Abstract
Bacteremia caused by nontyphoidal strains of Salmonella is endemic among African children. Case-fatality rates are high and antibiotic resistance increasing, but no vaccine is currently available. T cells are important for clearance of Salmonella infection within macrophages, but in Africa, invasive Salmonella disease usually manifests in the blood and affects children between 4 months and 2 y of age, when anti-Salmonella antibody is absent. We have previously found a role for complement-fixing bactericidal antibody in protecting these children. Here we show that opsonic activity of antibody and complement is required for oxidative burst and killing of Salmonella by blood cells in Africans. Induction of neutrophil oxidative burst correlated with anti-Salmonella IgG and IgM titers and C3 deposition on bacteria and was significantly lower in African children younger than 2 y compared with older children. Preopsonizing Salmonella with immune serum overcame this deficit, indicating a requirement for antibody and/or complement. Using different opsonization procedures, both antibody and complement were found to be necessary for optimal oxidative burst, phagocytosis and killing of nontyphoidal Salmonella by peripheral blood cells in Africans. Although most strains of African nontyphoidal Salmonella can be killed with antibody and complement alone, phagocytes in the presence of specific antibody and complement can kill strains resistant to killing by immune serum. These findings increase the likelihood that an antibody-inducing vaccine will protect against invasive nontyphoidal Salmonella disease in African children.
Keywords: bacteremia, bactericidal antibody, immunity, opsonic activity, vaccines
Infection with nontyphoidal strains of Salmonella (NTS), in particular Salmonella enterica serovar Typhimurium (S. Typhimurium) are a major cause of invasive bacterial disease, which typically manifest as bacteremia and meningitis, among African children (1–3). In some African countries such as Malawi, NTS is the commonest cause of bacteremia (1). Among children younger than 5 y of age in Kenya, the estimated minimum incidence of NTS bacteremia is 175 per 100,000 (2). Case-fatality rates exceed 20% and 50% for NTS bacteremia (3) and meningitis (4), respectively. Antibiotic resistance is an increasing problem (1) and currently no vaccine is available. There are well recognized clinical associations with young age, malaria, anemia, malnutrition, and HIV infection (1–3). A better knowledge of the mechanisms of immunity that protect against fatal infection from invasive strains of NTS in Africans is required both for understanding why invasive NTS disease is endemic in Africa and for developing an effective vaccine against NTS.
Animal studies have long implicated cellular mechanisms as being of key general importance for immunity to Salmonella (5, 6). This is explained in part by Salmonellae being facultative intracellular organisms and strains that are incapable of intracellular survival are avirulent (7). The importance of oxidative burst for protection against Salmonella in man is demonstrated by the high susceptibility of patients with chronic granulomatous disease to severe Salmonella infections (8, 9). Likewise, phox-knockout mice are very sensitive to Salmonella infections (10). Further evidence for the importance of cellular immunity in protecting against Salmonella in man is shown by the high frequency of severe Salmonella infections in individuals with defects in the IL-12/23–IFN-γ axis (11, 12), IFN-γ from CD4+ lymphocytes and NK cells being important for activating macrophages to produce an oxidative burst. Both in Africa and worldwide, individuals with HIV/AIDS, especially those with low CD4+ lymphocyte counts (13), are particularly vulnerable to fatal NTS infection (1, 14–16).
NTS bacteremia most commonly occurs in African children younger than 2 y of age (1, 2, 17), and we found a relative sparing of infants younger than 4 months of age in Malawi (18). This period coincides with the loss of maternal antibody and lack of production of antibody by the child’s own immune system. We have recently shown an important role for antibody-mediated complement-dependent killing in protecting children younger than 2 y against invasive African strains of NTS, a protection that is not dependent on cells (18). Antibody can also protect against Salmonellae through opsonization of these bacteria promoting uptake and killing by phagocytic cells, thereby linking humoral and cell-mediated immunity against Salmonella. An important role for the opsonic mechanism is suggested by studies on murine salmonellosis. Although mouse complement has limited bactericidal activity (19, 20), adoptive transfer studies (21, 22) and studies in T cell–deficient mice (23, 24) demonstrate the importance of antibody in immunity against Salmonella. Antibody is required for phagocytosis, oxidative burst function, and cellular killing of Salmonella by mouse macrophages (25).
In the present study, we investigate oxidative burst function in relation to age, antibody levels, and complement deposition on a typical invasive African strain of nontyphoidal Salmonella among healthy children in Malawi. We go on to investigate the relative importance of both antibody and complement for oxidative burst, phagocytosis, and killing of invasive NTS by peripheral blood cells in Africans.
Results
Opsonization of Nontyphoidal Salmonella Is Required for Normal Oxidative Burst Function in Blood Neutrophils from African Children.
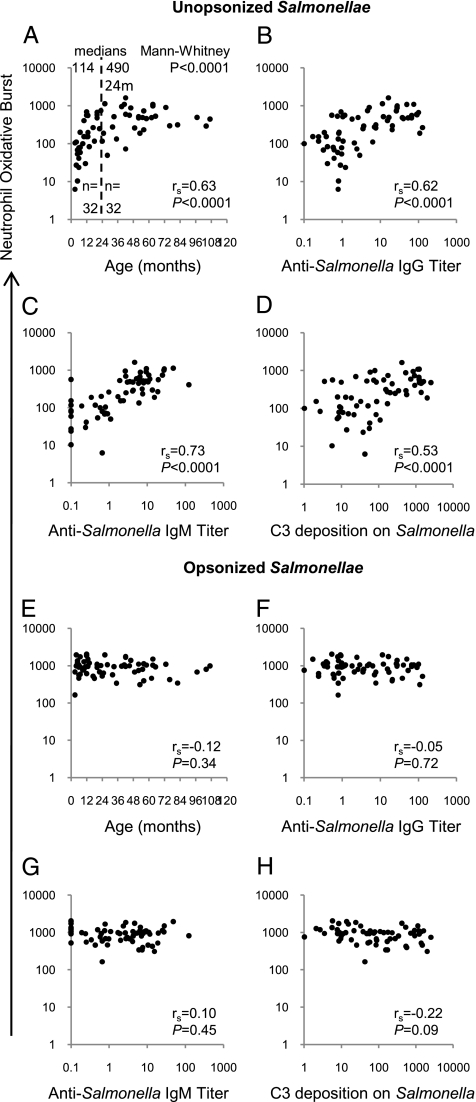
We first investigated the effect of age on oxidative burst function of neutrophils in whole peripheral blood samples obtained from 64 healthy Malawian children in response to stimulation with the invasive Malawian S. Typhimurium isolate D23580 (ref. 26; see also http://www.sanger.ac.uk/Projects/Salmonella/). Oxidative burst induced by Salmonellae in the absence of extrinsic opsonins correlated with age (rs, 0.63; 95% CI, 0.46–0.76; P < 0.0001), the median oxidative burst in children younger than 24 months being significantly lower than that in children older than 24 months (medians, 114 U and 490 U; Mann-Whitney P < 0.0001; Fig. 1A). Oxidative burst also correlated with serum anti–S. Typhimurium D23580 IgG titer (rs, 0.62; 95% CI, 0.44–0.75; P < 0.0001; Fig. 1B), anti-D23580 IgM titer (rs, 0.73; 95% CI, 0.59–0.83; P < 0.0001; Fig. 1C), and deposition of the C3 component of complement on Salmonella measured in a separate assay using autologous serum prepared from each blood sample (rs, 0.53; 95% CI, 0.32–0.68; P < 0.0001; Fig. 1D).
Fig. 1.
Oxidative burst in neutrophils in whole blood from Malawian children following stimulation with Salmonellae. Neutrophil oxidative burst to unopsonized (A–D) and preopsonized (E–H) S. Typhimurium D23580 compared with (A and E) age of the child, (B and F) anti–S. Typhimurium D23580 IgG titer, (C and G) anti-D23580 IgM titer, and (D and H) C3 deposition on D23580. Each point corresponds to blood from one child (N = 64).
These findings suggest that oxidative burst function is dependent on opsonization of Salmonella by antibody binding and/or complement deposition on Salmonellae. All blood samples had normal levels of total and alternative pathway hemolytic complement activity (18), indicating that complement in the absence of antibody is insufficient for full oxidative burst. As we have shown that antibody is required for complement-deposition on invasive African strains of Salmonella (18), complement in the absence of specific antibody is unlikely to be opsonic. To confirm the need for opsonization for induction of an oxidative burst, we stimulated blood from these children with S. Typhimurium D23580 preopsonized with exogenous immune human serum. The neutrophils in all 64 blood samples produced a strong oxidative burst response (median, 956 U) which was independent of the age of the child (Fig. 1E), blood sample titer of anti-D23580 IgG (Fig. 1F) and anti-D23580 IgM (Fig. 1G) or C3 complement deposition on Salmonella by serum prepared from each blood sample (Fig. 1H).
Therefore, the correlation between age and oxidative burst induced by unopsonized Salmonellae is a result of low or absent levels of anti-Salmonella antibody in the blood of younger children and not to a deficiency of the oxidative burst mechanism in the leukocytes of these children. Preopsonization with immune serum provided exogenous antibody and complement. Oxidative burst induced in peripheral blood neutrophils by Salmonellae in the absence of external opsonins correlated with antibody-dependent complement-mediated serum bactericidal activity (rs, 0.44; 95% CI, 0.22–0.62; P = 0.0002; Fig. S1). Hence, African children will tend to have either both modalities of antibody-mediated immunity against NTS or neither. This is likely to reflect common targets on Salmonella for both bactericidal and opsonic antibody.
Complement and Antibody Are Required for Induction of Oxidative Burst Response in Neutrophils and Monocytes in Peripheral Blood of Africans by Strains of Invasive NTS Irrespective of Susceptibility to Killing by Antibody and Complement Alone.
Next, we investigated the effect of different opsonization procedures on the ability of Malawian adult peripheral blood cells to produce an oxidative burst to Salmonella. Not all strains of invasive African NTS are susceptible to antibody-dependent complement-mediated killing by immune serum (i.e., immune serum–resistant strains), so we were interested in whether antibody-dependent oxidative burst was equivalent when induced by immune serum-resistant strains and immune serum-sensitive strains. To do this, we selected a immune serum-resistant strain of S. Typhimurium, D26104, as a second isolate to compare with immune serum-sensitive S. Typhimurium strain D23580 (Fig S2).
Both Salmonella strains were opsonized with fresh African adult immune serum, heat-inactivated immune serum, African child serum lacking antibody to Salmonella, C6-deficient serum with antibodies to Salmonella, and total IgG from immune serum. To prevent opsonization by endogenous antibody and complement from the peripheral blood samples, blood cells were washed twice in RPMI medium before each assay.
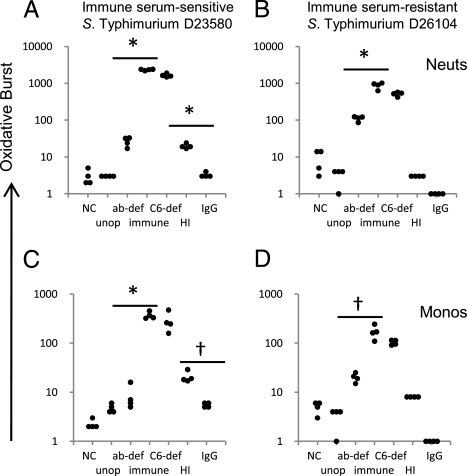
Optimal oxidative burst in neutrophils and monocytes occurred in response to stimulation with both strains of NTS opsonized with fresh immune serum. As expected, the magnitude of the response in neutrophils was greater than that in monocytes (Fig. 2), as blood had been taken from healthy adults at rest, and so monocytes were unlikely to be activated. Only background levels of oxidative burst occurred with unopsonized Salmonellae, confirming an absolute requirement for opsonization. Oxidative burst levels in response to stimulation with Salmonellae opsonized with antibody-deficient serum were significantly reduced compared with bacteria opsonized with immune serum (t test, P = 0.002 to P < 0.0001), confirming the need for antibody for an optimal oxidative burst response.
Fig. 2.
Effect of different opsonization procedures on oxidative burst activity in African adult washed peripheral blood phagocytic cells following stimulation with Salmonellae. Oxidative burst in neutrophils (A and B) and monocytes (C and D) following stimulation with immune serum–sensitive S. Typhimurium D23580 (A and C) and immune serum–resistant S. Typhimurium D26104 (B and D). NC, negative control, no stimulant; unop, unopsonized Salmonellae; ab-def, Salmonellae opsonized with anti-Salmonella antibody-deficient serum; immune, opsonized with immune serum; C6-def, opsonized with C6-deficient serum; HI, opsonized with heat-inactivated serum; IgG, opsonized with IgG purified from immune serum; Monos, monocytes; Neuts, neutrophils. Points in each group of four are from separate experiments. *P ≤ 0.0001; †P < 0.002.
Oxidative burst was absent in response to Salmonellae opsonized with purified IgG, suggesting an absolute requirement for complement in the opsonization process. As opsonization with C6-deficient serum produced the same oxidative burst response as fresh serum, terminal pathway complement activity is not required. Opsonization of D23580 with heat-inactivated immune serum led to a reduced, but detectable oxidative burst compared with opsonization with purified IgG (t test, P = 0.002 and P < 0.0001), although there was no response in a similar experiment using D26104.
To confirm that both antibody and complement are both required for oxidative burst function in response to NTS, we used five paired Malawian adult whole immune sera and purified IgG prepared from these sera to opsonize Salmonellae. All sera contained antibody to O-antigen of LPS and to other outer membrane molecules/non–O-antigen components of LPS (Fig. S3). Whereas there was normal oxidative burst with Salmonellae opsonized with each serum, none occurred with Salmonellae opsonized with IgG alone (Fig. S4) or with two commercial normal human Ig preparations. The same result was obtained when the diarrhogenic laboratory S. Typhimurium strain SL1344 (http://www.sanger.ac.uk/Projects/Salmonella/) was used instead of invasive African strain D23580 (Fig. S5).
Antibody and Complement Are Also Required for Optimal Phagocytosis of Invasive NTS by Peripheral Blood Cells from Africans.
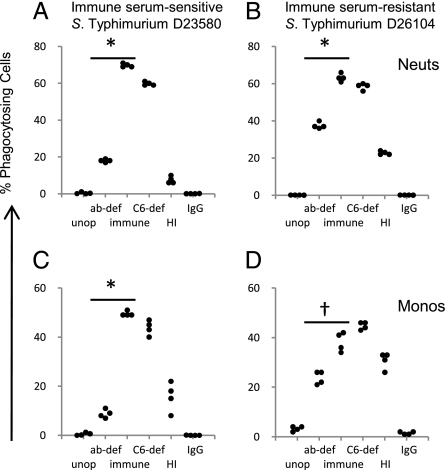
As bacteria need to be in the intracellular compartment for oxidative burst to be effective, we looked at opsonization and phagocytosis of Salmonellae. Phagocytosis is often a necessary intermediary step for triggering oxidative burst activity, and we investigated the effect of the different opsonization protocols on phagocytosis of immune serum–sensitive and immune serum–resistant strains of NTS by washed neutrophils and monocytes from adult African blood. The largest percentages of phagocytosing neutrophils and monocytes occurred with Salmonellae opsonized with fresh immune serum and C6-deficient serum, whereas unopsonized Salmonellae and Salmonellae opsonized with IgG resulted in negligible phagocytosis. Reduced percentages of phagocytosing cells occurred with antibody-deficient serum (t test, P = 0.001 to P < 0.0001) and heat-inactivated serum compared with bacteria opsonized with immune serum (Fig. 3). The insufficiency of antibody alone in effective opsonization of Salmonellae for phagocytosis by blood cells was confirmed using the five paired adult Malawian sera and IgG preparations and two normal human Ig preparations. Opsonization with all IgG preparations resulted in minimal phagocytosis in contrast to phagocytosis by approximately 40% to 70% of neutrophils and monocytes when serum was used (Fig. S6). Hence, without antibody and complement for opsonization, there is negligible phagocyte oxidative burst activity and phagocytosis of Salmonellae.
Fig. 3.
Effect of different opsonization procedures on phagocytosis of Salmonellae by African adult washed peripheral blood cells. Percentage of washed neutrophils (A and B) and monocytes (C and D) phagocytosing immune serum–sensitive S. Typhimurium D23580 (A and C) and immune serum–resistant S. Typhimurium D26104 (B and D). Abbreviations as for Fig. 2. Points in each group of four are from separate experiments. *P < 0.0001; †P < 0.01.
Both Antibody and Complement Are Necessary for Cellular Killing of Invasive NTS by Peripheral Blood from Africans.
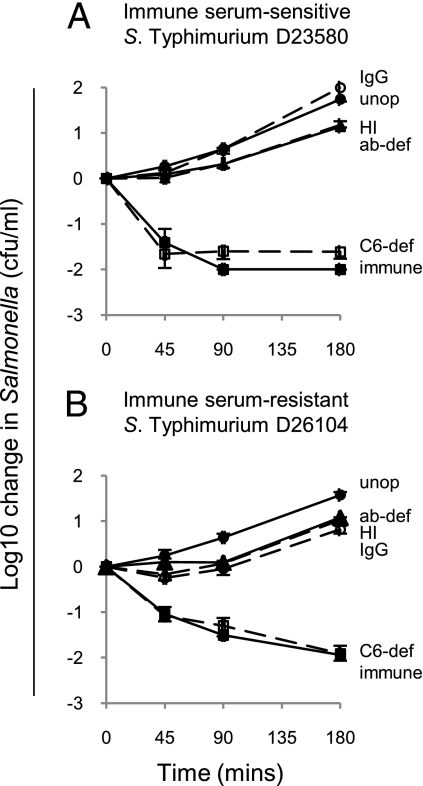
It was crucial to understand whether differences in oxidative burst and phagocytosis with different opsonization regimes paralleled differences in killing of NTS by peripheral blood cells. In order not to adversely affect Salmonellae viability by the opsonization process itself, we limited opsonization to 20 min at room temperature. Suspensions of washed peripheral blood cells were then inoculated with opsonized Salmonellae at 105 cfu/mL and Salmonella-killing monitored over 3 h at 37°C. Both immune serum–sensitive and immune serum–resistant strains of Salmonellae were killed with an approximate 100-fold reduction in Salmonellae at 3 h (Fig. 4). Similar killing was observed when blood cells were lysed before plating on agar for viable count determination, indicating that the reduction in Salmonellae was not a result of internalization of bacteria within phagocytes. No killing occurred with RPMI medium instead of washed blood cells, indicating that killing is cell-mediated and that the opsonization process itself did not cause Salmonella killing. Similar killing levels occurred with Salmonellae opsonized with C6-deficient serum compared with normal immune serum confirming a lack of cell-free complement-dependent bactericidal activity in the assay.
Fig. 4.
Effect of different opsonization procedures on killing of Salmonellae by African adult washed peripheral blood cells. In vitro killing of (A) serum-sensitive S. Typhimurium D23580 and (B) serum-resistant S. Typhimurium D26104 at 45, 90, and 180 min. Negative values correspond with a decrease in viable Salmonellae compared with the initial concentration of 105 cfu/mL Abbreviations as for Fig. 2: Unop (filled circle, solid line); ab-def (filled triangle, solid line); immune (filled square, solid line); C6-def (empty square, dashed line); HI (empty triangle, dashed line); IgG (empty circle, dashed line). Data are mean ± SD of three experiments.
Unopsonized Salmonellae and Salmonellae opsonized with antibody alone were not killed by blood cells paralleling the absence of phagocytosis and oxidative burst activity observed with these preparations of Salmonella. No killing occurred with Salmonellae opsonized with antibody-deficient serum and heat-inactivated serum, indicating that low levels of phagocytosis and oxidative burst seen with these opsonization protocols were insufficient for Salmonella killing. Again, we used Salmonellae opsonized with the five adult Malawian sera and IgG preparation and two normal human Ig preparations, this time to confirm that opsonization of Salmonellae with antibody alone does not result in peripheral blood cell killing of these bacteria. As for oxidative burst and phagocytosis, cell killing only occurred in the presence of antibody and complement, and opsonization with antibody alone was insufficient for this to occur (Fig. S7).
Discussion
These findings indicate a second mechanism of antibody-dependent killing of invasive African nontyphoidal strains of Salmonella in peripheral blood of Africans that complements the cell-free bactericidal activity of antibody previously described against immune serum-sensitive strains of Salmonella (18). The importance of cell-mediated immunity to Salmonella in man is indicated by the increase susceptibility to Salmonella infections in individuals who are deficient in cellular immune mechanisms (8, 9, 11, 12). Our previous work in Malawi found an epidemiological association between lack of Salmonella-specific antibody and incidence of NTS bacteremia in African children (18). By demonstrating the need for antibody for oxidative burst, phagocytosis, and peripheral blood phagocyte killing of Salmonellae, the current study serves to link antibody and cell-mediated immunity in protecting African children against Salmonella. Our findings indicate that blood phagocytes from even the youngest African children have a fully functional oxidative burst mechanism, but often lack specific opsonizing antibody required to activate this mechanism against Salmonella.
Therefore, African children with antibody to Salmonella can deal with these bacteria through the dual cell-free and cell-dependent mechanisms described, but those who lack antibody are deficient in both humoral and effective cellular immunity to Salmonella (Fig. S1). These findings are consistent with a previous study on the bactericidal and opsonic role of antibody and complement against the diarrhogenic laboratory LT2 strain of S. Typhimurium (27). Therefore, our findings further help explain the high mortality seen with invasive Salmonella disease in young African children (3, 4). More positively, this work increases the expectation that an antibody-inducing vaccine will protect African children against fatal and recrudescent Salmonella infection. Bactericidal antibody only acts on Salmonellae while the bacteria are in the extracellular compartment and Salmonellae are adapted for intracellular survival (7). In contrast, opsonic antibody facilitates killing of internalized Salmonellae by phagocytes, although antibody still needs to bind Salmonellae before phagocytosis. Even if Salmonellae avoid opsonization by directly entering into macrophages in gut-associated lymphoid tissue, the hematogenous spread of Salmonellae from one macrophage to another in the reticuloendothelial system (23) exposes them to bactericidal and opsonic antibody.
As many as 10% of invasive NTS strains in Malawi are resistant to complement-mediated killing by immune serum (18). A major concern with a vaccine that only induces bactericidal antibody is that serum-resistant strains would not be affected by such antibody. A second concern is that strain replacement could occur following the introduction of a bactericidal antibody–inducing vaccine against NTS with serum-resistant NTS becoming dominant. Hence we investigated oxidative burst, phagocytosis, and cellular killing of an African immune-serum resistant and immune-serum sensitive strain of NTS. That opsonic antibody facilitates cellular killing of both strains of NTS is reassuring. It is therefore important that a vaccine against NTS induces both bactericidal and opsonizing antibody.
The mechanisms that allow Salmonellae to survive within phagocytes (7) protect against oxidative burst with genes in Salmonella pathogenicity island 2 inhibiting colocalization of reactive oxygen intermediates at the phagolysosome (28) and sod genes encoding scavenger proteins that target reactive oxygen intermediates (29). It is perhaps surprising that despite such adaptations, oxidative burst is highly effective against Salmonellae. An equilibrium is most likely established between host and pathogen whereby opsonizing antibody gives the host the advantage, whereas in the absence of antibody, T helper 1 cytokines (11, 12), or functional oxidative burst mechanism (8, 9), intracellular Salmonellae survive. Only small numbers of bacteria need avoid cell killing in this intracellular niche to establish latent infection that can subsequently reactivate leading to recrudescence of invasive disease, as commonly observed in HIV-infected African adults (14) and subjects with defective T helper 1 immunity (11).
The critical requirement for complement in the opsonic process is striking. There is negligible phagocytosis, oxidative burst, or cellular killing by African peripheral blood cells of invasive African strains of S. Typhimurium opsonized with antibody alone. Use of peripheral blood enabled us to investigate blood cell killing of Salmonellae, together with phagocytosis and oxidative burst by monocytes and neutrophils simultaneously. Our findings are consistent with previous work in developed countries using isolated neutrophils from human blood (27, 30) and human cultured macrophages (31). These studies demonstrated that antibody and complement are required for phagocytosis (27, 30), oxidative burst (31), and cellular killing (27) of laboratory strains of S. Typhimurium. Antibody and complement have also been shown to be necessary for killing of enterococci by human neutrophils (32).
Interestingly, work on mouse macrophages has found that antibody alone can enhance phagocytosis, oxidative burst, and killing of S. Typhimurium (25). The reason for the different requirement for complement in opsonization of NTS in humans and mice is not clear, although a study using C1q-deficient mice suggests a role for complement in protecting mice against S. Typhimurium in vivo (33). Salmonella infections have occasionally been described in patients with primary complement deficiencies (34, 35). Studies in mice have also indicated a role for phagocyte inducible NO synthase (36) and antimicrobial peptides (37) in protection against Salmonella.
The clinical implications for African children who lack complement, either through genetic defects or consumption, are serious and potentially have the same effect on protection against Salmonella as the absence of specific antibody. The clinical association between malaria and NTS bacteremia in African children is well recognized (38, 39), yet the underlying mechanisms of this association are poorly understood. Our findings suggest that the high levels of complement consumption in malaria, particularly in severe malarial anemia (40), could underlie this association, together with direct inhibition of oxidative burst activity resulting from ingestion of malaria-parasitized red blood cells or hemozoin by phagocytes (41).
To develop a vaccine against NTS for Africa, it is necessary to identify the targets of opsonizing antibodies that protect against Salmonella infection. Recent work in a mouse model suggests that these include porin outer membrane proteins (23, 24), and previous work has also indicated a role for the O-antigen of lipopolysaccharide (42). Whether antibody to these targets is protective in man is currently not known. We found antibody to both O-antigen of LPS and other Salmonella outer membrane molecules in immune African sera. Ideally, surface molecules will be identified that are targets of both opsonizing and bactericidal antibody facilitating both extracellular and intracellular killing of Salmonellae. Our findings provide further evidence for the likely feasibility of an antibody-producing vaccine to protect African children against fatal NTS bacteremia.
Materials and Methods
Study Population.
Blood samples were from 64 healthy Malawian children (median age, 23.5 months) attending surgical outpatient clinics in Blantyre, Malawi, and from healthy HIV-uninfected adults. No individual had a history of known Salmonella infection or vaccination. Whole blood was anticoagulated with sodium heparin at 4 IU/mL and used in study assays within 4 h or allowed to clot in plain tubes at room temperature for 1 to 2 h followed by serum separation and freezing in aliquots at −80°C. When required, endogenous antibody and complement were removed from whole blood by washing twice with RPMI and resuspending cells at the initial volume with RPMI.
Salmonellae.
Two clinical isolates of invasive nontyphoidal Salmonella from bacteremic Malawian children were used. D23580 is an immune serum–sensitive and D26104 an immune serum–resistant strain of S. Typhimurium. Further details are in SI Materials and Methods. All Salmonella preparations were in midlog growth. For oxidative burst and phagocytosis assays, bacteria were heat-killed at 56 °C for 30 min, and for cell killing assays, viable bacteria were used. For the phagocytosis assay, Salmonellae were labeled with FITC by incubating 1010/mL bacteria with FITC at 25 μg/mL for 20 min at 37°C and then washing twice with PBS solution.
Materials.
Unless otherwise stated, chemicals and reagents were obtained from Sigma.
Sera and Ig Preparations.
Antibody-deficient serum was from Malawian children and lacked antibody to and bactericidal activity against S. Typhimurium D23580. Immune serum was from Malawian adults and effected “normal killing” of S. Typhimurium D23580 (18). C6-deficient serum was from a patient with genetic deficiency of C6. Heat-inactivated serum was immune serum that had been incubated at 56 °C for 30 min. Further details are in SI Materials and Methods. Total IgG was extracted from immune serum and prepared using Streptococcal Protein G HiTrap Columns (GE Healthcare) as previously described (18). Human normal Ig for i.v. administration was from CSL Behring and for s.c. or intramuscular use was from Baxter.
Opsonization Procedures.
Opsonization was by incubating Salmonellae in serum or IgG (at 10 g/L in PBS solution) for 20 min at room temperature at a ratio of 1:10 and a final concentration of 109/mL for heat-killed bacteria and 106/mL for viable bacteria. Following opsonization, viable bacteria for killing assays were used immediately.
Oxidative Burst Assay.
This was a modification of the Bursttest assay (Becton Dickinson), which quantitatively measures oxidative burst activity in neutrophils and monocytes by flow cytometry by determining oxidation of dihydrorhodamine to rhodamine. Heparinized whole blood or washed cells were stimulated with heat-killed Salmonellae at 2 × 108/mL for 10 min at 37°C according to the manufacturer’s instructions before incubating with dihydrorhodamine. Following red cell lysis and fixation, leukocytes were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Neutrophils and monocytes were gated according to light scatter characteristics. Rhodamine fluoresces in the FL1 channel and oxidative burst activity was measured according to the manufacturer’s instructions as geometric mean fluorescence intensity from the FL1 histogram (arbitrary units).
Phagocytosis Assay.
FITC-labeled Salmonellae (10 μL of 109/mL) were added to paired aliquots of 50 μL washed peripheral blood cells cooled to 0 °C with one aliquot then incubated at 37 °C in a water bath for 10 min while the other (negative control) remained at 0 °C. Trypan blue (50 μL 0.16%) was added to each sample to quench the fluorescence of noninternalized bacteria. Cells were washed with PBS solution, resuspended in 1 mL FACS lysing solution (Becton Dickinson) to lyse red cells and fix leukocytes, and then incubated at room temperature for 20 min in the dark. Following further PBS solution washes, the cells were resuspended in 20 μg/mL propidium iodide before analysis by flow cytometry. Leukocytes were discriminated from free bacteria, unlysed red cells and cell fragments using an FL2 (propidium iodide) histogram and neutrophils and monocytes distinguished by light scatter characteristics. The percentage of each cell type that had phagocytosed Salmonellae was determined from the FL1 (FITC) histogram.
Salmonella Killing Assays.
Killing of Salmonellae by washed peripheral blood cells was performed using 90 μL washed cells and 10 μL of Salmonellae to give a final concentration of 105/mL bacteria. Samples were incubated on a rocker plate at 20 rpm at 37 °C and numbers of viable Salmonellae determined after 45, 90, and 180 min by serial dilution on Luria Bertani agar. When required, blood cells were lysed with saponin immediately before viable count determination. Serum bactericidal assays were performed in the same way using serum instead of washed peripheral blood cells and Salmonellae at a final concentration of 106/mL (18).
Salmonella Antibody Assays and Complement Assays.
Anti-Salmonella IgG and IgM antibody titers and complement C3 deposition on S. Typhimurium D23580 were by flow cytometry as previously described (18). Further details and details of the fluorescent bead–based immunoassay for antibodies to S. Typhimurium LPS and functional complement assays are in SI Materials and Methods.
Statistical Methods.
The Spearman approach was used for estimation of correlation with 95% CIs. The Mann-Whitney U test was used to compare neutrophil oxidative burst activity in children younger than 2 y with those older than 2 y. Comparisons of data from experiments using different opsonization procedures were made using a Student t test.
Ethical Approval.
The study was approved by the College of Medicine Research and Ethics Committee, College of Medicine, University of Malawi, and the ethics committee of the Liverpool School of Tropical Medicine. Informed consent was obtained from all participants or their parents or guardians.
Supplementary Material
Acknowledgments
We thank the parents, guardians, and children who participated in this study. We are grateful to Mr. Paul Pensulo and nurses Meraby Funsani and Grace Mwimaniwa for their help in recruiting children and Professor Elizabeth Molyneux and Professor Eric Borgstein and the staff at Queen Elizabeth Central Hospital, Beit Cure International Hospital, and the Malawi–Liverpool–Wellcome Trust Clinical Research Programme for their assistance. We thank Dr. Robert Kingsley (Wellcome Trust Sanger Institute) for providing the galE- mutant of S. Typhimurium D23580. This work was supported by a PhD studentship from the Training Committee of the Malawi–Liverpool–Wellcome Trust Clinical Research Programme (to E.N.G.), a Tropical Research Fellowship (to C.A.M.) and a Programme Grant (to M.E.M.) from the Wellcome Trust, and a Clinical Research Fellowship from GlaxoSmithKline (to C.A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.L.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910497107/DCSupplemental.
References
- 1.Gordon MA, et al. Epidemics of invasive Salmonella enterica serovar Enteritidis and S. enterica Serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 2.Berkley JA, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 3.Graham SM, et al. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux EM, et al. The outcome of non-typhoidal salmonella meningitis in Malawian children, 1997-2006. Ann Trop Paediatr. 2009;29:13–22. doi: 10.1179/146532809X401980. [DOI] [PubMed] [Google Scholar]
- 5.Mackaness GB, Blanden RV, Collins FM. Host-parasite relations in mouse typhoid. J Exp Med. 1966;124:573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanden RV, Mackaness GB, Collins FM. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966;124:585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114:555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus GM, Neu HC. Agents responsible for infection in chronic granulomatous disease of childhood. J Pediatr. 1975;86:415–417. doi: 10.1016/s0022-3476(75)80975-9. [DOI] [PubMed] [Google Scholar]
- 10.Mastroeni P, et al. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLennan C, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante J, et al. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Gilks CF. Acute bacterial infections and HIV disease. Br Med Bull. 1998;54:383–393. doi: 10.1093/oxfordjournals.bmb.a011695. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MA, et al. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 15.Gilks CF, et al. Life-threatening bacteraemia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet. 1990;336:545–549. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 16.Levine WC, Buehler JW, Bean NH, Tauxe RV. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J Infect Dis. 1991;164:81–87. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Sigaúque B, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan CA, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GC. The complementary activity of mouse-serum. J Immunol. 1943;46:319–323. [Google Scholar]
- 20.Marcus S, Esplin DW, Donaldson DM. Lack of bactericidal effect of mouse serum on a number of common microorganisms. Science. 1954;119:877. doi: 10.1126/science.119.3103.877. [DOI] [PubMed] [Google Scholar]
- 21.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham AF, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 24.Gil-Cruz C, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci USA. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uppington H, et al. Effect of immune serum and role of individual Fc gamma receptors on the intracellular distribution and survival of Salmonella enterica serovar Typhimurium in murine macrophages. Immunology. 2006;119:147–158. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kingsley RA, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genomes Res. 2009;19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaio MF, Rowland H. Bactericidal and opsonizing effects of normal serum on mutant strains of Salmonella typhimurium. Infect Immun. 1985;49:647–653. doi: 10.1128/iai.49.3.647-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Torres A, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 29.De Groote MA, et al. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vreede RW, Marcelis JH, Verhoef J. Antibodies raised against rough mutants of Escherichia coli and Salmonella strains are opsonic only in the presence of complement. Infect Immun. 1986;52:892–896. doi: 10.1128/iai.52.3.892-896.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Château MT, Caravano R. The oxidative burst triggered by Salmonella typhimurium in differentiated U937 cells requires complement and a complete bacterial lipopolysaccharide. FEMS Immunol Med Microbiol. 1997;17:57–66. doi: 10.1111/j.1574-695X.1997.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 32.Harvey BS, Baker CJ, Edwards MS. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992;60:3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warren J, et al. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70:551–557. doi: 10.1128/iai.70.2.551-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan BP, Walport MJ. Complement deficiency and disease. Immunol Today. 1991;12:301–306. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 35.Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984;63:243–273. [PubMed] [Google Scholar]
- 36.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 39.Bronzan RN, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 40.Nyakoe NK, Taylor RP, Makumi JN, Waitumbi JN. Complement consumption in children with Plasmodium falciparum malaria. Malar J. 2009;8:7. doi: 10.1186/1475-2875-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarzer E, et al. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson DC, Robbins JB, Szu SC. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–4686. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.