Abstract
In this study, we show the crucial roles of lipid signaling in long-term depression (LTD), that is, synaptic plasticity prevailing in cerebellar Purkinje cells. In mouse brain slices, we found that cPLA2α knockout blocked LTD induction, which was rescued by replenishing arachidonic acid (AA) or prostaglandin (PG) D2 or E2. Moreover, cyclooxygenase (COX)–2 inhibitors block LTD, which is rescued by supplementing PGD2/E2. The blockade or rescue occurs when these reagents are applied within a time window of 5–15 min following the onset of LTD-inducing stimulation. Furthermore, PGD2/E2 facilitates the chemical induction of LTD by a PKC activator but is unable to rescue the LTD blocked by a PKC inhibitor. We conclude that PGD2/E2 mediates LTD jointly with PKC, and suggest possible pathways for their interaction. Finally, we demonstrate in awake mice that cPLA2α deficiency or COX-2 inhibition attenuates short-term adaptation of optokinetic eye movements, supporting the view that LTD underlies motor learning.
Keywords: PLA2, prostaglandin, Purkinje, arachidonic, OKR
Although lipid signaling is implicated in diverse biological functions, its importance in synaptic mechanisms has become increasingly evident (1 –3). In the first step of lipid signaling, phospholipase A2 (PLA2) acts on membrane phospholipids to release arachidonic acid (AA) and certain lysophospholipid mediators. We previously reported that, among the more than 20 PLA2 subtypes so far identified (2, 4), five subtypes are located in the cerebellum, three of which (cPLA2α, sPLA2IIA, and iPLA2) are expressed with different patterns of localization in Purkinje cells (PCs) (5). It has also been reported (6 –8) that PLA2 is involved in cerebellar postsynaptic long-term depression (hereafter referred to as LTD) that is a persistent attenuation of L-α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor (AMPAR)–mediated transmission in parallel fiber (PF) synapses on PCs (9). In the second step of lipid signaling, AA is converted by cyclooxygenases (COXs) to prostaglandins (PGs) (10) or by lipoxygenases (LOs) to hydroxy fatty acids and leukotrienes (11). In the present study on mouse cerebellar slices, using genetic and pharmacological manipulations, we show the involvement of these enzymes and their products in LTD induction.
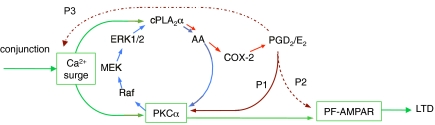
In LTD induction, the conjunction of PF and climbing fiber (CF) impulses evokes a strong Ca2+ surge in PC dendrites. This is caused by a supralinear addition of CF impulse–induced Ca2+ entry through voltage-gated Ca2+ channels and PF impulse–induced Ca2+ release from the intracellular store via metabotropic glutamate receptor type 1 (8, 12, 13). A Ca2+-sensitive protein kinase C subtype (PKCα) (14) is thus activated and in turn phosphorylates AMPARs at serine 880 of the GluR2 C terminus (15, 16). The phosphorylated AMPARs are subsequently dissociated from anchoring proteins and eliminated from the synaptic membrane via endocytosis, thus causing LTD. In neurochemistry, AA activates PKC, which in turn activates a kinase cascade sequentially involving Raf, MEK, ERK1/2, and finally PLA2, which produces AA (Fig. 1, blue arrows) (17 –19). Hence, after the Ca2+ surge, self-regeneration may develop by positive feedback through this closed loop, leading to a rapid, intense activation of PKCα that switches the status of PF-AMPARs from a basal state to the persistently depressed state for LTD (8, 20).
Fig. 1.
Signal transduction pathways activated by intracellular Ca2+ surge and leading to LTD. Green lines, major pathways. Blue arrows, loop connections. Red arrows, cPLA2α–COX-2 cascade. Brown lines (P1, P2, P3), possible pathways downstream of PGD2/E2.
In this study, we demonstrate the critical requirement of cPLA2α-COX-2 cascade for LTD induction. Incorporating the cascade and its downstream pathways, we modify the LTD-inducing signal transduction model (Fig. 1). Furthermore, to test the current hypothesis that LTD underlies motor learning, we examine whether the deficiency of cPLA2α or the i.p.administration of a COX-2 inhibitor affects the adaptation of optokinetic eye movement response (OKR) in awake mice (21). Part of the present findings have been reported preliminarily.*
Results
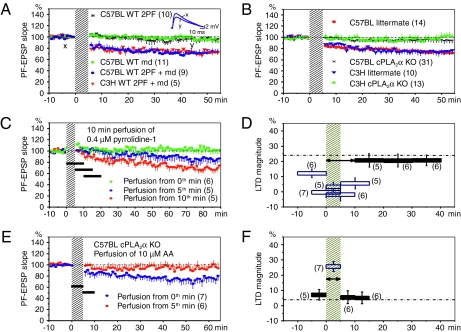
For slice experiments, we used six mouse types: (i) wild-type C3H/HeN strain (abbreviated as C3H WT), (ii) wild-type C57BL/6J strain innately being sPLA2IIA−/− (C57BL WT) (22), (iii) cPLA2α−/− C3H/HeN strain (C3H KO), (iv) cPLA2α−/− sPLA2IIA−/− C57BL/6J strain (C57BL KO), (v) littermate of C3H KO (C3H littermate), and (vi) littermate of C57BL KO (C57BL littermate). C57BL/6Cr WT mice were also used for testing OKR. In acute slices obtained at PW5-12, we recorded from PCs with patch pipettes in a current clamp configuration. We recorded PF stimulation–evoked excitatory postsynaptic potentials (PF-EPSPs) (Fig. 2A, Inset). Various reagents (Table S1) were applied to PCs by intracellular infusion through a patch pipette or bath applied to reach PCs under observation by extracellular perfusion. LTD was induced by conjunction (Cj) of double-shock PF stimulation and current pulse–induced membrane depolarization (200 ms, 0.8–1.2 nA), repeated at 1 Hz for 5 min (23). Cj induced LTD similar to that induced by PF-CF conjunction (Table S2). PFs were stimulated with double pulses (each 0.1 ms in duration) paired at 50-msec intervals timed in such a way that the first pulse fell 30 ms later than the onset of each depolarizing pulse. Separate application of double-shock PF stimulation or membrane depolarization induced no appreciable LTD (Fig. 2A). LTD developed during the initial 10 min and was followed by a slow phase continuing for another 40 min. As a convention in this study, “LTD magnitude” represents the average decrease in PF-EPSP slopes at 41–50 min relative to their average during the 5 min preconjunction period, and is indicated as mean ± SEM % (number of cells tested) in Table S2 (values given in the text are mean values only). LTD magnitudes in slices obtained from C3H WT and C57BL WT mice at PW 5–6 are 26.3 and 29.2%, respectively (Fig. 2A and Table S2), showing no significant difference (Student t test, P = 0.234). Hence, we conclude that sPLA2IIA, innately lacking in the genotype of the C57BL strain but present in that of the C3H strain (22), does not significantly contribute to LTD.
Fig. 2.
LTD and its blockade by cPLA2α KO or inhibitors. (A) Averaged time course of LTD. Ordinate, rising slope of PF-EPSPs relative to the average of five measurements during the 5-min preconjunction period. Error bars attached either above or below the plotted points, SEM. 2PF, double-shock PF stimulation; md, membrane depolarization; 2PF + md, conjunction. In brackets, number of PCs included in the plot. Obliquely shaded band indicates period of conjunction. (Inset) Specimen records of PF-EPSPs obtained from a C57BL WT PC before and after conjunction (x, y). (B) Comparison of LTD induction between KO and littermate mice. (C) Ten-minute perfusion of pyrrolidine-1 at various times relative to conjunction. Horizontal bars indicate perfusion time. (D) LTD magnitude vs. perfusion time of pyrrolidine-1. Error bars in D as well as F represent SD. Horizontal broken line was determined under 0.01% DMSO perfusion for 20 min overlapping conjunction (24.3%). One-way ANOVA and Dunnett posthoc test show that open squares, not filled ones, deviate from the control line significantly at P < 0.01 (two sided). Double-headed arrow indicates time window in which cPLA2α is involved in LTD. (E) Rescue of LTD by 5-min perfusion of 10 μM AA in cPLA2α KO PCs. Two sets of data are plotted for 5-min AA perfusions overlapping or immediately following conjunction. (F) Rescuing effect of AA in cPLA2α KO PCs. Horizontal broken line indicates LTD magnitude in cPLA2α KO PCs.
LTD Blockade by cPLA2α KO or Inhibitors.
In C3H KO and C57BL KO mice, the basic electrophysiological properties of PCs at postnatal weeks (PWs) 5–12 were comparable to those in littermate mice. Nevertheless, we regularly failed to induce LTD in either of these two strains of mutant mice (Fig. 2B). In contrast, in C3H and C57BL littermate mice, PCs showed substantial LTD (25.7% and 26.3%, respectively). The failure in LTD induction and lack of cPLA2α in the two mutants strongly indicate that cPLA2α is required for LTD induction.
Pyrrolidine-1, a cPLA2α-specific inhibitor (24, 25), effectively blocked LTD induction. Under infusion of 0.4 μM pyrrolidine-1 dissolved in 0.01% DMSO, the LTD magnitude decreased to 4.7% as compared with 27.8% attained under infusion of 0.01% DMSO alone (Fig. S1 A and B). Mapping with 5- or 10-min perfusions of pyrrolidine-1 revealed its time-limited effect on LTD (Fig. 2C). As summarized in Fig. 2D, LTD suppression is maximal when the perfusion of pyrrolidine-1 overlaps or immediately succeeds the conjunction, and diminishes when the inhibitor perfusion is more delayed. A modest effect of the inhibitor perfusion preceding conjunction could be due to a delay in washing out the inhibitor. Statistically significant decreases in the LTD magnitude (open squares in Fig. 2D) are associated with the inhibitor perfusions covering the 10-min period after the onset of conjunction (indicated by double-headed arrow). A similar 10-min window was also observed using 2 μM manoalide, a nonspecific PLA2 inhibitor (6) (Fig. S1C). These observations indicate that, to induce LTD, cPLA2α should be active during the 10-min period after the onset of conjunction.
Infusion of bromoenol lactone (BEL), an iPLA2-specific inhibitor, at 10 μM is known to substantially potentiate AMPA-mediated synaptic transmission in hippocampal CA1 pyramidal neurons (26), but a similar infusion of BEL has no appreciable effect on the PF-EPSPs or LTD in WT PCs (Fig. S1G). The LTD magnitude of 28.0% obtained during BEL infusion is identical to that obtained during the infusion of 0.01% DMSO used to dissolve BEL (27.8%). It is evident that cPLA2α, but neither sPLA2IIA nor iPLA2, is required for LTD induction.
Rescue of LTD by AA.
In C57BL KO PCs, 5-min perfusion of AA overlapping the conjunction rescued LTD (Fig. 2E). The LTD magnitude is restored to 22.4% by 5 μM AA and 23.5% by 10 μM AA (Fig. S1D). In C3H KO PCs also, the LTD magnitude is restored to 23.0% by 10 μM AA. The time course of the restored LTD in these cells (Fig. 2E) is approximately the same as that in littermate PCs (Fig. 2B). These observations support the view that cPLA2α contributes to LTD induction by providing AA as its product. Notably, AA restored LTD only when applied within the 5-min conjunction (Fig. 2F).
Unexpectedly, perfusion of 5 or 10 μM AA only weakly rescued the LTD blocked by pyrrolidine-1 (Fig. S1E); the LTD magnitude was 10.0% with 10 μM AA, only slightly larger than 4.7% without AA (t test, P = 0.004). However, 10 μM AA rescued more effectively the LTD blocked by the infusion of 2 μM manoalide (Fig. S1F) as reported previously (6); the LTD magnitude increased to 16.1%. The reason why AA only weakly restores the LTD blocked by pyrrolidine-1 is unclear. This inhibitor is competitive and does not appear to interfere with the PG production downstream of AA as examined in various types of cells (24, 25). Nevertheless, one may suspect that some interference with the LTD-inducing signal transduction in PCs occurs.
Whereas 5–10 μM exogenous AA rescues LTD blocked by cPLA2α KO or manoalide, exogenous AA does not enhance LTD in WT PCs. This is expected if endogenous AA causes LTD maximally. Exogenous AA even slightly decreased the LTD magnitude (Fig. S1H), which may be due to a product inhibition that an excess of produced unsaturated fatty acids including AA exerts back on PLA2 (27). It is also notable that bath application of AA alone, 5 μM for 20-min or 10 μM for 10-min (without Cj), induces no appreciable LTD-like depression (6.9%; Table S2).
LTD Blockade by COX-2 Inhibitors.
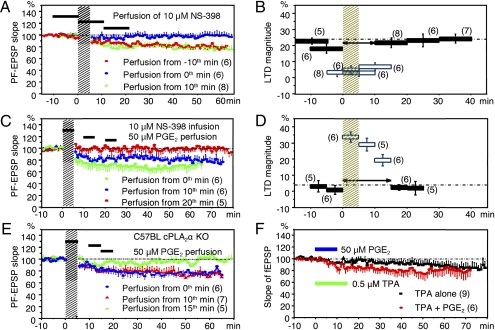
Of the eight different inhibitors of COXs and LOs applied by infusion, three COX-2 inhibitors, 0.5 μM DuP 697, 30 μM nimesulide, or 10 or 30 μM NS-398, consistently decreased LTD magnitudes to 2.7–4.0% (Table S2 and Fig. S5A). Indomethacin at 1.0 μM attenuated LTD to 6.6%, but at 0.5 μM, LTD remained at 21.3%. Indomethacin inhibiting COX-1 more sensitively than COX-2 might block LTD as a COX-2 inhibitor. SC-560 inhibiting COX-1, MK-886 inhibiting the 5-LO–activating protein, Baicalein inhibiting 12-LO, or (1-thienyl) ethyl 3,4-dihydroxy-benzylidene-cyanoacetate (TED) inhibiting 5-LO, 12-LO, or 15-LO did not significantly affect LTD. Bath-applied COX-2 inhibitors also blocked LTD, as shown for 10 μM NS-398 (Fig. 3A ). Mapping with 5- or 10-min perfusions of NS-398 (Fig. 3B) indicates that, to induce LTD, COX-2 should be active during the 10-min period following the onset of conjunction.
Fig. 3.
Blockade of LTD by COX-2 inhibitor and rescue by PGD2/E2. (A) LTD blockade by 10-min perfusions of COX-2 inhibitor (NS-398) at various times relative to conjunction. (B) Profile of time window mapped with 5- or 10-min perfusions of NS-398, illustrated similarly to Fig. 2D. (C) Rescue by 5-min perfusions of PGE2 of the LTD that was blocked by infusion of NS-398. (D) Mapping the receptiveness of LTD induction mechanisms to 5-min perfusion of PGE2, similar to Fig. 2F. (E) LTD rescued by 5-min perfusion of PGE2 in cPLA2α KO PC. (F) Synergy between perfused TPA and PGE2 in inducing LTD.
Because 30 μM NS-398 was reported to depress the induction of Ca2+ spikes in hippocampal neurons (1), we examined in three ways whether COX-2 inhibitors could have such an effect in PCs. First, we blocked fast Na+ spikes by tetrodotoxin (TTX) perfusion (28) and counted the number of slow Ca2+ spikes evoked by depolarizing rectangular currents (0.8–1.2 nA, 1-s duration) (Fig. S2 A–E). COX-2 inhibitors did not affect the average number so obtained. Second, complex spikes by stimulating CFs were evoked under perfusion of a COX-2 inhibitor (Fig. S2 F–I). No significant changes were detected in the rising slope of the first spike, which reflects Ca2+ influx (29). Third, we measured the Ca2+ surge by imaging techniques (Materials and Methods) and confirmed that none of the three COX-2 inhibitors affected the conjunction-evoked Ca2+ surge (Fig. S3).
Rescue of LTD by PGs.
COX-2 converts AA to PGG2 by enzymatic oxidation, then to PGH2 by peroxidase reaction. PGH2 is in turn converted to PGD2, PGE2, PGF2α, and PGI2 (10). In initial trials, we used PGs at 50 μM because PGE2 at this relatively high concentration produces maximal potentiation of LTP in neocortical neurons (3); but later we also tried lower concentration (discussed below). A crucial finding in this study is that perfusion or infusion of PGD2 or PGE2 effectively rescues LTD blocked by COX-2 inhibitors (Fig. 3C and Fig. S4 A and B). The rescued LTD magnitude (21.2%) is close to that attained under infusion of 0.2% ethanol used to dissolve PGs (Table S2 and Fig. S5B). The restoring effects of 50 μM PGD2 (to 21.6%) and PGE2 (to 25.9%) against NS-398 (4.5%) were comparable to each other, and the concurrent infusion of both PGD2 and PGE2 did not add to the effect of each (21.6%). We confirmed a similar rescuing effect of 50 μM PGD2/E2 infusions against another COX-2 inhibitor, 0.5 μM DuP 697 (Table S2 and Figs. S4A and S5). It is notable that 50 μM PGD2/E2 infused into WT PCs does not significantly affect LTD, the magnitude of which remained at 21–24% (Table S2). This is to be expected if endogenous PGD2/E2 sufficiently induces LTD.
LTD blocked by NS-398 infusion is also rescued by 5-min perfusion of 50 μM PGE2 during a time window lasting for 15 min (Fig. 3 C and D). We confirmed that the rescuing effect of PGE2 is apparent at 5 μM (28.5%) or even at 0.33 μM (22.5%) but not at 0.1 μM (5.1%) (Fig. S4B). NS-398–blocked LTD is also rescued by 0.33 μM PGD2 (22.8%). Furthermore, we found that 50 μM PGD2/E2, but not 50 μM PGF2α/I2, effectively rescued LTD (to 20.0–21.1%) blocked by the null absence of cPLA2α (Fig. 3E, Fig. S5B, and Table S2). The rescue occurred during a 15-min window (Fig. 3D and Fig. S4C). These results indicate that cPLA2α-AA-COX-2-PGD2/E2 mediates a major signal transduction pathway to LTD.
Nevertheless it should be noted that, even when the cascade was disrupted dually by a combination of the null absence of cPLA2α and COX-2 inhibition under 10 μM NS-398 infusion, exogenous AA at 10 μM still restored LTD to 26.2% (Fig. S4D). This observation suggests that, under certain conditions, AA is able to lead to LTD not only via PGD2/E2 but also via the direct activation of PKCα (Fig. 1).
Activation and Inhibition of PKC.
To trace the pathway downstream of PGD2/E2, the following observations are informative. An activator of PKC, 12-O-tetradecanoylphorbol 13-acetate (TPA), is known to chemically induce LTD (9). With 0.5 μM TPA perfusion for 15 min, this effect was marginal (8.8%) but was facilitated to a full extent (20.9%) by the concomitant perfusion of 50 μM PGE2 (Fig. 3F). This observation suggests that TPA and PGE2 jointly act on PKCα. Notably, however, PGD2/E2 is unable to rescue the LTD blocked by a specific PKC inhibitor; infusion of 100 nM Gö 6976 depressed LTD to 4.2%, which remained at −1.4–3.5% during PGD2/E2 perfusion (Fig. S4E and Table S1). These observations suggest that, to induce LTD, PGD2/E2 acts upstream, but not downstream, of or in parallel with PKCα (Fig. 1 and Discussion).
Testing of Prostanoid Receptors.
PGE2 activates four E-types of prostanoid receptor (EP1, EP2, EP3, and EP4) (30). In visual cortical neurons, EP2 and EP3 up-regulate and down-regulate the level of cAMP and thereby decrease and increase LTP, respectively (3). The cerebellum highly expresses EP1 mRNA and the EP1 protein (31), but only poorly expresses EP2, EP3, and EP4. PGD2 has also been reported to bind specifically to the rat brain synaptic membrane (32). In this study, specific antagonists for EP1 (ONO-8713, SC-19220, SC-51089, or SC-51322), EP3 (ONO-AE3-240), EP4 (ONO-AE3-208), or a nonspecific one for EP1, EP2, and D-type prostanoid receptors (AH-6809) were infused into PCs, but none of them blocked LTD (Fig. S4 F, G, and H, Table S2, and Discussion).
Effect of cPLA2α Knockout and COX-2 Inhibition on OKR Adaptation.
OKR gain was measured in awake mice using a sinusoidally oscillating patterned screen (Materials and Methods and SI Text). After measuring the basal OKR gain (Go), the screen oscillation continued for 1 h to induce an adaptive increase in the gain (ΔG). This procedure does not change the OKR phase, which will not be discussed. We estimated the “adaptation rate” (ΔG/Go) as a measure of the short-term OKR adaptation. As examined in C57BL cPLA2α KO and littermate mice, both at PW12, the general behavior was seemingly normal, and no significant difference was detected in Go between KO (0.37 ±0.01 SEM, n = 17) and littermates (0.36 ± 0.01, n = 16; Student t test, P = 0.711). However, a significantly low adaptation rate, 7.5 ± 2.7% (n = 17), was discovered in KO mice as compared with 15.9 ± 2.9% (n = 16) in littermates (P = 0.045).
Six C57BL/6Cr WT mice at PW11-13 were also subjected to 1 h OKR adaptation. Each mouse was tested at intervals of 4–15 days with three different i.p.injections, that is, nimesulide at 1 or 3 mg/kg body weight, or the vehicle (1% DMSO in PBS). Nimesulide at 1 mg/kg body weight is known to minimally interfere with PGE2 production in the brain, and at 3 mg/kg body weight, maximally for 2 h (33). Nimesulide injection at 3 mg/kg body weight did not affect general behavior, but the 1-h screen rotation that started 45 min after injection revealed substantially low adaptation rates, 8.9% ± 1.4%, as compared with 17.5% ± 0.6% similarly obtained after the administration of the vehicle (repeated-measures ANOVA and Dunnett posthoc test, P = 0.0341). A similar injection of 1 mg/kg body weight nimesulide did not significantly decrease the adaptation rate (14.2% ± 3.3%, P = 0.4748). We found that, during the 45-min resting time after 3 mg/kg body weight nimesulide injection, the OKR gain increased slightly but significantly from 0.39 ± 0.01–0.44 ± 0.03 (P = 0.023). However, because a similar increase from 0.38 ± 0.01–0.41 ± 0.01 (P = 0.032) was also induced by injection of the vehicle alone, it might not be a specific effect of COX-2 inhibition. Nimesulide thus depresses OKR adaptation dose-dependently without affecting basal OKR dynamics.
Discussion
cPLA2α-COX-2 Cascade.
In this study, we highlight the crucial involvement of the cPLA2α–COX-2 cascade in LTD induction. A conjunction-evoked strong surge of intracellular Ca2+ ions would activate cPLA2α that is Ca2+ sensitive. A recent GFP/Oregon Green 488-imaging experiment on tissue-cultured mouse PCs (34) demonstrated that a puff application of 30 μM glutamate induced a marked increase in intracellular Ca2+ concentration via P-type voltage-gated Ca2+ channels, which in turn caused a rapid redistribution of GFP-tagged cPLA2α from the cytosol to the somatic and dendritic Golgi compartments. In Chinese hamster ovary cells, Ca2+-stimulated cPLA2 translocates to the nuclear envelope and endoplasmic reticulum but not to the plasma membrane (35). Translocation to any membranous structure can be considered to enable cPLA2α to readily access glycerophospholipid substrates. cPLA2α would also be activated via GTP-binding-protein-coupled receptors (36) and via phosphorylation of the serine residue by extracellular signal-regulated protein kinase (ERK)1/2) (18, 19, 37).
The colocalization of COX-2 with cytosolic PLA2 on the plasma membrane and within punctate intracellular regions of monkey PCs (38) conforms to their functional association in the cascade. A functional coupling between cPLA2α and COX-2 is also indicated by the significant down-regulation of COX-2 expression and PGE2 formation in cPLA2α KO mouse brain (39). The COX-2 gene is inducible by numerous factors (10). The basal expression of COX-2 in the brain is rapidly enhanced after a seizure or a cold swim imposing acute stress on a rat (40). In the cerebral neocortex, COX-2 is expressed postsynaptically in excitatory neurons (41), and in the hippocampus, COX-2 inhibitors impair cellular functions including LTP (1). In visual cortical neurons, exogenous PGE2 facilitates LTP (3). In PCs, however, the functional involvement of COX-2 is more specific; its inhibitors minimally affect the basal properties of the membrane, Ca2+ spike excitability or complex spikes (Figs. S1 and S2) except for the LTD blockade. Moreover, exogenous PGD2/E2 does not affect normal LTD (Fig. S5B).
Signal Transduction Pathway for LTD.
The following five lines of previous (8, 13, 20) and present observations support the loop model (Fig. 1, blue arrows). (i) Inhibitors for PKC, MEK, ERK1/2, or PLA2 block conjunction-induced LTD. (ii) Inhibitors for PKC, ERK1/2, or PLA2 block LTD induced by a photorelease of Ca2+ ions. (iii) Chemical LTD induction by TPA is depressed by a MEK inhibitor. (iv) Inhibitors for PKC or PLA2 block LTD induced by a photorelease of MEK-CA that directly activates ERK1/2. (v) In the absence of endogenous AA due to cPLA2α knockout exogenous AA rescues LTD (Fig. 2E). Therefore, we retain the loop connections in our model.
However, the cPLA2α-COX-2 cascade must be incorporated in the model (red arrows in Fig. 1) based on another five lines of present observations, as follows. (i) In null deficiency of cPLA2α, LTD still occurs as long as PGD2/E2 is supplemented (Fig. 3E). (ii) When a COX-2 inhibitor blocks LTD, this is rescued by PGD2/E2 (Fig. 3 C and D). (iii) Under a combination of COX-2 inhibition and null deficiency of cPLA2α, AA still rescues LTD (Fig. S4D). (iv) PGD2/E2 facilitates activation of PKC by TPA to induce chemical LTD (Fig. 3F). (v) PGD2/E2, however, is unable to rescue LTD blocked by the inhibition of PKC (Fig. S4E). Hence, as indicated by P1 in Fig. 1, it is likely that PGD2/E2 acts upstream of PKC jointly with AA. Activation of PKC by PGE2 is known in a certain cell type (42). However, the possibility that PGD2/E2 acts on AMPARs in parallel with PKC (P2 in Fig. 1) cannot be excluded presently when a prostanoid receptor(s) acting downstream of PGD2/E2 remains unidentified. The prostanoid receptor(s) that might be involved in LTD induction could be unique because it should be equally receptive to PGD2 and PGE2 and also should be resistant to currently used EP1-4 or DP antagonists. A third possibility to consider is that PGD2/E2 affects LTD by activating Ca2+ channels or Ca2+ release from intracellular stores (P3 in Fig. 1) as reported to occur in certain types of cell (43, 44). If this occurs in PCs, COX-2 inhibitors may then depress the conjunction-evoked Ca2+ surge and thereby lead to LTD blockade. This possibility, however, is excluded under the present experimental conditions (Results and Figs. S1 and S2).
The time windows lasting for 5–15 min are observed at all steps of the cascade (Figs. 2 D and F and 3 B and D and Figs. S3C and S4C). However, these may not necessarily be determined by mechanisms inherent to each step; they may merely reflect a bottleneck set downstream of the cascade. In fact, a PKC inhibitor effectively blocks LTD only when applied within 20 min after the onset of conjunction (8). Any event initiated upstream of PKCα will not lead to LTD induction unless it successfully passes this 20-min bottleneck. Yet, the length of the time window may vary reflecting specific conditions at each step such as different accessibilities of applied reagents to their targets in PCs or speeds of their reactions. The process that finally sets the 20-min bottleneck at PKCα or downstream is yet to be determined, but most likely is the dissociation of a stable AMPAR population from the cytoskeleton via phosphorylation. This process could be sensitive to a PKC inhibitor while it proceeds during the 20 min, but will become insensitive after the dissociation is completed. The freed AMPARs will then be eliminated from the synaptic membrane by endocytosis. Kinetics of the endocytosis and associated exocytosis could determine the time course of the development of LTD (45).
Motor Learning.
Whether LTD actually underlies motor learning is a matter of discussion. In a number of knockout mutants tested so far, the loss of LTD detected in slice conditions parallels a disturbance in motor learning (21), or, in a certain case, enhanced LTD parallels an enhanced motor learning (46). However, in a fragile-X mental retardation mouse model, enhanced LTD accompanies impaired motor learning (47). Moreover, a drug (T588) that blocked LTD in slice or in vivo in anesthetized rats failed to affect motor learning when administered orally (48). The present observation that a COX-2 inhibitor blocked both LTD induction and OKR adaptation supports the hypothesis that LTD underlies motor learning.
Materials and Methods.
Animals.
C3H/HeN and C57BL/6J WT mice (all male) were purchased from Japan SLC Co. and C57BL/6Cr mice from Japan Crea Co. cPLA2αKO mice having C57BL and C3H backgrounds were raised in T. Shimizu’s laboratory at the University of Tokyo (22). Both male and female were used in KO and littermate mice, but there was no appreciable difference in LTD between them. All of the mice were maintained in an environment-controlled, 12-h light, 12-h dark room, and received water and food ad libitum. Experiments on KO mice were conducted with the researchers blinded to the genotype. We followed the Guidelines for the Care and Use of Laboratory Animals of RIKEN. The Wako Animal Experiment Committee of RIKEN approved our experimental plan.
In Vitro Cerebellar Slices.
A cerebellum was ectomized under ether anesthesia. Sagittal slices (0.3-mm thick) were obtained from the vermis using a slice maker (Microslicer DTK-1000, Dosaka, Japan) in tetrodotoxin (1 μM)–containing saline and kept at room temperature (23–25°C) for at least 1 h in normal saline. The recording chamber was perfused at a rate of 2 mL/min. In lobule VIII under visual control, a whole-cell clamp pipette (3–4 MΩ) was attached to the soma of a PC. The pipette contained (in mM) 134 K-gluconate, 6 KCl, 4 NaCl, 10 Hepes, 0.2 EGTA, 4 MgATP, 0.3 Tris-GTP, and 14 phosphocreatine (pH 7.25). Two types of perfusates were used. Solution A is standard Krebs-Henseleit Ringer containing (in mM) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 25.0 NaHCO3, 1.18 KH2PO4, 1.19 MgSO4, and 11.0 glucose. Solution B is ACSF containing (in mM) 125 NaCl, 2.5 KCl, 2 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, 1.0 MgSO4, and 10.0 glucose; the pH was adjusted to pH 7.3. These perfusates were added with 100 μM picrotoxin, equilibrated with 95% O2 and 5% CO2 gas, and warmed to maintain the temperature of the perfusion chamber at 30–31°C. Solution A was used in most of the present experiments, but we confirmed that LTD occurred similarly in A and B solutions despite certain quantitative differences noted in membrane resistance or PF- or CF-EPSPs (Table S2).
Pharmaceutical Agents.
Table S1 lists the presently used drugs. ONO-8713, ONO-AE3-240, and ONO-AE3-208 were provided through the courtesy of ONO Pharmaceutical Co., Ltd. AA was dispersed in the perfusates by vigorous vortex mixing and ultrasonication. The used concentrations of these drugs were determined by referring to published reports, IC50 values, and the results of our provisional tests (Fig. S2B and Table S1).
OKR measurement.
To induce OKR, a checked-pattern screen was sinusoidally oscillated by 15° peak-to-peak at 0.17 Hz around an awake mouse with the head fixed in the prone position. Eye movements were recorded using an infrared television camera (SI Text). For i.p.injection, nimesulide was dissolved in DMSO, which was then diluted by PBS to make up 100 μL solution containing DMSO at 1%. Immediately before injection, the solution was freshly prepared, warmed to 60 °C, and then gradually cooled to 39°C.
Ca2+ Imaging.
In a slice, after the formation of whole-cell configuration, Oregon Green 488 BAPTA-1 was infused into a PC for more than 20 min. The Ca2+ surge was measured using a confocal laser scanning microscope (FLUOVIEW FV1000, Olympus) before, during, and after 5-min conjunction (Fig. S3). Perfusion of a COX-2 inhibitor started 5 min before the onset of conjunction (additional details in SI Text).
Supplementary Material
Acknowledgments
S.N. and M.I. were supported by grants from the RIKEN Brain Science Institute. T.S. was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915020107/DCSupplemental.
References
- 1.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu T. Lipid mediators in health and diseases. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 3.Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J Neurosci. 2006;26:10209–10221. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami M, Kudo I. Phospholipase A2 . J Biochem. 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 5.Shirai Y, Ito M. Specific differential expression of phospholipase A2 subtypes in rat cerebellum. J Neurocytol. 2004;33:297–307. doi: 10.1023/B:NEUR.0000044191.83858.f7. [DOI] [PubMed] [Google Scholar]
- 6.Linden DJ. Phospholipase A2 controls the induction of short-term versus long-term depression in the cerebellar Purkinje neuron in culture. Neuron. 1995;15:1393–1401. doi: 10.1016/0896-6273(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds T, Hartell NA. Roles for nitric oxide and arachidonic acid in the induction of heterosynaptic cerebellar LTD. Neuroreport. 2001;12:133–136. doi: 10.1097/00001756-200101220-00034. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 10.O’Banion MK. Cyclooxygenase-2: Molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 11.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 12.Wang SSH, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K, et al. Ca2+ requirements for cerebellar long-term synaptic depression: Role for a postsynaptic leaky integrator. Neuron. 2007;54:787–800. doi: 10.1016/j.neuron.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCalpha confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–594. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 17.Shinomura T, Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: Its possible implications. Proc Natl Acad Sci USA. 1991;88:5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo S, Launey T. ERKs regulate PKC-dependent synaptic depression and declustering of glutamate receptors in cerebellar Purkinje cells. Neuropharmacology. 2003;45:863–872. doi: 10.1016/s0028-3908(03)00210-7. [DOI] [PubMed] [Google Scholar]
- 19.Ito-Ishida A, Kakegawa W, Yuzaki M. ERK1/2 but not p38 MAP kinase is essential for the long-term depression in mouse cerebellar slices. Eur J Neurosci. 2006;24:1617–1622. doi: 10.1111/j.1460-9568.2006.05055.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda S, Schweighofer N, Kawato M. Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. J Neurosci. 2001;21:5693–5702. doi: 10.1523/JNEUROSCI.21-15-05693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo S, et al. Dual involvement of G-substrate in motor learning revealed by gene deletion. Proc Natl Acad Sci USA. 2009;106:3525–3530. doi: 10.1073/pnas.0813341106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong DA, Kita Y, Uozumi N, Shimizu T. Discrete role for cytosolic phospholipase A(2)α in platelets: Studies using single and double mutant mice of cytosolic and group IIA secretory phospholipase A(2) J Exp Med. 2002;196:349–357. doi: 10.1084/jem.20011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22:763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 24.Seno K, et al. Pyrrolidine inhibitors of human cytosolic phospholipase A(2) J Med Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- 25.Ghomashchi F, et al. A pyrrolidine-based specific inhibitor of cytosolic phospholipase A2α blocks arachidonic acid release in a variety of mammalian cells. Biochim Biophys Acta Biomembranes. 2001;1513:160–166. doi: 10.1016/s0005-2736(01)00349-2. [DOI] [PubMed] [Google Scholar]
- 26.St-Gelais F, Ménard C, Congar P, Trudeau LE, Massicotte G. Postsynaptic injection of calcium-independent phospholipase A2 inhibitors selectively increases AMPA receptor-mediated synaptic transmission. Hippocampus. 2004;14:319–325. doi: 10.1002/hipo.10176. [DOI] [PubMed] [Google Scholar]
- 27.Bonventre JV. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992;3:128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- 28.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokni D, Yarom Y. State-dependence of climbing fiber-driven calcium transients in Purkinje cells. Neuroscience. 2009;162:694–701. doi: 10.1016/j.neuroscience.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: Properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 31.Candelario-Jalil E, et al. Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: Accumulation in Purkinje cells of the cerebellum. J Mol Neurosci. 2005;27:303–310. doi: 10.1385/JMN:27:3:303. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T, Yamashita A, Hayaishi O. Specific binding of prostaglandin D2 in rat brain synaptic membrane. Occurrence, properties, and distribution. J Biol Chem. 1982;22:13570–13575. [PubMed] [Google Scholar]
- 33.Steiner AA, Li S, Llanos-Q J, Blatteis CM. Differential inhibition by nimesulide of the early and late phases of intravenous- and intracerebroventricular-LPS-induced fever in guinea pigs. Neuroimmunomodulation. 2001;9:263–275. doi: 10.1159/000054289. [DOI] [PubMed] [Google Scholar]
- 34.Mashimo M, Hirabayashi T, Murayama T, Shimizu T. Cytosolic PLA2(α) activation in Purkinje neurons and its role in AMPA-receptor trafficking. J Cell Sci. 2008;121:3015–3024. doi: 10.1242/jcs.032987. [DOI] [PubMed] [Google Scholar]
- 35.Schievella AR, Regier MK, Smith WL, Lin L-L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30740–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 36.Axelrod J, Burch RM, Jelsema CL. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: Arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988;11:117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- 37.Lin L-L, et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 38.Pardue S, Rapoport SI, Bosetti F. Co-localization of cytosolic phospholipase A2 and cyclooxygenase-2 in rhesus monkey cerebellum. Brain Res Mol Brain Res. 2003;116:106–114. doi: 10.1016/s0169-328x(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 39.Bosetti F, Weerasinghe GR. The expression of brain cyclooxygenase-2 is down-regulated in the cytosolic phospholipase A2 knockout mouse. J Neurochem. 2003;87:1471–1477. doi: 10.1046/j.1471-4159.2003.02118.x. [DOI] [PubMed] [Google Scholar]
- 40.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hüll M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: Possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J Neurochem. 2001;79:950–958. doi: 10.1046/j.1471-4159.2001.00652.x. [DOI] [PubMed] [Google Scholar]
- 43.Mochizuki-Oda N, Mori K, Negishi M, Ito S. Prostaglandin E2 activates Ca2+ channels in bovine adrenal chromaffin cells. J Neurochem. 1991;56:541–547. doi: 10.1111/j.1471-4159.1991.tb08183.x. [DOI] [PubMed] [Google Scholar]
- 44.Asbóth G, Phaneuf S, Europe-Finner GN, Tóth M, Bernal AL. Prostaglandin E2 activates phospholipase C and elevates intracellular calcium in cultured myometrial cells: Involvement of EP1 and EP3 receptor subtypes. Endocrinology. 1996;137:2572–2579. doi: 10.1210/endo.137.6.8641211. [DOI] [PubMed] [Google Scholar]
- 45.Earnshaw BA, Bressloff PC. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J Neurosci. 2006;26:12362–12373. doi: 10.1523/JNEUROSCI.3601-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi T, et al. Enhancement of both long-term depression induction and optokinetic response adaptation in mice lacking delphilin. PLoS One. 2008;3:e2797. doi: 10.1371/journal.pone.0002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koekkoek SKE, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Welsh JP, et al. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc Natl Acad Sci USA. 2005;102:17166–17171. doi: 10.1073/pnas.0508191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.