Abstract
Under fasting conditions, increases in circulating concentrations of pancreatic glucagon maintain glucose homeostasis through induction of gluconeogenic genes by the CREB coactivator CRTC2. Hepatic CRTC2 activity is elevated in obesity, although the extent to which this cofactor contributes to attendant increases in insulin resistance is unclear. Here we show that mice with a knockout of the CRTC2 gene have decreased circulating glucose concentrations during fasting, due to attenuation of the gluconeogenic program. CRTC2 was found to stimulate hepatic gene expression in part through an N-terminal CREB binding domain that enhanced CREB occupancy over relevant promoters in response to glucagon. Deletion of sequences encoding the CREB binding domain in CRTC2 −/− mice lowered circulating blood glucose concentrations and improved insulin sensitivity in the context of diet-induced obesity. Our results suggest that small molecules that attenuate the CREB–CRTC2 pathway may provide therapeutic benefit to individuals with type 2 diabetes.
Keywords: gluconeogenesis, obesity, CREB, CRTC2, insulin
During fasting, increases in hepatic gluconeogenesis ensure energy balance for glucose-dependent tissues such as brain and the red blood cell compartment. Hepatic glucose production is elevated in type 2 diabetes, reflecting decreases in insulin signaling that otherwise inhibit the gluconeogenic program (1–3).
Increases in circulating glucagon are thought to trigger gluconeogenic gene expression in part through the cAMP-dependent phosphorylation of the transcription factor CREB and through the dephosphorylation of its cognate coactivator CRTC2 (4–6). In parallel, decreases in circulating insulin concentrations during fasting also stimulate gluconeogenic genes by the dephosphorylation of the forkhead activator FOXO1 (7).
Localized in the cytoplasm under basal conditions through a phosphorylation-dependent association with 14-3-3 proteins, CRTC2 shuttles to the nucleus following its dephosphorylation at Ser171, where it mediates induction of cellular genes by binding to the bZIP domain of CREB over relevant promoters (8, 9). The importance of CRTC2 for gluconeogenic gene expression is supported by RNAi-mediated knockdown studies, where acute depletion of CRTC2 lowered hepatic glucose production in fasted mice and by overexpression studies in which phosphorylation-defective, active CRTC2 increases circulating glucose levels under both fasting and feeding conditions (4, 6, 10).
To determine the importance of the CRTC2–CREB association for induction of the gluconeogenic program, we characterized mice lacking the conserved N-terminal CREB binding domain of CRTC2. We found that gluconeogenic gene expression and hepatic glucose production were reduced in CRTC2-deficient mice during fasting and in the setting of insulin resistance. Our results support an important role for CRTC2 in mediating effects of fasting signals on hepatic gluconeogenesis.
Results
Exposure to cAMP agonist promotes CRTC2 dephosphorylation and nuclear entry (Fig. 1A). Following its translocation, CRTC2 is recruited to CREB binding sites on the PEPCK promoter as measured by chromatin immunoprecipitation (ChIP) assay (Fig. 1B). Although CREB binding is readily detected over this promoter under basal conditions, exposure to FSK further enhances CREB occupancy.
Fig. 1.
CRTC2 increases CREB promoter occupancy in response to fasting signals. (A) Immunoblot (Upper) and immunocytochemical (Lower) analysis showing CRTC2 dephosphorylation and nuclear translocation in primary hepatocytes exposed to forskolin (FSK). Scale bar, 5 um. (B) Chromatin immunoprecipitation (ChIP) assay of CREB (Upper) and CRTC2 (Lower) occupancy over the human PEPCK promoter in HEK293T cells exposed to FSK (black bars) or vehicle (white bars) (n = 3, P < 0.05; SEM). Location of CREB binding sites indicated. (C) Gel mobility shift assay of CREB and GST-CRTC2 proteins using a 32P-labeled double-stranded CRE oligonucleotide. Protein–DNA complexes and free probe indicated. (D) (Upper Left) Schematic diagram showing organiziation of wild-type or mutant CRTC2 vectors lacking either the N-terminal CREB binding domain (aa 1–50) or central regulatory region (aa 51–539) indicated. (Upper Right) Immunoblot with anti-flag epitope antiserum showing relative expression of each CRTC2 polypeptide in transfected cells. (Lower) Transient assay of HEK293T cells transfected with cAMP responsive EVX-luc reporter vector and exposed to FSK as indicated (n = 3, P < 0.05; SEM).
CRTCs associate with CREB through a conserved, 50–amino acid N-terminal CREB binding domain (CBD), which specifically recognizes the bZIP region of CREB (11). The importance of the CREB bZIP domain for DNA binding led us to test whether its association with the CBD increases CREB binding to relevant promoters. In gel mobility shift assays, a GST (GST)–CRTC2 (aa 1–120) polypeptide containing the CBD exhibited no DNA binding activity, but it enhanced binding of CREB to a double-stranded CRE oligonucleotide probe 4-fold (Fig. 1C and Fig. S1). In keeping with these effects, overexpression of wild-type CRTC2 enhanced the activity of a cAMP responsive (EVX-luciferase [luc]) reporter in HEK293T cells exposed to FSK, but a mutant CRTC2 lacking the CBD did not (Fig. 1D). By contrast, a CRTC2 mutant lacking only the central regulatory region, but containing the CBD and C-terminal transactivation domains, exhibited near wild-type activity.
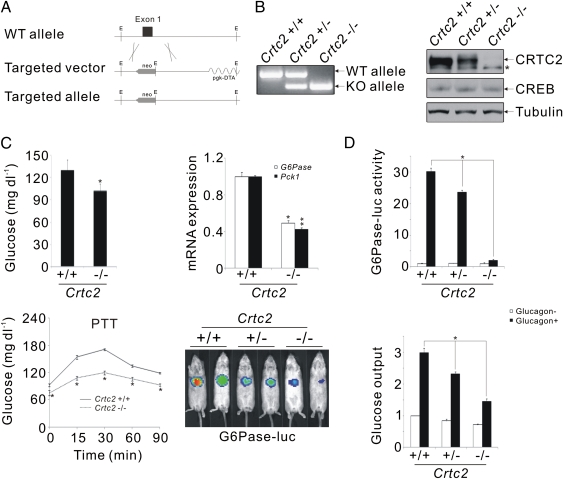
We deleted sequences encoding the N-terminal CREB binding domain (CBD), having shown that this domain increases CREB occupancy over relevant binding sites in the PEPCK promoter (Fig. 2A). Full-length CRTC2 protein was detected in tissues from wild-type but not CRTC2 KO mice (Fig. 2B). Truncated CRTC2 polypeptides were also absent from CRTC2−/− hepatic extracts by immunoblot assay with a C-terminally directed CRTC2 antiserum (aa 454–607; Fig. S2). CRTC2−/− animals were born at the expected Mendelian frequency; they were indistinguishable from control littermates at birth (Fig. S2).
Fig. 2.
Fasting hypoglycemia in CRTC2−/− mice. (A) Schematic diagram of wild-type and mutant CRTC2 alleles following homologous recombination with targeting vector lacking exon 1 sequences. (B) (Left) PCR analysis showing CRTC2 fragments generated from wild-type, heterozygous, or homozygous CRTC2 mutant mice. (Right) Immunoblot of CRTC2 protein amounts in hepatic extracts from from wild-type and CRTC2 mutant mice. *Nonspecific band. CRTC2 antiserum was developed against aa 454–607 of mouse CRTC2. (C) (Top) Circulating glucose concentrations (Left) and hepatic mRNA amounts for gluconeogenic genes (Right; G6Pase, PEPCK) in wild-type and CRTC2−/− mice (n = 10, P < 0.05; SEM). (Lower) pyruvate tolerance testing (n = 5, P < 0.05; SEM) (Left) and G6Pase-luc reporter activity (Right) in fasted wild-type and CRTC2 mutant mice. (D) G6Pase reporter activity (Top) and glucose output (Bottom) from primary hepatocytes (wild-type, CRTC2+/−, CRTC2−/−) under basal conditions and following exposure to glucagon (n = 3, P < 0.05; SEM).
Previous studies showing that RNAi-mediated knockdown of CRTC2 in liver promotes fasting hypoglycemia (4, 6) prompted us to examine effects of CRTC2 gene disruption on glucose homeostasis. Relative to wild-type controls, CRTC2−/− mice maintained lower fasting blood glucose concentrations on a normal chow diet (Fig. 2C, Upper Left). Gluconeogenic capacity, measured by pyruvate tolerance testing (PTT), was also reduced in CRTC2 mutant males (Fig. 2C, Lower Left). Correspondingly, hepatic mRNA amounts for G6Pase and PEPCK1 were down-regulated in fasted CRTC2−/− mice compared with controls (Fig. 2C, Upper Right). Consistent with a transcriptional effect, hepatic glucose-6-phosphatase (G6Pase)–luc reporter activity was also reduced in fasted CRTC2−/− mice compared with control littermates (Fig. 2C, Lower Right). The effects of CRTC2 on hepatic glucose output in CRTC2−/− mice appear to be cell autonomous because glucose output and G6Pase reporter activity were also down-regulated in primary cultures of CRTC2−/− hepatocytes compared with wild-type cells exposed to glucagon (Fig. 2D).
Chronic increases in hepatic glucose production are thought to contribute to the development of type 2 diabetes because they stimulate compensatory increases in insulin secretion that ultimately lead to islet cell failure (3). In line with their reduced hepatic glucose production, CRTC2 mutant mice had lower ad libitum circulating concentrations of insulin; circulating triglycerides and cholesterol were also down-regulated (Fig. 3A and Fig. S3). Correspondingly, whole body insulin sensitivity was increased in CRTC2−/− mice by glucose and insulin tolerance testing (Fig. 3B). Although they experienced similar weight gain, CRTC2−/− mice remained relatively insulin sensitive under high-fat diet (HFD) conditions, when insulin resistance is typically increased (Fig. 3 C and D and Fig. S3). Taken together, these results suggest that CRTC2 contributes to the development of insulin resistance in part through its effects on hepatic gluconeogenesis.
Fig. 3.
CRTC2−/− mice have enhanced insulin sensitivity under high-fat diet conditions. (A) Circulating triglyceride, cholesterol, and insulin concentrations in wild-type and CRTC2 mutant mice on a normal chow (NC) diet (n = 15, P < 0.05; SEM). (B) Whole-body insulin sensitivity of NC-fed wild-type and CRTC2 mutant mice by IP glucose and insulin tolerance testing (GTT, ITT) (n = 5, P < 0.05; SEM). (C) Effect of high-fat diet (HFD) feeding on weight gain, circulating insulin concentrations, and whole-body insulin sensitivity (GTT, ITT) of wild-type and CRTC2 mutant mice (n = 5, P < 0.05; SEM). (D) Quantitative PCR analysis of gluconeogenic gene expression in wild-type and CRTC2 mutant mice under NC or HFD conditions (n = 5, P < 0.05; SEM).
We examined whether CRTC2 re-expression is sufficient to rescue gluconeogenic gene expression in CRTC2−/− hepatocytes. Exposure to glucagon increased G6Pase and PEPCK mRNA amounts in wild-type but not CRTC2−/− hepatocytes; adenoviral delivery of CRTC2 restored PEPCK and G6Pase mRNA induction by glucagon in CRTC2−/− cells (Fig. 4A). In keeping with these effects, exposure to glucagon also increased CREB occupancy over G6Pase and PEPCK promoters in wild-type cells but not in CRTC2−/− hepatocytes (Fig. 4C). In addition, adenoviral CRTC2 expression also rescued effects of glucagon on CREB occupancy over G6Pase and PEPCK promoters in CRTC2−/− cells (Fig. 4 B and C), confirming the ability for CRTC2 to enhance CREB binding to target promoters.
Fig. 4.
CRTC2 increases CREB occupancy over gluconeogenic genes. (A) Q-PCR analysis of gluconeogenic gene expression in wild-type or CRTC2−/− primary hepatocytes under basal conditions and following exposure to glucagon (n = 3, P < 0.05; SEM). Effect of adenoviral CRTC2 or green fluorescent protein (GFP) expression shown. (B and C) ChIP assays of CRTC2 (B) and CREB (C) occupancy over G6Pase and PEPCK promoters in wild-type or CRTC2−/− hepatocytes (n = 3, P < 0.05; SEM). Exposure to glucagon indicated. Effect of adenoviral CRTC2 expression relative to control (Ad-GFP) shown. (D) Model for activation of the gluconeogenic program by CREB and CRTC2 during fasting. Increases in circulating glucagon trigger CRTC2 dephosphorylation and nuclear entry. Binding of nuclear CRTC2 to CREB increases CREB binding to relevant promoters.
Discussion
During fasting, increases in circulating pancreatic glucagon promote glucose homeostasis by stimulating the gluconeogenic program in liver. Hepatic glucose output is up-regulated in insulin resistance, when it contributes to chronic increases in circulating glucose levels and ultimately to islet cell failure and type 2 diabetes. Our results suggest that CRTC2 contributes significantly to the development of insulin resistance through its effects on hepatic CREB activity in this setting.
Although hepatic glucose production was reduced in our CRTC2−/− mice, Kaestner et al. observed minimal effects on circulating glucose concentrations in their CRTC2−/− animals under normal chow conditions (12). In addition to possible differences in mouse strains, we note that the mutant CRTC2 gene in their study, lacking exons 4–11, is capable of generating an in-frame polypeptide containing the N-terminal CREB binding domain fused to the C-terminal transactivation domain. Although the expression levels of this CRTC2 product are unknown, we found that a similar truncated CRTC2 polypeptide lacking the central regulatory domain exhibits near wild-type activity on a CRE-luc reporter (Fig. 1D), potentially explaining why hepatic glucose production is relatively unaffected in that study.
To avoid residual regulatory contributions from a mutant CRTC2 allele, we deleted sequences encoding the N-terminal CREB binding domain (CBD), which we show here increases CREB occupancy over relevant binding sites in the PEPCK promoter. Although CREB has been shown to modulate cellular gene expression in response to a variety of stimuli, the extent to which these increases reflect changes in promoter occupancy has been unclear (13). In a recent study, calcium and cAMP agonists were found to enhance CREB occupancy independently of their effects on CREB phosphorylation (14). Our results suggest that such increases in CREB binding are likely transmitted through recruitment of CRTC2 to relevant sites. Because it interacts directly with residues in the bZIP domain, the CREB binding domain of CRTC2 may increase CREB occupancy by stabilizing its secondary structure. Future studies should reveal whether CRTC2 mediates these effects on specific target gene subsets, depending on the nucleotide sequence or location of CREB binding sites on cAMP responsive promoters.
Materials and Methods
Mice.
Mice were housed in a temperature-controlled environment under 12-h light/dark cycle conditions with free access to water and standard chow diet (Lab diet 5001). For high-fat diet feeding experiments, standard chow was replaced with chow containing 60% calories from fat. To obtain CRTC2−/− mice, the CRTC2 targeting vector was constructed by replacing sequences in exon 1 of the CRTC2 gene with a phosphoglycerol kinase (pgk) neomycin cassette for positive selection and a pgk-diphtheria toxin-A cassette for negative selection. The CRTC2 targeting vector was linearized and electroporated into R1 embryonic stem cells, and G418-resistant clones were screened for homologous recombination by Southern blot analysis. Targeted clones were injected into 129-derived blastocysts to generate chimeric mice. Heterozygous mice were backcrossed with C57BL/6J for 2 generations and then intercrossed (het × het) to obtain homozygous CRTC2−/− mice confirmed by PCR-based genotyping. Experiments were performed with CRTC2 −/− mice and their CRTC2 +/+ littermates. Genotyping of the wild-type CRTC2 allele was performed using primer pair: 5′-gctgggtatattgggacagg-3′ and 5′-gccagaggccacttgtgtag-3′, which generates a 391-bp product. Primers for detection of the disrupted CRTC2 allele were 5′-ctgggaagcaagaaaccaag-3′ and 5′-gccagaggccacttgtgtag-3′, which gave rise to a 222-bp product.
Live Imaging.
Mice were imaged as described (15). Mice were injected intraperitoneally with glucagon (100 mg·kg−1; Sigma) or vehicle. Before imaging, mice were injected intraperitoneally with 50 mg·kg−1 Nembutal (Abbott Laboratories) and 100 mg·kg−1 firefly D-luciferin (Biosynth AG). Mice were imaged on the IVIS 100 Imaging System (Boston Scientific) and analyzed with Living Image software (Xenogen) 1 h after glucagon injection.
In Vivo Analysis.
Mouse tissues were sonicated and centrifuged, and supernatants were reserved for β-gal activity, protein determinations, and SDS–PAGE analysis. Blood glucose values were determined using a LifeScan automatic glucometer. Glucose tolerance tests (GTT) were performed by glucose i.p.administration (1 g·kg−1) after overnight fasting. Insulin tolerance tests were performed by i.p. injection of human regular insulin (1 U·kg−1) after 5 h fasting. For pyruvate challenge experiments, mice were fasted overnight and injected i.p. with pyruvate (2 g·kg−1). Serum triglycerides and ketones were evaluated by cardio-check analyzer. Serum cholesterol (Biovison), insulin (Mercodia), and leptin (Millipore) levels were measured according to the manufacturer’s instructions.
Immunoblot, Immunostaining, and Gel Shift Analysis.
Immunoblot and immnuostaining assays were performed as previously described (15). Recombinant CREB and GST-CRTC2 (aa 1–120) were isolated as described previously (15, 16). CRTC2 antiserum was developed against residues 454–607 of mouse CRTC2 (8). For gel shift assays, binding reactions were in buffer containing 12 mM Hepes, pH 7.9, 12% glycerol, 1 mM EDTA, 75 mM KCl, 5 mM MgCl2, with 0.5 mg·ml−1 BSA, 2 mM TCEP, 25 μg·ml−1 poly dI:dC, and protease inhibitors. Proteins were incubated with 32P end-labeled somatostatin promoter duplex oligo for 30 min at room temperature before electrophoresis on nondenaturing 4% polyacrylamide gels with 2.5% glycerol in TBE buffer.
Cell Culture, Luciferase Activity, and Glucose Output.
HEK293T (ATCC) cells were cultured in DMEM containing 10% FBS (HyClone), 100 mg·ml−1 penicillin–streptomycin. Mouse primary hepatocytes were isolated and cultured as previously described (15). Transient assays were performed as previously described using adenoviral (Ad-G6Pase-luc) reporters for primary hepatocytes and plasmid based reporters (EVX-luc) for HEK293T cells (4, 6, 10). Cells were exposed to glucagon (100 nm) or FSK (10 μM) for 4 h, and luciferase activities were normalized to β-galactosidase activity from adenoviral or plasmid-encoded RSV β-gal. Glucose output from primary hepatocytes was determined enzymatically, after 1-h collection in glucose-free M199 media, supplemented with 10 mM lactate and 1 mM pyruvate (15).
Chromatin Immunoprecipitation and Quantitative PCR.
HEK293T cells or mouse primary hepatocytes were treated with forskolin (10 μM) or glucagon (100 nM). Cells were cross-linked on the plates with 0.75% formaldehyde and chromatin prepared essentially as described (8). For CREB and CRTC2 IPs, rabbit polyclonal antibody raised against respective antigens (11) was used along with rabbit IgG for negative controls. After removing crosslinks, DNA was extracted using phenol-chloroform, and CREB-target promoters were quantified using SYBR green real-time PCR. All signals were normalized to input chromatin signals. For quantitative PCR to test gene expression, total cellular RNAs from whole liver or primary hepatocytes were extracted using the RNeasy kit (Qiagen). mRNA levels were measured as previously described (15).
Statistical Analyses.
All studies were performed on at least three independent experiments. Results are reported as mean and SEM. The comparison of different groups was carried out using a two-tailed unpaired Student’s t test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank members of the Montminy laboratory for helpful discussions. This work was supported by National Institutes of Health Grants R01-DK083834 and R01-DK049777, by the Clayton Foundation Laboratories for Medical Research, and by the Helmsley Foundation. M.M. is supported by the Keickhefer foundation; K.V. is supported by the Department of Biomedicine, University of Bergen; and Y.W. is supported by a mentor-based fellowship from the American Diabetes Association.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914897107/DCSupplemental.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 3.Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 4.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 5.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saberi M, et al. Novel liver-specific TORC2 siRNA corrects hyperglycemia in rodent models of type 2 diabetes. Am J Physiol Endocrinol Metab. 2009;297:E1137–E1146. doi: 10.1152/ajpendo.00158.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Bittinger MA, et al. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 11.Conkright MD, et al. TORCs: Transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Le Lay J, et al. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 2009;10:55–62. doi: 10.1016/j.cmet.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols M, et al. Phosphorylation of CREB affects its binding to high and low affinity sites: Implications for cAMP induced gene transcription. EMBO J. 1992;11:3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccio A, et al. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez GA, Menzel P, Leonard J, Fischer WH, Montminy MR. Character-ization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991;11:1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.