhood pro-B cell acute lymphoblastic leukemia. Oncogene. 2001;20:3969–3978. doi: 10.1038/sj.onc.1204515. [DOI] [PubMed] [Google Scholar]
- 28.Jan S-T, Mao C, Vassilev A, Navara CS, Uckun FM. COBRA-1. A rationally-designed epoxy-THF containing compound with potent tubulin depolymerizing activity as a novel anticancer agent. Bioorg Med Chem Lett. 2000;11:1193–1197. doi: 10.1016/s0960-894x(00)00212-2. [DOI] [PubMed] [Google Scholar]
- 29.Young RM, et al. Mouse models of non-Hodgkin’s lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113:2508–16. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldi A, et al. Genomic and expression profiling identifies the B cell associated PTK Syk as a possible therapeutic target in mantle cell lymphoma. Brit J Haematol. 2006;132:303–16. doi: 10.1111/j.1365-2141.2005.05883.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wossning T, et al. Deregulated Syk inhibits differentiation and induces growth factor independent proliferation of pre-B cells. J Exp Med. 2006;13:2829–2840. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobessi S, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B-cells. Leukemia. 2009;23:686–97. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 34.Buchner M, et al. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009;69:5424–32. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 35.Maia S, et al. Gene expression profiling identifies BAX-delta as a novel tumor antigen in acute lymphoblastic leukemia. Cancer Res. 2005;65(21):10050–8. doi: 10.1158/0008-5472.CAN-05-1574. [DOI] [PubMed] [Google Scholar]
- 36.Fine BM, et al. Gene expression patterns associated with recurrent chromosomal translocations in acute lymphoblastic leukemia. Blood. 2004;103(3):1043–9. doi: 10.1182/blood-2003-05-1518. [DOI] [PubMed] [Google Scholar]
- 37.Holleman A, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351(6):533–42. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 38.Kopantzev Y, Heller M, Swaminathan N, Rudikoff S. IL-6 mediated activation of STAT3 bypasses Janus kinases in terminally differentiated B lineage cells. Oncogene. 2002;21:6791–800. doi: 10.1038/sj.onc.1205815. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Kurosaki T, Corey SJ. Engagement of the B cell antigen receptor activates STAT through lyn in a Jak-independent pathway. Oncogene. 2007;26:2851–2859. doi: 10.1038/sj.onc.1210092. [DOI] [PubMed] [Google Scholar]
- 40.Wen X, et al. Kinase activation of the non-receptor tyrosine kinase Etk/BMX alone is sufficient to transactivate STAT-mediated gene expression in salivary and lung epithelial cells. J Biol Chem. 1999;274:38204–10. doi: 10.1074/jbc.274.53.38204. [DOI] [PubMed] [Google Scholar]
- 41.Saharinen P, et al. The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase delta. Blood. 1997;90:4341–53. [PubMed] [Google Scholar]
- 42.Nelson KL, et al. Activation of STAT3 by the c-Fes protein tyrosine kinase. J Biol Chem. 1998;273:7072–7. doi: 10.1074/jbc.273.12.7072. [DOI] [PubMed] [Google Scholar]
- 43.Laird AD, et al. Src family kinase activity is required for signal transducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2:461–9. [PubMed] [Google Scholar]
- 44.Lund TC, et al. The Src-family kinase Lck can induce STAT3 phosphorylation and DNA binding activity. Cell Signal. 1999;11:789–96. doi: 10.1016/s0898-6568(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, et al. Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene. 2006;25:4904–12. doi: 10.1038/sj.onc.1209501. [DOI] [PubMed] [Google Scholar]
- 46.Fan H, Rothstein TL. Lymphokine dependence of STAT3 activation produced by surface immunoglobulin cross-linking and by phorbol ester plus calcium ionophore treatment in B cells. Eur J Immunol. 2001;31:665–71. doi: 10.1002/1521-4141(200102)31:2<665::aid-immu665>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Su L, Rickert RC, David M. Rapid STAT phosphorylation via the B cell receptor. Modulatory role of CD19. J Biol Chem. 1999;274:31770–4. doi: 10.1074/jbc.274.45.31770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu D-M, Uckun FM. Calpain inhibitor II induces caspase-dependent apoptosis in human acute lymphoblastic leukemia and non-Hodgkin’s lymphoma cells as well as some solid tumor cells. Clin Cancer Res. 2000;6:2456–2463. [PubMed] [Google Scholar]
- 49.He J, et al. Lysosome is a primary organelle in B cell receptor-mediated apoptosis: An indispensable role of Syk in lysosomal function. Genes Cells. 2005;10:23–35. doi: 10.1111/j.1365-2443.2004.00811.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang C. Syk and Lyn are involved in radiation-induced signaling, but inactivation of Syk or Lyn alone is not sufficient to prevent radiation-induced apoptosis. J Biochem. 1995;118:33–8. doi: 10.1093/oxfordjournals.jbchem.a124888. [DOI] [PubMed] [Google Scholar]
- 51.Han W, et al. Role of BLNK in oxidative stress signaling in B cells. Antioxid Redox Sign. 2001;3:1065–73. doi: 10.1089/152308601317203576. [DOI] [PubMed] [Google Scholar]
- 52.Nakayama J, et al. BLNK suppresses pre-B cell leukemogenesis through inhibition of JAK3. Blood. 2009;113:1483–1492. doi: 10.1182/blood-2008-07-166355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jumaa H, et al. Deficiency of the adaptor BLNK/SLP-65 in pre-B cell acute lymphoblastic leukemia. Nature. 2003;423:452–456. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 54.Qin S, Ding J, Kurosaki T, Yamamura H. A deficiency in Syk enhances ceramide- induced apoptosis in DT40 lymphoma B cells. FEBS Lett. 1998;427(1):139–43. doi: 10.1016/s0014-5793(98)00383-4. [DOI] [PubMed] [Google Scholar]
- 55.Jiang K, et al. Regulation of Akt-dependent cell survival by Syk and Rac. Blood. 2003;101:236–44. doi: 10.1182/blood-2002-04-1251. [DOI] [PubMed] [Google Scholar]
- 56.Mahajan S, et al. Transcription factor STAT5A is a substrate of Bruton’s tyrosine kinase in B cells. J Biol Chem. 2001;276:31216–3122. doi: 10.1074/jbc.M104874200. [DOI] [PubMed] [Google Scholar]
- 57.Malaviya R, Vassilev A, Uckun FM. 2,4,6-Trihydroxy-alpha-p-methoxyphenylacetophenone (Compound D-58) is a potent inhibitor of allergic reactions. Am J Ther. 2001;8(6):417–424. doi: 10.1097/00045391-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Simmons D, Melendy T, Usher D, Stillman B. Simian virus 40 large T antigen binds to topoisomerase I. Virology. 1996;222:365–374. doi: 10.1006/viro.1996.0433. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh S, et al. Specificity of a-cyano-b-hydroxy-b-methyl-N-[4-(Trifluoromethoxy)Phenyl]-Propenamide as an inhibitor of the epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 1999;5:4264–4272. [PubMed] [Google Scholar]
- 60.Mahajan S, et al. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton’s tyrosine kinase (BTK), LFM-A13 [a-Cyano-b-hydroxy-b-methyl-N-(2,5-dibromophenyl)propenamide] J Biol Chem. 1999;274:9587–9599. doi: 10.1074/jbc.274.14.9587. [DOI] [PubMed] [Google Scholar]
- 61.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5573–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.