Abstract
Cholera is an acute intestinal infection caused by the bacterium Vibrio cholerae. In order for V. cholerae to cause disease, it must produce two virulence factors, the toxin-coregulated pilus (TCP) and cholera toxin (CT), whose expression is controlled by a transcriptional cascade culminating with the expression of the AraC-family regulator, ToxT. We have solved the 1.9 Å resolution crystal structure of ToxT, which reveals folds in the N- and C-terminal domains that share a number of features in common with AraC, MarA, and Rob as well as the unexpected presence of a buried 16-carbon fatty acid, cis-palmitoleate. The finding that cis-palmitoleic acid reduces TCP and CT expression in V. cholerae and prevents ToxT from binding to DNA in vitro provides a direct link between the host environment of V. cholerae and regulation of virulence gene expression.
Keywords: AraC, crystal structure, pathogenesis, oleic acid, palmitoleic acid
Vibrio cholerae is a Gram-negative bacterium and the causative agent of the acute intestinal infection known as cholera. Upon entry into the host intestine, V. cholerae induces a transcriptional cascade resulting in the expression of the master virulence regulator, ToxT. ToxT directly activates the expression of the two primary virulence factors of V. cholerae, the toxin-coregulated pilus (TCP) and cholera toxin (CT) (1–3) and also autoregulates its own expression from the tcp promoter (4, 5). ToxT is a member of the AraC-family of transcriptional regulators which are defined by a 100 amino acid region of sequence similarity that forms an independently folding DNA-binding domain (DBD) containing two helix-turn-helix (HTH) motifs (6). Members fall into three functional groups depending on the types of genes that they regulate. Those members that regulate carbon metabolism, such as AraC of E. coli, are active as dimers and respond to small effector molecules that bind to the N-terminal domain of the protein. Those members that are involved in the stress response, such as SoxS, Rob, and MarA typically function as monomers. The third group is involved in regulating virulence gene expression and includes Rns of enterotoxigenic E. coli (7), BfpT (PerA) of enteropathogenic E. coli (8), and ExsA of Pseudomonas aeruginosa (9), amongst numerous others. These regulators may respond to physical cues such as temperature and pH and it is not yet known if they function primarily as monomers or dimers. To date, of the large number of known AraC-family proteins (PROSITE, PS01124 (6)), only two full-length structures, MarA (10) and Rob (11), have been solved.
ToxT activates the transcription of many promoters, including those that control the tcp and ctx operons, via binding to degenerate thirteen base-pair sequences called toxboxes. These sequences are organized either singly, or in direct or inverted repeat configurations (12) such that ToxT can function at promoters to positively regulate gene transcription as either a monomer or a dimer depending on the structure of the promoter (13, 14). The activity of ToxT is known to be sensitive to bile, a mixture of many molecules including saturated fatty acids (SFAs), unsaturated fatty acids (UFAs), salts, and cholesterol that is secreted into the intestine from the gall bladder (15, 16). In the presence of bile, and more specifically bile components oleic, linoleic acid, or arachidonic acid, transcription of ctxA and tcpA by V. cholerae is drastically reduced, leading to the suggestion that ToxT function is inhibited by UFAs found in bile (16).
In this study, the full-length structure of ToxT is determined and shown to be similar in overall structure with the regulator AraC. Unexpectedly, the structure revealed the regulatory domain of ToxT was bound to a ligand, the sixteen-carbon fatty acid cis-palmitoleate. The addition of cis-palmitoleate to V. cholerae culture media reduced ctx and tcp expression by 6–8 fold similar to what was previously observed with oleic acid (16) and both UFAs prevented the sequence specific DNA-binding of ToxT in vitro. These results suggest a model in which the presence of UFAs reduce virulence gene expression by directly binding to ToxT and altering the conformation of the protein such that it is less competent to bind to DNA and less likely to form stable dimers.
Results and Discussion
Structure of Full-Length ToxT and Comparison with Other AraC-family Members.
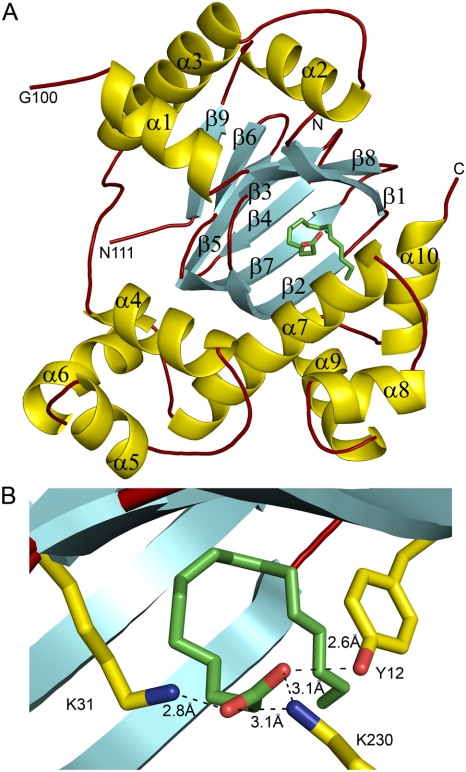
We have solved the 1.9 Å resolution crystal structure of ToxT (Fig. 1A, Table S1, PDB ID 3GBG). The crystal contained one monomer of ToxT per asymmetric unit, with each monomer containing two domains. The N-terminal domain (amino acids 1–160) is comprised of three α-helices (helix α1–α3) and a nine stranded β-sandwich (strand β1–β9) forming a “jelly roll” or “cupin-like” fold (17) containing a binding pocket enclosed by residues Y12, Y20, F22, L25, I27, K31, F33, L61, F69, L71, V81, and V83 from the N-terminal domain and residues I226, K230, M259, V261, Y266, and M269 from the C-terminal domain (Fig. S1). The volume of this predominantly hydrophobic pocket is 780.9 Å3 as calculated by the program CASTp (18). The pocket contains a sixteen-carbon fatty acid bound such that its negatively charged carboxylate head group forms salt bridges with both K31 from the N-terminal domain and K230 from the C-terminal domain (Fig. 1B; see discussion below). Following a short linker (amino acids 161–169), the C-terminal domain (170–276) is made up of two HTH DNA-binding motifs (the more N-terminal HTH1 and the more C-terminal HTH2) linked by a relatively long α-helix, helix α7. The interface between the two domains has an area of ∼2000 Å2 and is very polar, with few hydrophobic interactions (Fig. S2).
Fig. 1.
The structure of ToxT. (A) Ribbon diagram of ToxT showing α-helices (gold), β-strands (cyan), and loops (dark red). The bound cis-palmitoleate is shown in stick form with carbons in green and oxygens in red. The N and C termini are indicated. Helices and strands are numbered according to their topological connectivity in the full-length protein. Note that residues 101–110 are disordered in the structure, as indicated by the loop ends on the left side of the molecule. (B) Close up of the cis-palmitoleate binding region showing interactions of the carboxylate head group with side chains.
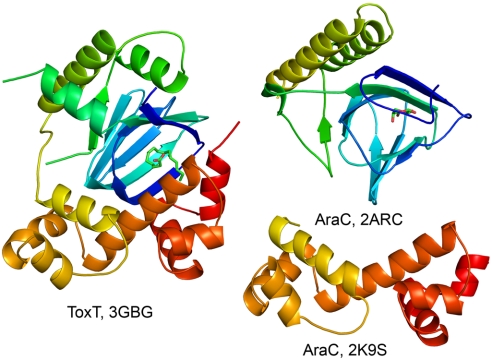
Structures of other AraC-family members are limited to three members: AraC, in which the N- (19) and C-terminal (20) domain structures have been determined separately (Fig. 2), MarA, which contains only a DNA-binding domain (10), and Rob, which, in contrast to ToxT and AraC, contains an N-terminal DNA-binding domain and a C-terminal regulatory domain (11). Both the MarA and Rob structures have been cocrystallized with DNA. The C-terminal domain of Rob, like the N-terminal domains of ToxT and AraC, is composed of several helices and β-sheets forming a binding pocket. While the structure of Rob contains no ligand, the N-terminal domain of AraC (PDB ID 2ARC) has been determined with arabinose bound in the β-sandwich in a position similar to the fatty acid in ToxT (Fig. 2).
Fig. 2.
Ribbon diagrams of ToxT and the N- and C-terminal domains of AraC. The models are colored by a rainbow effect with blue at the N terminus and red at the C terminus. The arabinose bound in the N-terminal domain of AraC is shown in stick form. PDB accession numbers are indicated for the three structures.
A comparison of full-length ToxT with existing high resolution structures using Distance ALIgnment (DALI) (21) and Secondary Structure Matching (SSM) (22) gave no significantly similar hits over the entire 276 amino acids. However, when the two domains are taken separately, the N-terminal domain of ToxT most closely resembles the N-terminal domain of AraC (for 126 α-carbons, the RMSD is 3.63 Å; PDB ID 1XJA (23)), while the C-terminal domain is most similar to the DBD of AraC (RMSD 2.12 Å for 92 α-carbons; PDB ID 2K9S (20)). ToxT and AraC have a very similar N-terminal topology (Fig. S3A) and other than the N-terminal arm of AraC (residues 7–17), all of the other secondary structural elements of these two proteins can be aligned (Fig. S3B).
N-Terminal Domain.
The fold of the N-terminal domain of ToxT is similar to AraC in that it contains eight antiparallel β-sheets (Fig. 1A) followed by helix α1 (19). However, ToxT is missing the N-terminal arm that is present in AraC that interacts with arabinose. Helix α1 and sheet β9 are linked by a disordered region between residues 101 and 110. Childers et al. have shown that alanine substitutions of four of these residues (M103, R105, N106, and L107) show either greatly enhanced ctxAp-lacZ expression or ≤ 10% expression of the ctxAp-lacZ and acfA-phoA fusions (24) demonstrating this region is important for virulence gene expression. Helix α3 of ToxT is analogous to the helix that allows for coiled-coil N-terminal dimerization in the AraC structure (19). Although ToxT is clearly a monomer in this structure and appears to bind to independent toxboxes as a monomer (13), certain promoters such as tcp, ctx, and tagA require ToxT dimerization on adjacent toxboxes for full activation (13, 14). In AraC, the coiled-coil is anchored at the ends by a triad of leucine residues providing stability (19). Although analogous leucine residues are not present in α3, if ToxT were to dimerize in a manner similar to that observed in AraC, complementary salt bridges would be formed between helix α3 residues such as D141, E142, K157, and K158 of one monomer and the same residues on the other monomer. In fact, Hsaio et al. have suggested that a D141G substitution is able to repress msh promoters as a monomer, but is unable to activate tcp (25).
A number of residues in the N-terminal domain have been shown to be important for ToxT mediated activation of virulence gene expression (24). As suggested by Childers et al., those involved in maintaining an N-terminal hydrophobic core (M32, W34, I35, L42, L60, L71, W117, L127, F147–148, and F151–152) are essential for protein folding and stability (24). Two surface exposed glutamates, E52 in β5, and E129 in α2, as well as S140, which lies in the loop between α2 and α3, have also been shown important for function (24) for reasons not illuminated by the structure.
Hung et al. have identified a small molecule inhibitor of ToxT, virstatin (26), that interferes with its ability to dimerize and activate transcription of the tcp and ctx promoters (14). They also demonstrated that a L114P substitution is virstatin resistant and suggested that it may favor a conformation that allows the protein to dimerize more efficiently (14). It is interesting to note that L114 lies in the vicinity of the unresolved residues (residues 101 and 110) (Fig. 1A) and substitution to a proline may result in a conformational change affecting the adjacent unresolved loop or N-terminal ligand binding pocket.
DNA-Binding Domain.
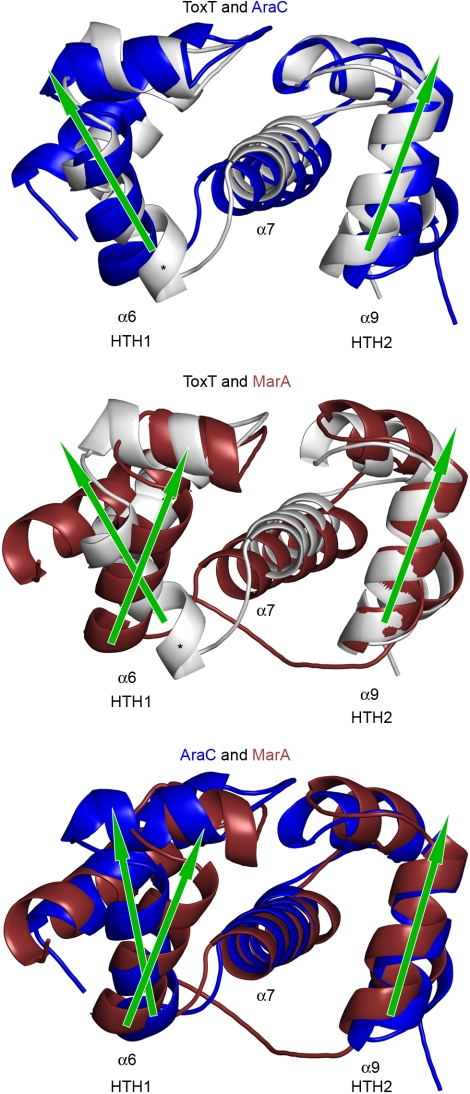
The DBD of ToxT is composed of seven α-helices. HTH1 is comprised of α5 and α6, HTH2 is comprised of α8 and α9, and they are connected by a central helix α7 (Fig. 1A). Helix α4 and helix α10 are involved in scaffolding and stability of HTH1 and HTH2 respectively. Pairwise SSM alignments performed by WinCoot (27, 28) of the DBD’s of ToxT (amino acids 170–273), AraC, and MarA, show consistently close alignments of HTH2, with greater variability in the orientation of HTH1 (Fig. 3). The DNA-bound structure of MarA demonstrates that it is possible for AraC-family members to utilize helices α6 and α9, oriented in a parallel manner, to bind consecutive major grooves on curved target DNA (10). This parallel arrangement is conserved in Rob; however the structure does not show both HTH motifs bound to major grooves (11). As suggested by Rodgers and Schleif (20), helix α6 of AraC, which is at a divergent angle with respect to helix α9, would likely have to undergo a conformational change in order to allow for consecutive major groove binding on target DNA. In ToxT, helix α6 is not only nonparallel with helix α9, but is also more distorted and bent when compared to what is observed in AraC (Fig. 3). Another difference in this domain is in the orientation of helix α7. In AraC and MarA, the orientation of helix α7 is virtually the same, whereas in ToxT helix α7 is orientated differently with respect to the other structures. As discussed below, the position of helix α7 is such that it could link the N-terminal binding pocket to conformational changes occurring in the DNA-binding domain.
Fig. 3.
Pairwise SSM alignments of the DBDs of ToxT (3GBG), AraC (2K9S), and MarA (1BL0). ToxT is represented in silver, AraC in blue, and MarA in dark red. Helix α7 and the DNA-binding helices α6 and α9 are labeled. Arrows indicate the relative direction each DNA-binding helix is pointing. The asterisk indicates the distorted section of helix α6 on ToxT.
Residues identified by Childers et al. in the C-terminal domain that are important for ToxT function include several in the cores of HTH1 and HTH2 (I174, V178, W186, W188, L206, V211, I217, F245, F251, and F255) (24) that are critical for proper folding and stability. There are also a number of surface exposed residues that could be involved in stabilizing the DBD (S175, R184, R221, S227, E233, K237, G244, and N260) (24). Finally, it appears that residues such as K203 (α6), R214 (α7), T253 (α9), and S257 (α9) are positioned to be directly involved in protein/DNA interactions.
A Fatty Acid is Present in ToxT and Influences its DNA-binding Activity.
UFAs such as arachidonic, linoleic, and oleic acid have previously been shown to strongly inhibit the expression of ToxT-activated genes, whereas SFAs such as palmitic and stearic acid do not (16). The structure of ToxT contains an almost completely buried and solvent inaccessible sixteen-carbon fatty acid bound to the pocket in the N-terminal domain (Fig. 1A and B, Fig. 2, Fig. S1A and B). The negative charge on the carboxylate head group hydrogen bonds with Y12 and forms salt bridges with K31 from the N-terminal domain and K230 from the C-terminal domain (Fig. 1B, Fig. S1D). NMR studies of chloroform/methanol extractions from pure ToxT samples indicate the presence of a long-chain, singly unsaturated fatty acid in a cis configuration (Fig. S4). Although the electron density ends after carbon sixteen of the hydrophobic chain, suggesting cis-palmitoleate, the ToxT structure could accommodate the two additional carbons of oleate (Fig. S1C). An Fo-Fc difference map calculated after refinement with oleate placed into the pocket shows strong negative density after carbon sixteen, further indicating that the bound molecule is cis-palmitoleate.
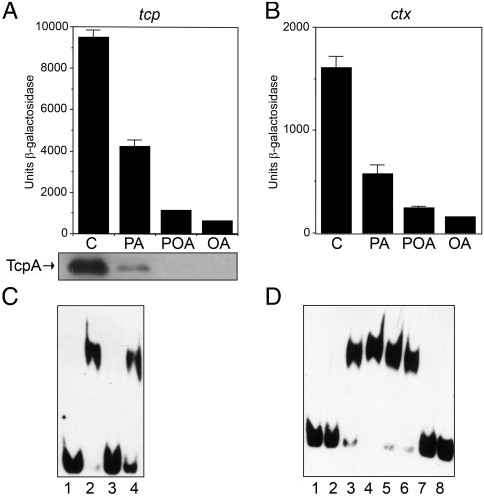
To address whether cis-palmitoleate was capable of influencing the activity of ToxT, different UFAs and SFAs were added to cultures of V. cholerae strains carrying transcriptional fusions to the tcp and ctx operons. We found that the expression of these operons were reduced between 6–8 fold with cis-palmitoleic acid and between 10–15 fold with oleic acid, whereas only a twofold reduction was observed with palmitic acid (Fig. 4A and B). As previous studies have shown that toxT transcription is unaffected by UFAs, it has been suggested that UFAs act on ToxT directly (16).
Fig. 4.
Effects of fatty acids on tcp and ctx expression. (A) and (B) β-galactosidase activity of tcp-lacZ and ctx-lacZ fusion constructs respectively. Cells were grown in LB pH 6.5 at 30 °C for 18 hours +/- the indicated fatty acids at 0.02% dissolved in methanol. The inset shows TcpA expression by Western blot under the same conditions in the corresponding lanes (C—control with methanol; PA—sodium palmitate; POA—palmitoleic acid; OA—oleic acid). (C) EMSA showing a specific interaction between ToxT and a 40-base-pair segment of the tcp promoter. All lanes contain 0.0025 μM labeled probe. Lane 1, free DNA; lane 2, 0.2 μM ToxT; lane 3, 0.2 μM ToxT with a 70-fold molar excess of cold competing DNA; lane 4, 0.2 μM ToxT with a 70-fold molar excess of a 42-base-pair nonspecific DNA cold competitor. (D) EMSA showing the effect of several fatty acids on ToxT/DNA interactions. All lanes contain 0.0025 μM labeled probe. Lane 1, free DNA; lane 2, 0.125 μM ToxT; lane 3, 0.2 μM ToxT; lane 4, 0.25 μM ToxT; lane 5, 0.25 μM ToxT with methanol; lane 6, 0.25 μM ToxT with 0.002% palmitic acid; lane 7, 0.25 μM ToxT with 0.002% palmitoleic acid; lane 8, 0.25 μM ToxT with 0.002% oleic acid.
EMSAs were performed, and a 100-fold molar excess of protein was shown to bind to a 40 base-pair probe containing two toxboxes from the tcp promoter in vitro (Fig. 4C, lane 2). This interaction is specific since it was completely inhibited by a 70-fold molar excess of specific competitor DNA (lane 3) but not by a 70-fold molar excess of nonspecific competitor DNA (lane 4). Addition of methanol or 0.002% palmitic acid to the reaction had no effect on ToxT binding (Fig. 4D, lanes 5 and 6). However, addition of 0.002% palmitoleic or oleic acid completely prevented ToxT from binding to DNA (Fig. 4D, lanes 7 and 8), consistent with the reduction of tcp and ctx transcription observed in the presence of these fatty acids (Fig. 4A and B). A control EMSA experiment with a different protein/DNA pair was also performed to show that unsaturated fatty acids do not block all protein/DNA interactions (Fig. S5).
As no fatty acids were added to any buffer or crystallization condition, the cis-palmitoleate most likely originated from the E. coli used as the protein expression strain. Indeed, cis-palmitoleic acid comprises 10.5% of the total fatty acid content in E. coli membranes, whereas oleic acid is absent (29). As it is expected that there would be very little free fatty acid in the cytoplasm of these bacteria, it is likely that ToxT bound cis-palmitoleate released from the membrane upon cell lysis. Other groups have seen similar phenomena such as the binding of cis-vaccenic acid by the pheromone-binding protein of Bombyx mori when purified from an E. coli expression system (29). Previous studies indicate that 23.5% of the fatty acid content of bile is oleic acid, and if cis-palmitoleic acid is present in bile, it is at a concentration of less than 0.5%. As both oleic and cis-palmitoleic acids are monounsaturated at the ninth carbon and as there is room in the ToxT structure to potentially accommodate the longer oleic acid, it is not surprising that both fatty acids can serve as a ligand for ToxT. However, given the abundance of oleic acid in bile when compared to cis-palmitoleic acid, it seems that oleic acid would be the natural ligand responsible for altering ToxT function in vivo.
A Structural Model for ToxT Activation.
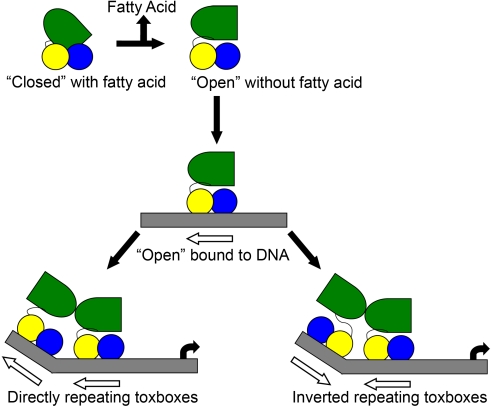
The finding that UFAs reduce the expression of tcp and ctx expression in V. cholerae and that they significantly reduce the ability of ToxT to bind to DNA in vitro suggests a model for the regulation of ToxT function via fatty acid binding (Fig. 5). In this model, when the bacteria are in the lumen of the intestine in the presence of fatty acids, the position of the carboxylate head group of the fatty acid bridging K31 from the N-terminal domain with K230 from the C-terminal domain (Fig. 1B) keeps ToxT in a “closed” conformation that is not capable of binding DNA (5). Restraint of K230, which is located at the C-terminal end of helix α7, would cause helix α7 to assume a position that pulls and distorts helix α6 into an orientation that is unfavorable for DNA-binding (Fig. 3). Once the bacteria have penetrated the mucus of the intestine where the concentrations of fatty acids are presumably reduced (15), charge-charge repulsion between K31 and K230 destabilizes the closed conformation, leading to an opening of the N- and C-terminal domains. In this “open” conformation, K230, helix α7, and helix α6 would no longer be restrained, and reorient into a conformation that is competent for DNA-binding. The EMSA data support this model, in which an equilibrium exists between fatty acid bound “closed” ToxT that cannot bind to DNA and fatty acid free “open” ToxT that can bind to DNA. While a 50-fold molar excess of ToxT over the probe is not sufficient to drive the binding equilibrium in the direction of the DNA-bound state (Fig. 4D, lane 2), increasing the concentration of ToxT (Fig. 4D, lanes 3 and 4) shifts the equilibrium in the direction of a protein/DNA complex. Addition of 0.002% palmitoleic or oleic acid then disrupts the protein/DNA complex by shifting the equilibrium back to the “closed” state, containing a protein/fatty acid complex, releasing it from DNA (Fig. 4D, lanes 7 and 8). As discussed above, a number of studies have suggested that ToxT dimerizes upon binding to adjacent toxboxes (12, 14, 26, 30). It is clear from the structure that side-by-side dimerization of “closed” ToxT on adjacent toxboxes would be difficult if not impossible due to steric constraints. However, we predict the “open” form of ToxT would be able to dimerize on adjacent toxboxes in either the direct or inverted orientations (Fig. 5).
Fig. 5.
Model for the regulation of ToxT by monounsaturated fatty acids. Fatty acid bound ToxT is in a “closed” conformation, which cannot bind to DNA. Release of the fatty acid results in an “open” conformation that can bind to DNA. In the “open” conformation the N-terminal domain is free to move in relation to the C-terminal domain, and is able to dimerize with another ToxT at an adjacent toxbox. The linker is sufficiently flexible to allow dimerization on DNA in either direct or inverted orientations.
These studies describe the high resolution structure of ToxT and provide evidence that UFAs decrease the ability of ToxT to interact with DNA. The presence of a UFA in ToxT provides a direct link between the host environment of V. cholerae and the regulation of virulence gene expression. Further insights into the mechanisms by which UFAs influence ToxT will contribute to our understanding of this critical regulatory step in the disease process. In addition, this structure provides a foundation for studying the mechanisms by which small molecules such as virstatin (14, 26) are able to inhibit ToxT function as well as the therapeutic potential of UFAs in the treatment of cholera.
Materials and Methods
ToxT Expression.
ToxT was purified using the IMPACT-CN fusion protein system (New England Biolabs). Full-length ToxT was cloned from Vibrio cholerae O395 and ligated into pTXB1 (New England Biolabs) to produce a toxT-intein/CBD (chitin binding domain) fusion construct. ToxT was expressed by autoinduction (31) in ZYM-5052 media using BL21-CodonPlus® (DE3)-RIL (Stratagene) E. coli. All Luria-Burtani broth (LB) agar plates and media contained 100 μg mL-1 carbenicillin and 25 μg mL-1 chloramphenicol. Selenomethionine ToxT was produced by growing the same E. coli strain in a minimal medium phosphate amino acids selenomethionine (PASM-5052) containing a mixture of 10 μg mL-1 methionine, 125 μg mL-1 selenomethionine, and 100 nanomolar (nM) vitamin B12.
Purification of ToxT.
Cells were harvested by centrifugation, resuspended in column buffer (20 mM Tris pH 8.0, 1 mM EDTA, and 500 mM NaCl), lysed via French press, and clarified by centrifugation. Chitin beads (New England Biolabs) were equilibrated with cold column buffer, mixed with the clarified supernatant, and incubated at 4 °C with gentle rocking. The chitin bead slurry was then loaded onto a gravity flow column, washed with 10 column volumes of column buffer, and equilibrated with five column volumes of cleavage buffer (20 mM Tris pH 8.0, 1 mM EDTA, and 150 mM NaCl). The intein with the CBD was cleaved from ToxT using cleavage buffer with 100 mM dithiothreitol (DTT) and left at 4 °C for 20 h. Eluant from the chitin column was then loaded onto a HiTrap sepharose packed (SP) fast flow (FF) cationic exchange column (GE) to separate the ToxT-intein/CBD fusion protein that coeluted with the native ToxT using a sodium chloride gradient. Pure fractions were pooled and concentrated to 1.75 mg mL-1 for crystallization.
Crystallization of ToxT.
ToxT was crystallized in hanging drops where 50% of the drop was ToxT in buffer from the cationic exchange column, 30% of the drop was 0.1 M Hepes pH 7.5 with 10% (w/v) PEG 8,000 (the mother liquor), and 20% of the drop was 36–40% 2-methyl-2,4-pentandiol (MPD) as an additive. ToxT crystals were transferred to a solution containing the mother liquor and 20% ethylene glycol as a cryoprotectant.
X-ray Data Collection.
A multiple anomalous dispersion (MAD) dataset from selenomethionine ToxT was collected on X6A in the National Synchotron Light Source at the Brookhaven National Laboratory, Long Island NY. A high resolution native data was collected on GM/CA-CAT in the Advanced Light Source at Argonne National Laboratory, Argonne IL. Data were indexed with X-ray Detector Software (XDS) (32), solved by Solve/Resolve (33, 34), refined with the Crystallography and NMR System (CNS) (35, 36), and the model was built using WinCoot (27, 28). A Ramachandran plot generated with Procheck (37, 38) shows 99.6% of residues in the most favored or additionally allowed regions and no residues in the disallowed regions.
Fatty Acid Extractions.
Fatty acids were extracted from samples of aqueous ToxT by the method of Bligh and Dyer (39). Samples were resuspended in methanol-d4 and used for NMR spectra. Positive controls of sodium palmitate (Sigma, P9767) and cis-palmitoleic acid (Fluka, 76169) were also dissolved in methanol-d4.
NMR Experiments.
All NMR experiments were acquired on a Bruker spectrometer operating at 600 MHz, utilizing a TCI cryoprobe. All data were collected at 25 °C. Spectral assignment utilized chemical shift comparison with values reported in the literature for fatty acids (40) and reference spectra obtained for samples of sodium palmitate and palmitoleic acid in the same experimental conditions. The assignment was confirmed by two dimensional homonuclear NMR experiments Total Correlation Spectroscopy (TOCSY) (41), mixing times of 60 and 120 ms, and Nuclear Overhauser Enhancement Spectroscopy (NOESY) (42), mixing time of 200 ms), and heteronuclear 1H-13C HMQC (43) and HMBC (44) experiments.
Electrophoretic Mobility Shift Assays.
Single-stranded, forty base-pair complimentary oligos (Operon) from the tcp promoter (5′GTGTTATTAAAAAAATAAAAAAACACAGCAAAAAATGACA) were end labeled with a biotin-conjugated dUTP using the Biotin 3′ End Labeling Kit (Pierce) following the manufacturer’s instructions and then annealed to form double-stranded fragments. EMSA’s were carried out using the LightShift Chemiluminescent EMSA Kit (Pierce) following the manufacturer’s instructions. Briefly, 2.5 picomole (pmole), 4 pmole, 5 pmole of ToxT were mixed with 50 femtomole (fmole) of double-stranded labeled DNA in a binding buffer (10 mM Tris pH 7.5, 1 mM EDTA, 100 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.3 mg mL-1 BSA, 150 μg herring sperm DNA, and 10% glycerol). Fatty acids were dissolved in methanol and added to a final concentration of 0.002% using the same volume of methanol as a control. To show specificity, a 70-fold molar excess of unlabeled double-stranded tcp fragment and a 70-fold molar excess of unlabeled nonspecific DNA (42 base pairs) were added as controls. Reactions were then incubated for 30 min at 30 °C and loaded on a 1x Tris Borate EDTA (TBE ) 6% polyacrylamide gel at 4 °C then transferred onto a positively charged membrane (Hybond XL, GE Healthcare) and detected by chemiluminescence. An EMSA experiment was conducted using the control reagents from the LightShift Chemiluminescent EMSA Kit following the manufacturer’s instructions while adding methanol and 0.02% fatty acids as indicated in Fig. S5.
β-galactosidase Assays.
β-galactosidase activity was determined by the method of Miller (45). The tcp-lacZ and ctx-lacZ strains MBN135 (46) and KSK218 (47) were grown for 18 h in LB media pH 6.5 at 30 °C. Either methanol or the indicated fatty acids were added to 0.02%.
Immunoblot Analysis.
Cell extracts from 18 h cultures grown as for the β-galactosidase assays were prepared and analyzed on 16% SDS-polyacrylamide slab gels. Proteins were visualized by transferring to nitrocellulose and probing with antiTcpA antibody (48) using the enhanced chemiluminescence (ECL) detection system (Amersham).
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants AI060031 and AI072661 (to F.J.K.), Grant AI039654 (to R.K.T.) and Grant AI41558 (to K.S.). M.G.C. was supported by the NSF (National Science Foundation) Research Experience for Undergraduates (REU) site DBI-0647279 and the Department of Defense Awards to Stimulate and Support Undergraduate Research Experience (ASSURE) Program. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. The GM/CA-CAT beamline has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Science (Y1-GM-1104). The use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. DE-AC02-06CH11357.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915021107/DCSupplemental.
References
- 1.Champion GA, Neely MN, Brennan MA, DiRita VJ. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 2.DiRita VJ, Parsot C, Jander G, Mekalanos JJ. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins DE, Nazareno E, DiRita VJ. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol. 1992;174:6974–6980. doi: 10.1128/jb.174.21.6974-6980.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RC, Taylor RK. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. doi: 10.1111/j.1365-2958.1995.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu RR, DiRita VJ. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J Bacteriol. 1999;181:2584–2592. doi: 10.1128/jb.181.8.2584-2592.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. Arac/XylS family of transcriptional regulators. Microbiol Mol Biol R. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munson GP, Scott JR. Binding site recognition by Rns, a virulence regulator in the AraC family. J Bacteriol. 1999;181:2110–2117. doi: 10.1128/jb.181.7.2110-2117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobe T, Schoolnik GK, Sohel I, Bustamante VH, Puente JL. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:963–975. doi: 10.1046/j.1365-2958.1996.531415.x. [DOI] [PubMed] [Google Scholar]
- 9.Hovey AK, Frank DW. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1995;177:4427–4436. doi: 10.1128/jb.177.15.4427-4436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee S, Martin RG, Rosner JL, Davies DR. A novel DNA-binding motif in MarA: The first structure for an AraC family transcriptional activator. Proc Natl Acad Sci USA. 1998;95:10413–10418. doi: 10.1073/pnas.95.18.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon HJ, Bennik MH, Demple B, Ellenberger T. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat Struct Biol. 2000;7:424–430. doi: 10.1038/75213. [DOI] [PubMed] [Google Scholar]
- 12.Withey JH, DiRita VJ. The toxbox: Specific DNA sequence requirements for activation of Vibrio cholerae virulence genes by ToxT. Mol Microbiol. 2006;59:1779–1789. doi: 10.1111/j.1365-2958.2006.05053.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellair M, Withey JH. Flexibility of Vibrio cholerae ToxT in transcription activation of genes having altered promoter spacing. J Bacteriol. 2008;190:7925–7931. doi: 10.1128/JB.00512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci USA. 2007;104:2372–2377. doi: 10.1073/pnas.0611643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuhmacher DA, Klose KE. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol. 1999;181:1508–1514. doi: 10.1128/jb.181.5.1508-1514.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee A, Dutta PK, Chowdhury R. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun. 2007;75:1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunwell JM, Khuri S, Gane PJ. Microbial relatives of the seed storage proteins of higher plants: Conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiol Mol Biol R. 2000;64:153–179. doi: 10.1128/mmbr.64.1.153-179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dundas J, et al. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34:W116–118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soisson SM, MacDougall-Shackleton B, Schleif R, Wolberger C. Structural basis for ligand-regulated oligomerization of AraC. Science. 1997;276:421–425. doi: 10.1126/science.276.5311.421. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers ME, Schleif R. Solution structure of the DNA binding domain of AraC protein. Proteins. 2009;77:202–208. doi: 10.1002/prot.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm L, Kaariainen S, Rosenstrom P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D . 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 23.Weldon JE, Rodgers ME, Larkin C, Schleif RF. Structure and properties of a truely apo form of AraC dimerization domain. Proteins. 2007;66:646–654. doi: 10.1002/prot.21267. [DOI] [PubMed] [Google Scholar]
- 24.Childers BM, et al. Identification of residues critical for the function of the Vibrio cholerae virulence regulator ToxT by scanning alanine mutagenesis. J Mol Biol. 2007;367:1413–1430. doi: 10.1016/j.jmb.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 25.Hsiao A, Xu X, Kan B, Kulkarni RV, Zhu J. Direct regulation by the Vibrio cholerae regulator ToxT to modulate colonization and anticolonization pilus expression. Infect Immun. 2009;77:1383–1388. doi: 10.1128/IAI.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 27.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 28.Lohkamp B, Emsley P, Cowtan K. Coot News. CCP4 Newsletter. 2005;42:3–5. [Google Scholar]
- 29.Oldham NJ, Krieger J, Breer H, Svatos A. Detection and removal of an artefact fatty acid from the binding site of recombinant Bombyx mori pheromone-binding protein. Chem Senses. 2001;26:529–531. doi: 10.1093/chemse/26.5.529. [DOI] [PubMed] [Google Scholar]
- 30.Prouty MG, Osorio CR, Klose KE. Characterization of functional domains of the Vibrio cholerae virulence regulator ToxT. Mol Microbiol. 2005;58:1143–1156. doi: 10.1111/j.1365-2958.2005.04897.x. [DOI] [PubMed] [Google Scholar]
- 31.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expres Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Kabsch W. Evaluation of single-crystal x-ray diffraction data from a position-sensitive detector. Journal of Applied Crystallography. 1988;21(6):916–924. [Google Scholar]
- 33.Terwilliger TC. Maximum-likelihood density modification. Acta Crystallogr D. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallogr D. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 36.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 37.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. Journal of Applied Crystallogr. 1993:283–291. [Google Scholar]
- 38.Morris AL, MacArthur MW, Hutchinson EG, Thornton JM. Stereochemical quality of protein structure coordinates. Proteins. 1992;12:345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- 39.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 40.Gunstone FD, Harwood JL, Padley FB. The Lipid handbook. 2nd Edition. New York: Chapman and Hall; 1994. pp. 516–517. [Google Scholar]
- 41.Bax A, Davis DG. MLEV-17 based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson. 1985;65:355–360. [Google Scholar]
- 42.Macura S, Huang Y, Suter D, Ernst RR. Two-dimensional chemical exchange and cross-relaxation spectroscopy of coupled nuclear spins. J Magn Reson. 1981;43:259–281. [Google Scholar]
- 43.Muller L. Sensitivity enhanced detection of weak nuclei using heteronuclear multiple quantum coherence. J Am Chem Soc. 1979;101:4481–4484. [Google Scholar]
- 44.Bax A, Summers MF. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J Am Chem Soc. 1986;108:2093–2094. [Google Scholar]
- 45.Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 46.Nye MB, Pfau JD, Skorupski K, Taylor RK. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J Bacteriol. 2000;182:4295–4303. doi: 10.1128/jb.182.15.4295-4303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skorupski K, Taylor RK. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci USA. 1997;94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun DX, Seyer JM, Kovari I, Sumrada RA, Taylor RK. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect Immun. 1991;59:114–118. doi: 10.1128/iai.59.1.114-118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.