Abstract
The exocrine pancreas plays an important role in endogenous zinc loss by regulating excretion into the intestinal tract and hence influences the dietary zinc requirement. The present experiments show that the zinc transporter ZnT2 (Slc30a2) is localized to the zymogen granules and that dietary zinc restriction in mice decreased the zinc concentration of zymogen granules and ZnT2 expression. Excess zinc given orally increased ZnT2 expression and was associated with increased pancreatic zinc accumulation. Rat AR42J acinar cells when induced into a secretory phenotype, using the glucocorticoid analog dexamethasone (DEX), exhibited increased ZnT2 expression and labile zinc as measured with a fluorophore. DEX administrated to mice also induced ZnT2 expression that accompanied a reduction of the pancreatic zinc content. ZnT2 promoter analyses identified elements required for responsiveness to zinc and DEX. Zinc regulation was traced to a MRE located downstream from the ZnT2 transcription start site. Responsiveness to DEX is produced by two upstream STAT5 binding sites that require the glucocorticoid receptor for activation. ZnT2 knockdown in the AR42J cells using siRNA resulted in increased cytoplasmic zinc and decreased zymogen granule zinc that further demonstrated that ZnT2 may mediate the sequestration of zinc into zymogen granules. We conclude, based upon experiments with intact mice and pancreatic acinar cells in culture, that ZnT2 participates in zinc transport into pancreatic zymogen granules through a glucocorticoid pathway requiring glucocorticoid receptor and STAT5, and zinc-regulated signaling pathways requiring MTF-1. The ZnT2 transporter appears to function in a physiologically responsive manner involving entero-pancreatic zinc trafficking.
Keywords: zinc, nutrition, pancreas, secretion, gene regulation
Mammalian zinc homeostasis is maintained through a balance between gastrointestinal absorption, tissue turnover, and biliary, pancreatic, and intestinal secretions into the intestinal lumen (1). Under conditions of normal dietary zinc intake, substantial amounts of zinc are released into the small intestine from the exocrine pancreas (2, 3). These secretions represent a major component of calculations used to establish the dietary zinc requirement for humans.
Over 85% of the pancreas is comprised of exocrine cells (4). Localization studies have shown that pancreatic zinc is concentrated in the granules of acinar cells, where digestive proenzymes are also stored prior to exocytosis through the apical membrane for entry into the intestinal lumen. Abnormal zinc metabolism has been reported after pancreatectomy (5–7). Zinc deficiency causes pancreatic acinar cells to become depleted of zinc (8, 9). Radiotracer studies have shown that the pancreas exhibits high rates of zinc turnover [reviewed in (10)]. The exocrine pancreas is a target organ of zinc toxicosis. Excessive dietary zinc alters acinar cell structure, and produces necrosis, causing depletion of zymogen granules and reduces digestive enzyme secretion (11–13). This sensitivity to zinc supports the need for tight regulation of pancreatic acinar cell zinc transport.
Accumulation and distribution of zinc within the cells are mediated by two zinc transporter families (SLC39A/ZIP and SLC30A/ZnT) (2, 14). We found that expression of zinc transporters ZnT1 and ZnT2 in pancreas showed progressive decreases during zinc depletion of mice (10). The experiments reported here demonstrate the localization of ZnT2 to zymogen granules of pancreatic acinar cells and up-regulation of the ZnT2 gene by zinc and glucocorticoids (GC). ZnT2 regulation by zinc involves the transcription factor MTF-1 and a downstream metal responsive element (MRE). Regulation by GC requires the glucocorticoid receptor (GR) and STAT5 acting in a trans pathway requiring upstream STAT5-response elements. The dual regulation of ZnT2 by dietary zinc and glucocorticoid hormone suggests this transporter is involved in zinc trafficking in pancreatic acinar cells at the level of zymogen granules. In this way, ZnT2 may contribute to the pathway for release of endogenous zinc into the gastrointestinal tract.
Results
Dietary Zinc Intake Regulates the Zinc Content and the Zinc Transporters ZnT1/ZnT2 of the Mouse Pancreas.
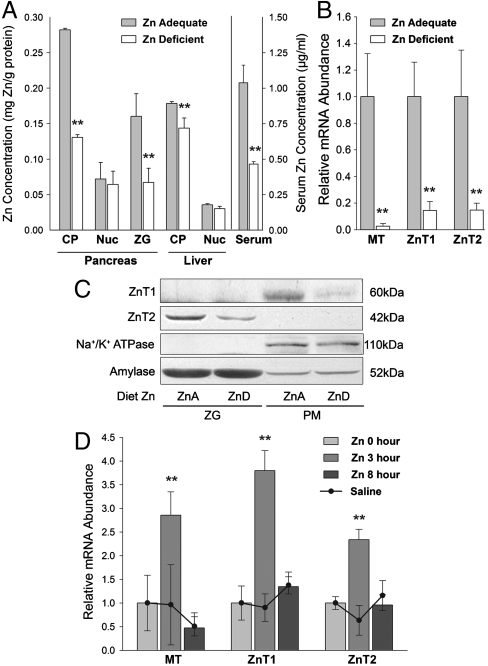
Mice fed a zinc-restricted diet developed signs of zinc deficiency, as shown by depressed serum zinc concentrations (Fig. 1A). To understand how zinc restriction affects the pancreas, the zinc concentrations in subcellular fractions were measured. Zinc restriction resulted in pancreatic cytoplasm and zymogen granules having less than half the amount of zinc found in mice fed the zinc adequate diet. In contrast, zinc concentrations of the crude nuclear fractions were not reduced by zinc restriction (Fig. 1A). By contrast, the cytoplasmic fraction from liver did not show the same magnitude of decrease, indicating that liver is resistant to zinc restriction, whereas the pancreas is not. Transcript levels of metallothionein (MT), ZnT1 and ZnT2 in pancreatic RNA from these mice are shown in Fig. 1B. These responses show that the sensitivity of both zinc transporter genes to the level of dietary zinc is comparable to that of MT, a well recognized zinc-regulated gene. Western blotting clearly showed a decrease of ZnT1 in the plasma membrane-enriched fraction during zinc restriction (Fig. 1C). The greater abundance of ZnT1 in the plasma membrane is consistent with the zinc efflux function of ZnT1 (15). Of particular interest is that ZnT2 was exclusively detected in the isolated zymogen granule fraction and showed a reduced expression in response to dietary zinc restriction (Fig. 1C). The two marker proteins for plasma membranes and zymogen granules, Na+/K+ ATPase and amylase, respectively, were unaffected by zinc restriction. Zinc given orally produced an increase in serum and pancreatic zinc concentrations and transient elevations in pancreatic MT, ZnT1, and ZnT2 mRNA. A full survey of ZnT and Zip transcripts is shown in Figs. S1 and S2, respectively. The response to an oral dose of 35 μg zinc/g body weight is shown in Fig. 1D. The changes in pancreatic, liver, and serum zinc concentrations are shown in Fig. S3. These results show both ZnT1 and ZnT2 are sensitive to elevated zinc intake supporting their role in endogenous zinc excretion to promote homeostasis.
Fig. 1.
Tissue and serum zinc concentrations and the expression of ZnT1 and ZnT2 in mouse pancreas. (A–C) Mice were fed either a zinc-adequate (ZnA) or a zinc-deficient diet (ZnD) for 21 days. (A) Cytoplasmic, nuclear, and zymogen granule compartments were isolated along with serum. Zinc is expressed as mg zinc/g protein or μg zinc/ml. (B,D) qPCR analysis of MT, ZnT1 and ZnT2 mRNA levels in pancreas. Values are relative mRNA normalized to 18S rRNA. (C) Abundance of ZnT1 and ZnT2 proteins in isolated zymogen granules (ZG) and plasma membranes (PM) of ZnA and ZnD mice were analyzed by Western blotting. Na+/K+ ATPase and amylase are loading controls for PM and ZG fractions, respectively. (D) Mice were given a dose of 35 μg zinc/g body weight or saline orally. MT, ZnT1, and ZnT2 mRNAs were measured 3 h and 8 h after gavage. n = 3–4.
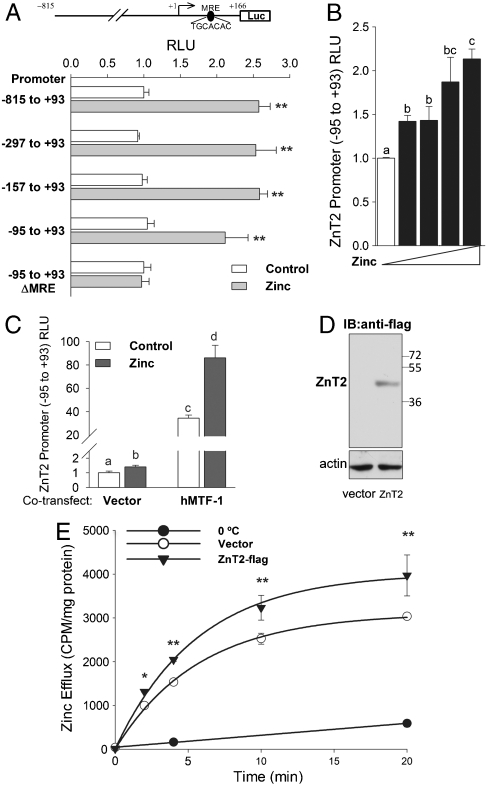
We cloned 0.9 kb of the mouse ZnT2 promoter (-815 to +93) and smaller fragments down to -95 to +93 into the pGL3-Basic vector. The constructs were then used to transfect HeLa and HEK 293 cells allowing us to study the zinc regulation of ZnT2. Each of the constructs tested produced approximately the same change in promoter activity upon addition of zinc (100 μM). The exception was when the MRE consensus was mutated (Fig. 2A). That mutation eliminated zinc regulation. A dose-dependent increase in ZnT2 promoter activity was found up to 120 μM zinc (Fig. 2B), which is consistent with MTF-1 regulation. In agreement with that finding was the response of the ZnT2 promoter construct to zinc when a vector expressing hMTF-1 was cotransfected in HEK 293 cells (Fig. 2C). Next, HeLa cells were transfected with a C-terminal flag-tagged ZnT2 or empty vector and were allowed to accumulate 65Zn. Overexpression was confirmed by Western blotting (Fig. 2D). The 65Zn efflux from preloaded cells was greater in the overexpressing cells (Fig. 2E). This finding is in agreement with a role for ZnT2 in zinc efflux.
Fig. 2.
ZnT2 promoter and transport activity in transfected cells in response to zinc. (A) HeLa cells were transfected with murine ZnT2 promoter constructs over the range (-815 to +93) to (-95 to +93) ligated into pGL3-Basic vector. One construct (-95 to +93) was also mutated at the MRE consensus sequence (+53 to +59). Luciferase activity was measured 48 h after transfection (values are relative luminescence units; firefly/renilla). Zinc (100 μM) was added for the last 24 h. (B) Activity of -95 to +93 promoter construct in response to 0, 20, 40, 80, or 120 μM Zn added for the last 24 h. (C) HEK293 cells were cotransfected with an hMTF-1 expression vector and the -95 to +93 ZnT2 promoter construct for 48 h with 100 μM Zn added for the last 24 h. (D,E) AR42J cells were transfected with a control or pCMV-ZnT2-flag vector for 48 h. (D) Western blot of total cell lysate showing ZnT2-flag overexpression. (E) Cells were preincubated with 65Zn for 24 h. Efflux of the 65Zn is expressed on a cpm/cell protein basis. Bars with different superscripts indicate the means are statistically different at P < 0.05.
ZnT2 Expression Is Up-Regulated by Glucocorticoids.
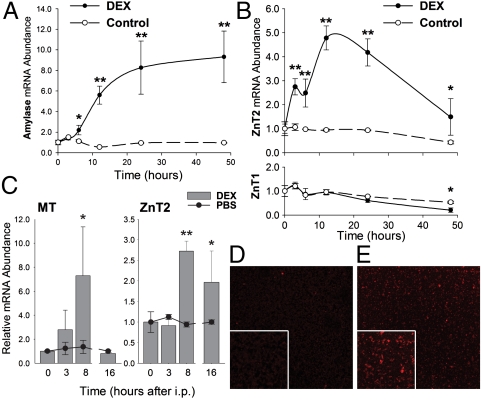
Rat AR42J pancreatic acinar cells were used as a model to further understand the regulation of ZnT1 and ZnT2 expression in pancreatic acinar cells. Dexamethasone (DEX) was added to the AR42J cell cultures to stimulate cell differentiation (16). An increase in amylase mRNA, a signature of acinar differentiation and secretory enzyme production, was observed following addition of DEX (Fig. 3A). Similarly, a strong up-regulation of ZnT2 mRNA was found upon DEX treatment (Fig. 3B, Upper Panel). Surprisingly, however, no change in ZnT1 expression was observed in response to DEX (Fig. 3B, Lower Panel), indicating selective GC regulation of the ZnT2 gene. Western analysis of the membrane fractions of AR42J cells confirmed ZnT2 was induced by DEX. To examine if ZnT2 is regulated by GC hormones in vivo, mice were given DEX by i.p. injection. Pancreatic ZnT2 mRNA expression was significantly up-regulated to nearly 2-fold by 8 h after the injection (Fig. 3C). Increased MT mRNA following DEX treatment was used as a positive control (17). DEX also produced a reduction in the zinc concentration of the pancreas consistent with enhanced zinc secretion (Fig. S4). Immunofluorescence analysis of isolated zymogen granules by confocal microscopy demonstrated ZnT2 was robustly induced by DEX (Fig. 3D vs. E).
Fig. 3.
Dexamethasone increases ZnT2 expression in rat AR42J cells and mouse pancreas. (A, B) AR42J cells were incubated with DEX for up to 48 h. ZnT1, ZnT2, and amylase mRNA levels were measured by qPCR. (C) Mice were injected i.p. with either DEX or saline and killed 3, 8, or 16 h thereafter. mRNA levels were measured by qPCR. (D, E) Confocal immunofluorescence microscopy of purified zymogen granules from pancreas of (D) saline or (E) DEX-treated mice. ZnT2 was detected with affinity-purified antibody. n = 3–4.
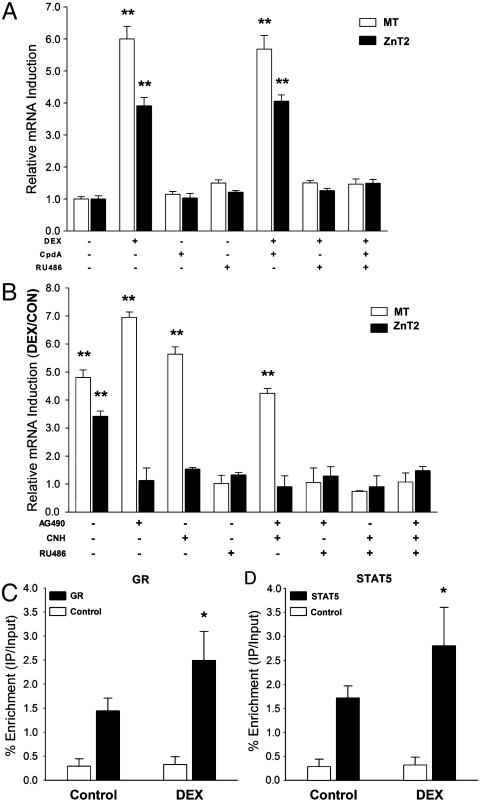
To understand the mechanism of ZnT2 regulation by DEX, the GC antagonist RU486 and CpdA, a newly discovered nonsteroidal selective GR agonist, were exploited to study the association of ZnT2 gene transcription with signaling via the GR. Having a higher binding affinity, CpdA competes with DEX for GR binding, and induces GR release from chaperones and nuclear translocation and transrepression of NF-κB-driven gene expression. CpdA exhibits no transactivation potential on GRE-driven gene transcription (18), and was not able to activate ZnT2 or MT gene expression in the AR42J cells (Fig. 4A). However, when the cells were treated with DEX and CpdA at the same time, the hormonal analog could still initiate expression of ZnT2 and MT, presumably via transactivation of GR. In contrast, the addition of the RU486 prevented DEX from stimulating the up-regulation of ZnT2 and MT (Fig. 4A). These results indicate DEX stimulates ZnT2 expression via transactivation of GR-driven gene expression, but is not associated with NF-κB activation. MT, a well characterized GC transactivation-regulated gene as mentioned above (17), was used as a positive control in these experiments.
Fig. 4.
Dexamethasone-induced ZnT2 expression in AR42J pancreatic acinar cells is mediated by STAT5 through a pathway that requires the glucocorticoid receptor. (A) Cells were cultured in the medium supplemented with or without DEX, CpdA, and/or RU486 for 12 h. (B) Cells were cultured with either the JAK2 inhibitor, AG490 or the STAT5 inhibitor (CNH) for 24 h, prior to DEX for 12 h. qPCR was used to measure MT and ZnT2 mRNA levels. (C,D) AR42J cells were treated with to DEX for 5 h, two-step cross-linking ChIP assays were performed with GR (C) or STAT5 (D) antibodies, and DNA enrichment was analyzed by qPCR. n = 3.
The responsiveness of ZnT2 expression to DEX in both AR42J cells and pancreas of the intact mouse led to an analysis of GRE’s in the upstream ZnT2 promoter region. Two half GRE sites were found, but there was an absence of a full GRE. Whereas these noncanonical half sites may impart GC regulation for some genes (19), we also examined the involvement of STAT5 and GR in transduction pathways for regulation of other GC-controlled genes (20–22). Two STAT5-REs were identified in the mZnT2 promoter (23). We used a recently developed STAT5-specific inhibitor [chromone-based nicotinyl hydrazone (CNH)] (24) and the widely used Janus kinase 2 (JAK2) inhibitor (AG490) to examine STAT5 involvement in ZnT2 activation by DEX. As shown in Fig. 4B, the inhibition of STAT5, alone or in combination with JAK2 inhibition, completely blocked DEX induction of ZnT2. Of note is that the DEX-induced increase in MT expression was not inhibited by either AG490 or the STAT5 inhibitor. Chromatin immunoprecipitation (ChIP) experiments, using GR and STAT5 antibodies, individually, revealed enhanced recruitment of both proteins to the ZnT2 promoter when DEX was added to the AR42J cells (Fig. 4C and D).
ZnT2 Transport Promotes Zinc Efflux from Pancreatic Acinar Cells.
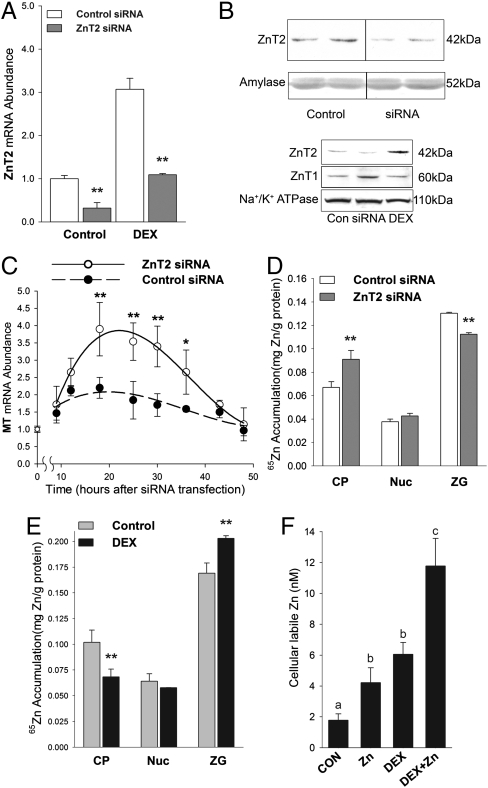
ZnT2 mRNA was effectively knocked down in AR42J cells by using ZnT2 siRNA. With either the presence or absence of DEX, ZnT2 mRNA was knocked down by about 70% at 48 h after ZnT2 siRNA transfection (Fig. 5A). These results show that there was no interaction between the two independent events, i.e. transcription initiation by GR and mRNA degradation by siRNA. Western analysis confirmed the knock down of ZnT2 protein (Fig. 5B). Effects of ZnT2 knockdown on intracellular zinc homeostasis in acinar cells were assessed following siRNA transfection. This knockdown should produce a transient zinc accumulation in the cytoplasm and activation of both ZnT1 and MT gene expression via zinc activation of the transcription factor MTF-1. ZnT2 knockdown increased the abundance of ZnT1 protein (Fig. 5B, Lower Panel). Furthermore, MT mRNA was found to increase with a peak around 24 h posttransfection (Fig. 5C). In agreement with the induction of MT, an increase (36%) in cytoplasmic (65Zn) zinc accumulation was observed with ZnT2 siRNA inhibition (Fig. 5D). Furthermore, 65Zn in the zymogen granules was decreased by 15% by ZnT2 siRNA inhibition. However, no change in 65Zn content was found in the crude nuclear fraction (Fig. 5D), suggesting specificity in cellular accumulation. These findings support the hypothesis that ZnT2 transports cytoplasmic zinc into zymogen granules. The same approach was also used in DEX-treated cells. DEX treatment decreased 65Zn recovered in the cytoplasmic fraction, but increased 65Zn accumulation in the zymogen granules (Fig. 5E). This implies a higher amount of zinc sequestrated in a secretory pathway. Finally intracellular labile zinc levels were measured fluorometrically. The results indicate DEX treatment increased labile zinc 3-fold, whereas treatment with 40 μM zinc increased labile zinc by 2-fold (Fig. 5F). Interestingly zinc and DEX produced an additive effect on labile zinc. These results imply that DEX produces an increase in labile zinc that is available for export from these cells.
Fig. 5.
Zinc trafficking in rat AR42J pancreatic acinar cells is influenced by ZnT2 expression. (A) Cells were transfected with either ZnT2 siRNA or control siRNA and cultured with or without DEX for 48 h. ZnT2 mRNA was measured by qPCR. (B) Western blots showing effect of ZnT2 siRNA inhibition on ZnT2 protein in AR42J cells. Control vs. ZnT2 siRNA treated cells (Upper Panels). Comparative effects of DEX and ZnT2 siRNA on ZnT1 vs. ZnT2 (Lower Panels). (C) Cells were transfected with ZnT2 siRNA and harvested at various times. MT mRNA was measured by qPCR. (D, E) 65Zn retention was used as a direct measure of zinc accumulation. Some cells were transfected with ZnT2 siRNA (D) and some were treated with DEX (E). After 48 h in the presence of 65Zn the cells cytoplasmic (CP), crude nuclear (Nuc), and zymogen granule (ZG) fractions were isolated. 65Zn content was measured and the specific activity was used to calculate zinc retention. (F) Cellular labile zinc was measured using a FluoZin 3-AM fluorescence assay. Cells were treated with zinc (40 μM) or DEX or both for 48 h. Values with a different superscript are significantly different at P < 0.05 or greater. n = 4.
Discussion
Zinc transporter expression in the pancreas is of interest because pancreatic secretions constitute an important component of mammalian zinc homeostasis (8, 9). ZnT1 and ZnT2 are highly expressed in the pancreas and are responsive to the dietary zinc supply (10). Here we demonstrate that ZnT2 is responsive to glucocorticoid hormone whereas ZnT1 is not. In addition, ZnT2 is localized to zymogen granules, whereas ZnT1 is associated with the plasma membrane of acinar cells. These differences in regulation and cellular location suggest differing roles in zinc trafficking by the exocrine pancreas. Specifically, we propose that zinc output from acinar cells follows two distinct pathways: Cell-to-ductal zinc efflux via the apical membrane, which is zinc-dependent and involves primarily ZnT1 for cellular efflux as well as zinc that is released along with digestive proenzymes from zymogen granules, where zinc is transported into the granules by ZnT2.
Because zymogen granules are the storage sites of digestive enzyme precursors (4), our finding that ZnT2 is localized to secretory zymogen granules suggests this transporter may ensure that sufficient zinc is available to maintain maximal activity of these digestive metalloenzymes. A reduction in activity of these enzymes is observed during dietary zinc restriction (25). Of note is that zymogen granules have an acidic intragranule pH (26), which is in line with the notion that ZnT2 favors acidic vesicles to produce its maximal transport activity. Our results with FluoZin-3 suggest that both zinc and DEX increase the intracellular pool of labile zinc in acinar cells. This labile zinc pool may be an important source of zinc that enters the pancreatic secretory pathway. The high MT content of acinar cells likely serves as a reservoir of labile zinc (27).
High zinc consumption has been shown in avian species, mice, and the pig to be detrimental to normal pancreatic exocrine function and produces atrophy (11–13). This sensitivity suggests secretory pathways of zinc loss, are essential for preventing premature pancreatic enzyme release, necrosis and atrophy. These signs of zinc toxicity appear similar to the autodigestion of pancreatitis that have been suggested to be due to abnormal calcium signaling within zymogen granules (28).
ZnT2 was first cloned from a rat kidney cDNA library by complementation and was found to be localized to acidic vesicles and to facilitate zinc sequestration in intracellular compartments (15). The expression of ZnT2 has been found to be regulated by zinc in certain tissues, e.g. pancreas, small intestine, prostate, and blood–brain barrier (10, 29–31). As shown in the current experiments, zinc regulation is conferred by the consensus MRE located at +53 to +59 of the murine ZnT2 gene, which when mutated ablates zinc responsiveness. The dramatic increase in expression of ZnT2 with MTF-1 overexpression strongly supports a MRE/MTF-1 mode of regulation involving this MRE site.
The pancreatic acinar AR42J cell model has been widely used to characterize effects of glucocorticoid hormones on secretory activity of the exocrine pancreas and regulation of the amylase gene (16, 32, 33). DEX treatment of AR42J cells induces a highly differentiated phenotype. The response to DEX is mediated by the GR binding to the GRE sequence located in the promoter of this gene. More recently, cell-specific and cell lineage-specific transcription factors have been shown to interact with the GR, thus modifying glucocorticoid-sensitive expression (20, 21). For the exocrine pancreas, pancreatic acinar transcription factor (PTF1) is a salient example (34). Database analyses of GRE-regulated genes reveal half GRE sites also confer GR interactions that are functional. The absence of either a half or full GRE within the first 1 kb of ZnT2 was perplexing in view of the responsiveness of this gene to DEX in AR42J cells and intact mice. However, ZnT2 does have the PTF-1 element essential for pancreas-specific expression. It was noted (Fig. 3A and B) that induction of ZnT2 was more rapid upon addition of DEX compared to the classical response of amylase suggesting a nontraditional GR-mediated mechanism was involved. The effect of GC on ZnT2 suggests that the up-regulation of ZnT2 by DEX is related to metabolic changes rather than the differentiation process per se, which would be expected to influence both ZnT1 and ZnT2. ZnT2 regulation via the GR was established here by demonstrating inhibition through RU486. This strongly suggests that the signaling pathway involves GR dimerization rather than the antiinflammatory pathway involving assembly of the NF-κB·GR complex. An alternative mechanism, compatible with GR dimerization is interaction of GR with STAT5, where phosphorylated STAT5 provides trans-regulation (20, 21). Two STAT5-response elements (STAT5-RE), with the consensus sequence  , are found upstream of the mZnT2 gene (23). The STAT5 inhibitor used here prevents the dimerization of phosphorylated STAT5 via the SH2 domain (24) and blocked ZnT2 induction by DEX (Fig. 4). Similarly, the JAK2 inhibitor AC490 fully blocked the induction of ZnT2 by DEX. By contrast, neither AG490 nor the STAT5 inhibitor prevented the induction of MT mRNA. This inhibition of the ZnT2 signal transduction pathway is consistent with trans regulation of ZnT2 and cis regulation of MT. Of note, RU486 blocked the induction of both ZnT2 and MT genes by DEX, which is consistent with GR dimerization. Of relevance is that STAT5-GR interactions are responsible for regulation of the β-casein gene by glucocorticoids and prolactin in mammary cells (22). Because both are lactogenic hormones, it is most relevant that ZnT2 has also recently been shown to be regulated by prolactin through a JAK2/STAT5 mechanism in mammary cells (23). The STAT5-RE’s discussed above are also termed gamma interferon activation site elements, particularly in reference to prolactin control of genes uniquely expressed in the mammary gland (35). Furthermore, STAT5-GR interactions have been documented to regulate transcription of glucocorticoid responsive genes in hepatocytes that are responsible for growth (36). To our knowledge, ZnT2 is the only gene expressed in pancreatic acinar cells that has been shown to be regulated by DEX via the STAT5-GR interaction.
, are found upstream of the mZnT2 gene (23). The STAT5 inhibitor used here prevents the dimerization of phosphorylated STAT5 via the SH2 domain (24) and blocked ZnT2 induction by DEX (Fig. 4). Similarly, the JAK2 inhibitor AC490 fully blocked the induction of ZnT2 by DEX. By contrast, neither AG490 nor the STAT5 inhibitor prevented the induction of MT mRNA. This inhibition of the ZnT2 signal transduction pathway is consistent with trans regulation of ZnT2 and cis regulation of MT. Of note, RU486 blocked the induction of both ZnT2 and MT genes by DEX, which is consistent with GR dimerization. Of relevance is that STAT5-GR interactions are responsible for regulation of the β-casein gene by glucocorticoids and prolactin in mammary cells (22). Because both are lactogenic hormones, it is most relevant that ZnT2 has also recently been shown to be regulated by prolactin through a JAK2/STAT5 mechanism in mammary cells (23). The STAT5-RE’s discussed above are also termed gamma interferon activation site elements, particularly in reference to prolactin control of genes uniquely expressed in the mammary gland (35). Furthermore, STAT5-GR interactions have been documented to regulate transcription of glucocorticoid responsive genes in hepatocytes that are responsible for growth (36). To our knowledge, ZnT2 is the only gene expressed in pancreatic acinar cells that has been shown to be regulated by DEX via the STAT5-GR interaction.
In the current experiments we have not evaluated the responsiveness of ZIP5 in pancreatic acinar cells. High ZIP5 mRNA levels have been reported for human total pancreatic RNA. Localization of ZIP5 to the basolateral surface of pancreatic acinar cell is sensitive to zinc levels (37). Zinc availability to pancreatic acinar cells, as produced by factors that control cellular uptake, will influence ZnT1 expression and the zinc responsive component of ZnT2 expression. It will require further studies to define which of these zinc transporters control rates of zinc excretion via the exocrine pancreas.
Studies that could reflect on the glucocorticoid responsive expression of ZnT2 and a role in pancreatic zinc secretion are available. Adrenal insufficiency increases serum zinc concentrations whereas administration of glucocorticoids, corticotropin, and the excess cortisol production in Cushing’s Syndrome decrease these concentrations [reviewed in (38)]. Hypercortisolemia and hypozincemia are associated with chronic alcoholism (39). Excess cortisol could accelerate pancreatic zinc release and produce a systemic zinc deficiency as observed in alcoholism. Radiotracer kinetic studies with humans reveal that carbohydrate-active steroids (glucocorticoids) may alter rate constants of the fecal excretion of zinc (38). Hypozincemia associated with glucocorticoid action has been related to induced synthesis of MT in rodents (40). GR involvement in pancreatic tissue organization and the differentiation of acinar cells, as well as enzyme and zymogen granule production is compatible with a role in ZnT2 regulation. Furthermore, the control zinc provides via MTF-1 responsive ZnT2 expression is consistent with a homeostatic role in endogenous zinc secretion. However, how the correlation of serum zinc and cortisol levels influences pancreatic ZnT2 expression requires further studies. To our knowledge ZnT2 may represent the first member of either the ZnT or Zip families to be glucocorticoid regulated.
Materials and Methods
Acinar Cells.
AR42J cells (rat pancreatoma, ATCC CRL 1492) was purchased from American Type Culture Collection and were maintained at 37 °C in Ham’s F-12K medium (Mediatech) with 0.1 mg/ml L-Glutamine, 15% FBS (Mediatech) and penicillin, streptomycin, and amphotericin B (Sigma). Cells at 0.5 × 106 cells/well were cultured for at least 48 h before treatments. Cells were treated with 100 nM DEX phosphate (Sigma) in culture medium for 48 h for differentiation induction. Some cultures also contained RU486 (33) or CpdA (18), both at 1 μM. Control cultures contained PBS or DMSO at comparable concentrations. In some experiments cells were also treated with a chromone-based STAT5 inhibitor (400 nM), (EMD Biosciences) (24) or a JAK2 inhibitor (50 μM) (AG490) (Thermo Fisher) (20). Protein concentrations were measured spectrophotometrically with Rc Dc reagents (BioRad).
Mice.
Male CD-1 mice, 25–30 g (Charles River) were individually housed and fed a AIN76-based diet (Research Diets), containing 0.85 mg Zn/kg diet or 30 mg Zn/kg diet for 21 d as described previously (10). The procedures with mice were approved by the University of Florida Institutional Animal Care and Use Committee. For details, see SI Methods.
Quantitative Real-Time PCR, Immunoblotting, and Immunofluorescence Analysis.
This analysis has been previously described in refs. 41 and 42. See SI Methods.
ZnT2 siRNA, ChIP, and Luciferase Reporter Assay.
SMART pool siRNA against rat ZnT2 (300 mg) or nontargeting siRNA (Dharmacon) were used to transfect AR42J cells with Hyperfect reagent (Qiagen). ChIP-IT express reagents (Active Motif) were used for ChIP, which followed the two-step cross-linking method with disuccinimidyl glutarate treatment prior to formaldehyde. GR (H300, P-20) and STAT5 (C-17, L-20) antibodies were from Santa Cruz Biotech. Luciferase assay used the Dual-Glo Luciferase System (Promega) following transfection into HeLa and HEK 293 T cells with FuGene HD (Roche). Luminescence from firefly luciferase were normalized to renilla after 24 h incubation with 100 μM ZnSO4. These methods have been described in detail earlier (42, 43). See SI Methods.
Labile Zinc Measurements and 65Zn Kinetics.
Cells in F-12K with 0.3% BSA were treated with N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylenediamine or sodium pyrithione and were incubated with 1 μM FluoZin 3-AM (Invitrogen). After 30 min fluorescence was measured at 535 nm (excitation 485 nm). The labile zinc concentration was calculated (44) using the KD of 15 nM. For 65Zn kinetics, cells were treated with ZnT2 siRNA or DEX for 24 h, then were placed in medium (3 μM Zn) containing 65Zn (0.075 μCi/ml; 10 μCi/nmol; Oak Ridge National Laboratory) for another 24 h. 65Zn content was measured by gamma ray spectrometry (30% counting efficiency) and zinc accumulation was calculated from the specific activity. For efflux measurements ZnT2 transfected cells were allowed to accumulate 65Zn from identical medium as above for 24 h, then after the cells were washed and placed in fresh medium 65Zn release into the medium was measured.
Statistical Analysis.
Results are expressed as mean ± SD from at least 3 independent experiments. Significance was determined by an unpaired 2-tailed Student’s t test or by two-way ANOVA with statistical significance set at P < 0.05. As indicated in each figure *P < 0.05, **P < 0.01.
Supplementary Material
Acknowledgments.
We thank T.B. Aydemir, S.-M. Chang, and M.D. Knutson for helpful discussions and C. Guzman for manuscript preparation. Fig. 6 was prepared by www.AASArts.com. This research was supported by National Institutes of Health Grant DK31127 and Boston Family Endowment Funds of the University of Florida Foundation.
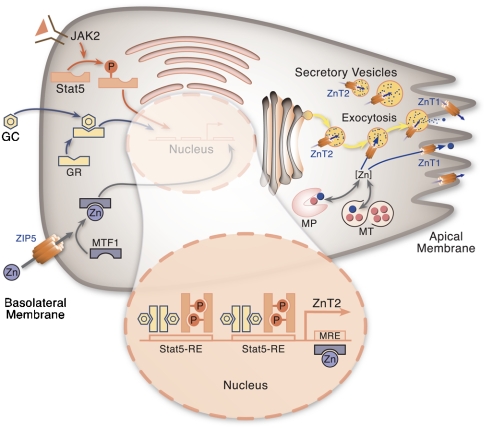
Fig. 6.
Proposed model of zinc transport and secretion in pancreatic acinar cells. Zinc influx is influenced by ZIP5 located at the basolateral plasma membrane. Sources of zinc for export are derived previously from the Golgi as metalloproteins (MP), metallothionein (MT), and the labile intracellular pool [Zn]. Zinc secretion at the apical plasma membrane is regulated through two different pathways. Zinc is transported into the ductal lumen of the pancreatic acinar cells through ZnT1 localized on the apical plasma membrane. Cytosolic zinc is sequestrated into zymogen granules by ZnT2 and is released during regulated exocytosis. Expression of ZnT1 and ZnT2 is mediated by MTF-1, depending on the intracellular zinc level. MT and ZnT2 expression is regulated by GC through the glucocorticoid receptor (GR). ZnT2 expression requires interaction of the GR with STAT5. ZnT2 expression is more likely to be associated with secretory stimulation of digestive enzymes.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914941107/DCSupplemental.
References
- 1.King J, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th Ed. Baltimore: Lippincott Williams and Wilkins; 2005. pp. 271–285. [Google Scholar]
- 2.Lichten LA, Cousins RJ. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 3.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick F, Jamieson FS. Structure–Function Relations in the Pancreatic Acinar Cell. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4th Ed. Vol 2. Burlington, MA: Elsevier Academic; 2006. pp. 1313–1315. [Google Scholar]
- 5.Matsuno S, Miyashita E, Ejiri T, Sato T. Zinc and magnesium output in pancreatic juice after pancreaticoduodenectomy. Tohoku J Exp Med. 1982;136(1):11–22. doi: 10.1620/tjem.136.11. [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Isaji S, Kawarada Y, Hibasami H, Nakashima K. Effect of zinc administration on pancreatic regeneration after 80% pancreatectomy. Pancreas. 1997;14(2):158–165. doi: 10.1097/00006676-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Smith ML, Fariss BL, Jennings PB. Serum zinc levels in sheep with experimental pancreatic abnormalities. Pancreas. 1986;1(1):20–23. doi: 10.1097/00006676-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan JF, Burch RE, Quigley JH, Magee DF. Zinc deficiency and decreased pancreatic secretory response. Am J Physiol. 1974;227(1):105–108. doi: 10.1152/ajplegacy.1974.227.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JF, et al. The zinc content of bile an pancreatic juice in zinc-deficient swine. Proc Soc Exp Biol Med. 1981;166(1):39–43. doi: 10.3181/00379727-166-41021. [DOI] [PubMed] [Google Scholar]
- 10.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101(40):14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Combs GF., Jr Effect of excess dietary zinc on pancreatic exocrine function in the chick. J Nutr. 1988;118(6):681–689. doi: 10.1093/jn/118.6.681. [DOI] [PubMed] [Google Scholar]
- 12.Kazacos EA, Van Vleet JF. Sequential ultrastructural changes of the pancreas in zinc toxicosis in ducklings. Am J Pathol. 1989;134(3):581–595. [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrielson KL, Remillard RL, Huso DL. Zinc toxicity with pancreatic acinar necrosis in piglets receiving total parenteral nutrition. Vet Pathol. 1996;33(6):692–696. doi: 10.1177/030098589603300608. [DOI] [PubMed] [Google Scholar]
- 14.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763(7):711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15(8):1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 16.Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Failla ML, Cousins RJ. Zinc accumulation and metabolism in primary cultures of adult rat liver cells. Regulation by glucocorticoids. Biochim Biophys Acta. 1978;543(3):293–304. doi: 10.1016/0304-4165(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 18.De Bosscher K, et al. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA. 2005;102(44):15827–15832. doi: 10.1073/pnas.0505554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkulov VM, Merkulova TI. Structural variants of glucocorticoid receptor binding sites and different versions of positive glucocorticoid responsive elements: Analysis of GR-TRRD database. J Steroid Biochem Mol Biol. 2009;115(1-2):1–8. doi: 10.1016/j.jsbmb.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Stoecklin E, Wissler M, Moriggl R, Groner B. Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional cooperation in the regulation of gene transcription. Mol Cell Biol. 1997;17(11):6708–6716. doi: 10.1128/mcb.17.11.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: A family affair. Trends Endocrinol Metab. 2008;19(9):331–339. doi: 10.1016/j.tem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner J, et al. Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of beta-casein gene transcription. J Biol Chem. 1997;272(33):20954–20960. doi: 10.1074/jbc.272.33.20954. [DOI] [PubMed] [Google Scholar]
- 23.Qian L, Lopez V, Seo YA, Kelleher SL. Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells. Am J Physiol-Cell Ph. 2009;297(2):C369–377. doi: 10.1152/ajpcell.00589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller J, Sperl B, Reindl W, Kiessling A, Berg T. Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem. 2008;9(5):723–727. doi: 10.1002/cbic.200700701. [DOI] [PubMed] [Google Scholar]
- 25.Hsu JM, Anilane JK, Scanlan DE. Pancreatic carboxypeptidases: Activities in zinc-deficient rats. Science. 1966;153(3738):882–883. doi: 10.1126/science.153.3738.882. [DOI] [PubMed] [Google Scholar]
- 26.De Lisle RC, Williams JA. Zymogen granule acidity is not required for stimulated pancreatic protein secretion. Am J Physiol. 1987;253(6 Pt 1):G711–719. doi: 10.1152/ajpgi.1987.253.6.G711. [DOI] [PubMed] [Google Scholar]
- 27.Krezel A, Maret W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J Am Chem Soc. 2007;129(35):10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- 28.Raraty M, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97(24):13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzi JP, Blanchard RK, Cousins RJ. Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J Nutr. 2001;131(1):46–52. doi: 10.1093/jn/131.1.46. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Elias V, Wong CP, Scrimgeour AG, Ho E. Zinc transporter expression profiles in the rat prostate following alterations in dietary zinc. Biometals. 2010;23:51–58. doi: 10.1007/s10534-009-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobilya DJ, Gauthier NA, Karki S, Olley BJ, Thomas WK. Longitudinal changes in zinc transport kinetics, metallothionein and zinc transporter expression in a blood–brain barrier model in response to a moderately excessive zinc environment. J Nutr Biochem. 2008;19(2):129–137. doi: 10.1016/j.jnutbio.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logsdon CD. Glucocorticoids increase cholecystokinin receptors and amylase secretion in pancreatic acinar AR42J cells. J Biol Chem. 1986;261(5):2096–2101. [PubMed] [Google Scholar]
- 33.Kaiser A, Stier U, Riecken EO, Rosewicz S. Glucocorticoid receptor concentration modulates glucocorticoid-regulated gene expression in rat pancreatic AR42J cells. Digestion. 1996;57(3):149–160. doi: 10.1159/000201329. [DOI] [PubMed] [Google Scholar]
- 34.Cockell M, et al. Binding sites for hepatocyte nuclear factor 3 beta or 3 gamma and pancreas transcription factor 1 are required for efficient expression of the gene encoding pancreatic alpha-amylase. Mol Cell Biol. 1995;15(4):1933–1941. doi: 10.1128/mcb.15.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decker T, Kovarik P, Meinke A. GAS elements: A few nucleotides with a major impact on cytokine-induced gene expression. J Interferon Cytokine Res. 1997;17(3):121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 36.Engblom D, et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 2007;21(10):1157–1162. doi: 10.1101/gad.426007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Kim BE, Petris MJ, Eide DJ. The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem. 2004;279(49):51433–51441. doi: 10.1074/jbc.M408361200. [DOI] [PubMed] [Google Scholar]
- 38.Henkin RI, Foster DM, Aamodt RL, Berman M. Zinc metabolism in adrenal cortical insufficiency: Effects of carbohydrate-active steroids. Metabolism. 1984;33(6):491–501. doi: 10.1016/0026-0495(84)90002-7. [DOI] [PubMed] [Google Scholar]
- 39.Menzano E, Carlen PL. Zinc deficiency and corticosteroids in the pathogenesis of alcoholic brain dysfunction—A review. Alcohol Clin Exp Res. 1994;18(4):895–901. doi: 10.1111/j.1530-0277.1994.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 40.Etzel KR, Shapiro SG, Cousins RJ. Regulation of liver metallothionein and plasma zinc by the glucocorticoid dexamethasone. Biochem Biophys Res Commun. 1979;89(4):1120–1126. doi: 10.1016/0006-291x(79)92124-7. [DOI] [PubMed] [Google Scholar]
- 41.Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukocyte Biol. 2009;86(2):337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liuzzi JP, Guo L, Chang SM, Cousins RJ. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am J Physiol-Gastr L. 2009;296(3):G517–523. doi: 10.1152/ajpgi.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol-Gastr L. 2009;296(4):G860–867. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352(2):222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.