Abstract
Mounting effective T cell responses is critical for eliciting long-lasting immunity following viral infection and vaccination. A multitude of inhibitory and stimulatory factors are induced following infection, and it is the compilation of these signals that quantitatively and qualitatively program the ensuing effector and memory T cell response. In response to lymphocytic choriomeningitis virus (LCMV) infection, the immunosuppressive cytokine IL-10 is rapidly up-regulated; however, how IL-10 is regulating what is often considered an “optimal” immune response is unclear. We demonstrate that IL-10 directly inhibits effector and memory CD4 T cell responses following an acutely resolved viral infection. Blockade of IL-10 enhanced the magnitude and the functional capacity of effector CD4 T cells that translated into increased and more effective memory responses. On the other hand, lack of IL-10 signaling did not impact memory CD8 T cell development. We propose that blockade of IL-10 may be an effective adjuvant to specifically enhance CD4 T cell immunity and protection following vaccination.
Keywords: T cell memory/ T cell programming, vaccination
T cell responses are critical for the clearance of viral infection. Following infection, antigen-presenting cells (APC) present viral antigens to T cells and, in the context of multiple cell-based and soluble factors, instruct virus-specific T cells to proliferate and acquire multiple antiviral and immune-stimulatory effector functions. Virus-specific CD8 T cells acquire the ability to lyse virally infected cells both directly through cell to cell killing and indirectly through the secretion of cytokines, including TNFα and IFNγ (reviewed in ref. 1). Simultaneously, virus-specific CD4 T cells proliferate and produce immunostimulatory cytokines (such as IL-2) that help to orchestrate the direction of the immune response, sustain and program memory CD8 T cell differentiation, and provide help to B cells for antibody production (2). Following clearance of the infection, effector virus-specific CD4 and CD8 T cells contract and, through a complex differentiation program, generate a stable memory T cell population able to rapidly respond and clear future infections with the same or similar virus (3). Early IL-2 signals are important for secondary CD8 T cell responses, and, once formed, T cell memory is sustained via homeostatic signals including the cytokines IL-7 and IL-15 (4, 5). Yet it is still poorly understood how different factors interact to initially instruct distinct immune responses and how the factors and signals that a T cell initially encounters are integrated to program long-term memory differentiation. Thus, the interplay between positive and negative regulatory signals toward memory development is largely unclear.
Viral replication triggers the production of multiple immune stimulatory factors that activate and program T cell responses. As a counterbalance, negative regulatory factors are also produced to control the magnitude of the antiviral immune response and attenuate T cell responses following viral clearance to prevent excessive immunopathology. Many immune and nonimmune cell types produce immunoregulatory factors shaping the immune environment. It is the compilation of these factors that dictates the quantity, quality, and direction of the ensuing T cell response. In addition to the specific factors produced, the amount and ratio of each factor with respect to others also play an important role producing distinct immune responses. Interestingly, it is during these initial APC:T cell interaction that information regarding the extent of T cell proliferation and contraction is largely programmed (3, 6–9). On the other hand, T cell functional responses are constantly being shaped by the current antigenic environment (10), suggesting that manipulating immune-regulatory factors could have the potential to significantly impact the development of T cell immunity and enhanced responses to vaccination.
Recently, the dominant role of negative immune-regulatory factors in suppressing T cell responses during chronic viral infections has been elucidated (11–14). Although not as high, these same factors are often also up-regulated in an acutely cleared viral infection (11–13). We recently demonstrated that increased IL-10 expression during persistent lymphocytic choriomeningitis virus (LCMV) infection leads to the suppression of T cell responses and, consequently, viral persistence. Interestingly, increased levels of IL-10 were also observed following acute LCMV infection, albeit much lower than those induced by persistent infection (12). However, following infection with an acutely cleared virus IL-10 does not impede productive T cell responses nor prevent elimination of infection. Thus, how IL-10 shapes the immune profile during an acute viral infection is unclear. IL-10 can be produced by and signal through many cells of the immune system, including dendritic cells (DC), B cells, macrophages, CD4 T cells, CD8 T cells, and innate and adaptive regulatory T cells (reviewed in refs. 15, 16). IL-10 aborts T cell responses when present during priming and can inhibit ongoing T cell activity (12, 13, 17, 18). IL-10 also acts directly on APC to prevent maturation, decrease stimulatory molecule expression (i.e., MHC class I and class II, B7-1, B7-2), and inhibit T cell activation (15, 18–20). IL-10 is generally suppressive to CD4 T cells, but toward CD8 T cells it can be stimulatory or suppressive depending on the situation (15). In response to bacterial infection, IL-10 was required for optimal CD8 T cell memory development (21), whereas in response to peptide stimulation, removal of IL-10 enhanced primary, but inhibited memory, CD8 T cell responses (22). On the other hand, IL-10 rapidly suppresses CD8 T cell activity during chronic HIV, hepatitis C virus (HCV), and LCMV infections (12, 13, 23–27). Thus, the stimulatory/inhibitory function of IL-10 appears to differ depending on the type of pathogen and the general antigenic environment.
In this study, we demonstrate that IL-10 suppresses optimal CD4 T cell memory development following acute LCMV infection. IL-10 decreases both the size and the functional capacity of effector and memory CD4 T cells and does so by directly acting on the CD4 T cell. In contrast, although initial effector virus-specific CD8 T cell responses were moderately increased in the absence of IL-10 signaling, the quantity and the quality of CD8 T cell memory development was unaffected by IL-10 signaling. Further, antibody blockade of IL-10 at the time of viral infection substantially enhanced memory CD4 T cell development, indicating the potential utility of blocking IL-10 as an adjuvant to vaccination to increase CD4 T cell memory.
Results
Early IL-10 Up-Regulation After LCMV Infection.
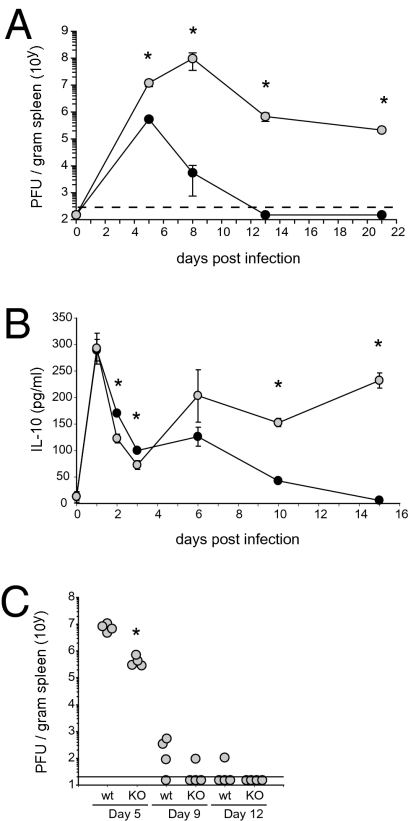
To determine the kinetics of IL-10 expression after viral infection, WT C57BL/6 mice were i.v. infected with LCMV-Armstrong (Arm). Infection with LCMV-Arm induces a robust CD4 and CD8 T cell response that results in viral clearance within 8–12 days (Fig. 1A; ref. 10). For comparison of IL-10 levels to a persistent LCMV infection that induces high levels of IL-10, mice were infected in parallel with LCMV-Clone 13 (LCMV-Cl 13). Infection with LCMV-Cl 13 leads to the rapid loss of T cell function and chronic infection (Fig. 1A; refs. 12, 13). Serum concentrations of IL-10 were similar one day following either LCMV-Arm or -Cl 13 infection and in both cases were dramatically elevated compared to uninfected animals (Fig. 1B), suggesting that the initial up-regulation of IL-10 is an inherent response to virus infection. IL-10 levels rapidly declined between days 2–5 after either LCMV-Arm or -Cl 13 infection. However, consistent with the role of IL-10 in promoting viral persistence, serum IL-10 levels continued to decline following LCMV-Arm infection, whereas they rebounded and remained elevated during persistent infection (Fig. 1B). Following the clearance of LCMV-Arm infection, IL-10 levels remained similar to those observed in uninfected mice through to the termination of the experiment (50 days post infection). Importantly, WT and IL-10−/− mice cleared LCMV-Arm infection in a similar timeframe, although virus clearance was slightly accelerated in the absence of IL-10 expression (Fig. 1C).
Fig. 1.
Virus infection rapidly induces IL-10 production. (A) Virus titers in the serum at the indicated day following i.v. infection with 2 × 106 PFU LCMV-Arm (black circles) or Cl 13 (gray circles). Each circle represents the average virus titer (PFU) ± SD at the indicated day after infection. The dashed line indicates the level of detection of the assay (200 PFU/g spleen). (B) IL-10 protein concentration in the serum of LCMV-Arm or Cl 13 infected mice. (C) Virus titers in the spleen of WT C57BL/6 and IL-10 KO mice after infection with 2 × 106 PFU LCMV-Arm. Each circle represents an individual mouse. Data are representative of four to five mice per group and two independent experiments.
IL-10 Suppresses CD4 but Not CD8 T Cells After an Acute Viral Infection.
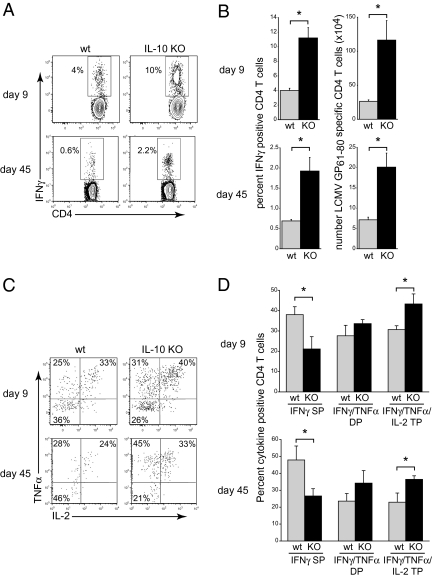
Based on the strong suppressive effect of IL-10 during persistent LCMV infection (12, 13), we used IL-10−/− mice to determine if the increased IL-10 expression after acute LCMV infection also exerted a suppressive effect on antiviral T cell responses. LCMV-Arm infection of WT C57BL/6 mice rapidly induced a robust and multifunctional CD4 T cell response (Fig. 2 A and B). Infection in the absence of IL-10 suppression resulted in a threefold increase in the frequency and a fivefold increase in the number of IFNγ producing virus-specific CD4 T cells (day 9; Fig. 2 A and B). In addition to suppressing CD4 T cell expansion, IL-10 signaling also decreased virus-specific CD4 T cell functional capacity. LCMV-Arm infection stimulated a higher percentage of IFNγ/TNFα and IFNγ/TNFα/IL-2 polyfunctional CD4 T cells 9 days after infection of IL-10−/− compared to WT mice (Fig. 2 C and D). The increased polyfunctionality of the cells translated into a significant decrease in the frequency of virus-specific CD4 T cells that solely produced IFNγ in response to stimulation (Fig. 2 C and D), suggesting that IL-10 signaling to some extent limits effector CD4 T cells differentiation into TNFα or IL-2 producing cells.
Fig. 2.
Enhanced CD4 T cell responses in IL-10 deficient mice. (A) WT or IL-10 KO mice were infected iv with 2 × 106 PFU LCMV-Arm, and IFNγ producing LCMV-GP61–80 specific CD4 T cell responses were quantified in the spleen on days 9 and 45 after infection. (B) Bar graphs indicate the average ± SD frequency and number of IFNγ producing LCMV-GP61–80 specific CD4 T cells on day 9 (Upper) and day 45 (Lower) after LCMV-Arm infection of WT (gray bars) or IL-10 KO (black bars) mice. (C) Flow plots illustrate TNFα and IL-2 expression by IFNγ+ LCMV-GP61–80 specific CD4 T cells on days 9 and 45 after LCMV-Arm infection. Note, cells in the Lower Left quadrant are IFNγ single positive (SP). (D) Bar graphs indicate the average ± SD frequency of IFNγ SP, IFNγ/TNFα double positive (DP), and IFNγ/TNFα/IL-2 triple positive (TP) LCMV-GP61–80 specific CD4 T cells. Data are representative of three to five mice per group and three independent experiments. *P < 0.05.
To establish whether the enhanced effector T cell responses observed in IL-10 deficient mice affected CD4 T cell memory formation, T cell responses were quantified 45 days following LCMV-Arm infection of WT or IL-10−/− mice. Memory virus-specific CD4 T cell responses developed in WT mice; however, infection in the absence of IL-10 signaling led to a threefold increase in both the percentage and the number of IFNγ-producing memory CD4 T cells compared to WT mice (Fig. 2 A and B). When compared, the level of contraction between the effector and memory responses were similar in WT and IL-10−/− mice (fourfold decrease in the number of IFNγ+ CD4 T cells in WT mice versus sixfold decrease in IL-10−/− mice), suggesting that consistent with the loss of IL-10 expression as infection is cleared (Fig. 1), the affect of IL-10 was during the initial phases of infection.
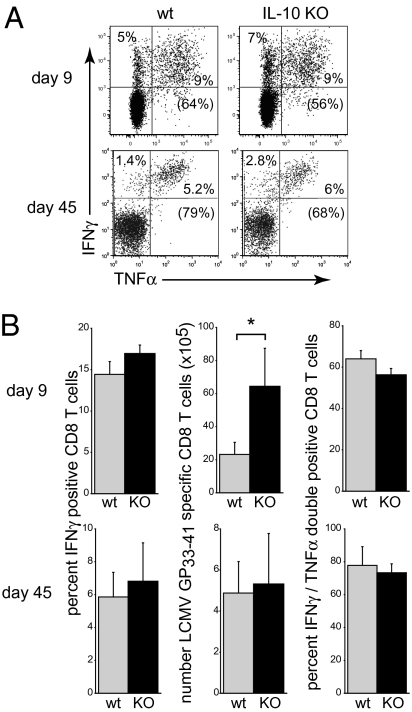
The finding that IL-10 suppressed CD4 T cell responses during acute LCMV infection suggested that it might similarly limit the full potential of CD8 T cell responses. Lack of IL-10 expression at day 9 following LCMV-Arm infection did not affect the frequency of IFNγ or IFNγ/TNFα double producing CD8 T cells, but, due to an increased amount of both CD8 T cells and splenocytes, IL-10 deficiency led to a threefold increase in the number of IFNγ producing CD8 T cells compared to WT mice (Fig. 3 A and B). Unlike CD4 T cells, virus-specific CD8 T cell memory responses were similar in WT and IL-10−/− mice, and the initial increase in the number of virus-specific CD8 T cells was not sustained into the memory phase (Fig. 3 A and B). Similarly, IL-10 expression did not increase the immunodominant LCMV- NP396–404- or subdominant GP276–286-specific CD8 T cell memory responses. Thus, IL-10 substantially limits both the quantity and the quality of virus-specific CD4 T cell response following acute LCMV infection although having no long-term impact on virus-specific CD8 T cells.
Fig. 3.
IL-10 minimally impacts virus-specific CD8 T cell responses. (A) The frequency of IFNγ and TNFα producing LCMV-GP33–41 specific CD8 T cells was quantified in the spleen of LCMV-Arm infected WT or IL-10 KO mice on days 9 and 45 after infection. Numbers in parentheses indicate the percentage of IFNγ and TNFα double-positive CD8 T cells. (B) Bar graphs indicate the average frequency and number (±SD) of IFNγ producing and IFNγ/TNFα double positive CD8 T cells. Data are representative of four mice per group and two independent experiments. *P < 0.05.
IL-10 Directly Inhibits Virus-Specific CD4 T Cells.
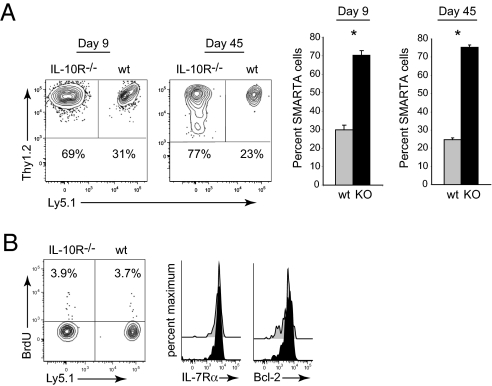
IL-10 acts on many of the cells in the immune system to modulate their function, which in turn could then indirectly affect CD4 T cell responses. To determine if the suppressive effect of IL-10 following acute infection was through direct targeting of the virus-specific CD4 T cells, we compared the responses of IL-10R+/+ and IL-10R−/− virus-specific CD4 T cells. To specifically analyze virus-specific CD4 T cells, we used T cell receptor transgenic, LCMV-specific CD4 (SMARTA) T cells. SMARTA cells recognize the immunodominant, I-Ab restricted LCMV-GP61–80 epitope (28). One thousand IL-10R+/+ WT SMARTA cells (Thy1.2+, Ly5.1+, Thy1.1−) and 1,000 IL-10R−/− SMARTA cells (Thy1.2+, Ly5.1−, Thy1.1−) were cotransferred into WT Thy1.1 congenic C57BL/6 hosts (Thy1.2−, Ly5.1−, Thy1.1+) that were then subsequently infected with LCMV-Arm. We avoided the problems associated with using large, nonphysiologic numbers of transferred transgenic T cells (29) by cotransferring a total of only 2,000 CD4 T cells. We and other groups have shown that SMARTA T cells behave similarly to their endogenous (i.e., host-derived) T cell counterparts, based on tetramer analysis and intracellular cytokine staining (30, 31). Similar to IL-10−/− mice, within 9 days following infection, IL-10R−/− SMARTA cells expanded to higher levels compared to WT SMARTA cells comprising 75% of the total SMARTA cell population (Fig. 4A). The 3:1 ratio of IL-10R−/− to WT IL-10R+/+ SMARTA cells was maintained into the memory phase (Fig. 4A), further indicating that the suppressive effect of IL-10 on CD4 T cells was during effector differentiation. IL-10+/+ and IL-10−/− SMARTA cells exhibited similar levels of homeostatic proliferation and phenotypically expressed high levels of memory associated proteins IL-7Rα and Bcl-2 (Fig. 4B), indicating that aside from increased amounts of cells, memory CD4 T cell differentiation does not appear altered in the absence of IL-10 signals.
Fig. 4.
IL-10 directly acts on virus-specific CD4 T cells. (A) Flow plots illustrate the frequency of WT (Thy1.2+, Ly5.1+) and IL-10R−/− (Thy1.2+, Ly5.1−) SMARTA on days 9 and 45 after LCMV-Arm infection. Bar graphs summarize the average ± SD frequency of WT (gray) and IL-10R KO (black) SMARTA cells. (B) Flow plots illustrate the frequency of WT and IL-10R KO SMARTA cells that have incorporated BrdU and histograms show the expression of IL-7Rα and Bcl-2 in WT (gray) and IL-10R KO (black) SMARTA cells on day 45 after infection. Data are representative of four mice per group and one to two experiments. *P < 0.05.
Antibody Blockade of IL-10 Enhances Virus-Specific CD4 T Cell Responses.
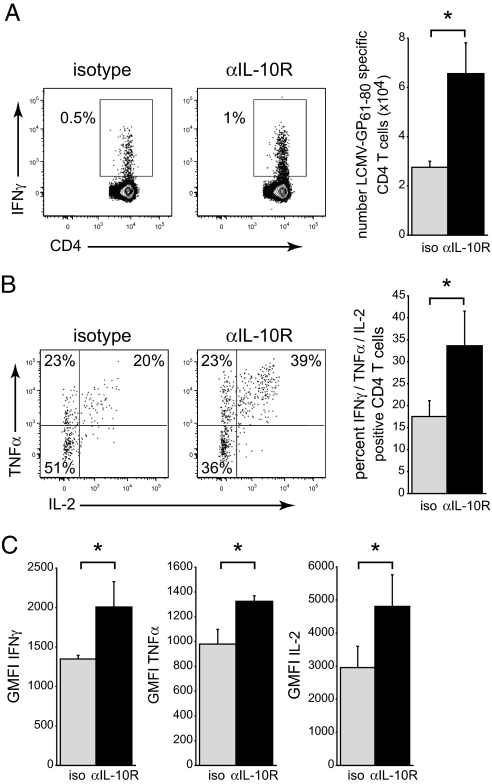
We next sought to determine the therapeutic potential of blocking IL-10 to enhance CD4 T cell immunity. The observation that IL-10 functions during the initial phase of CD4 T cell expansion suggests that therapeutic strategies to block IL-10 at this time point could induce a long-term increase in memory CD4 T cell development. We have previously established that antibody blockade of the IL-10R prevents the loss of T cell function associated with persistent LCMV infection, indicating the efficacy of IL-10R blockade to augment T cell function in vivo (12). C57BL/6 mice were either treated with an isotype control antibody or an anti-IL-10R blocking antibody before (day 0) and after (day 5) LCMV-Arm infection. Memory CD4 T cell responses were quantified 45–50 days after infection. Treatment with IL-10R blocking antibody resulted in a twofold increase in the frequency of IFNγ-producing CD4 T cells compared to isotype treated control mice and a 2.5-fold increase in the number of virus-specific CD4 T cells (Fig. 5A). Consistent with IL-10 working directly on CD4 T cells to suppress their accumulation, neither isotype nor anti-IL-10R antibody treatment affected the total number of splenocytes (2.3 × 107 ± 5 × 106 for isotype control spleen versus 2.7 × 107 ± 2.4 × 106 for anti-IL-10R treated mice) or the frequency of CD4 or CD8 T cells. Further, IL-10R blockade did not affect the frequency or number of LCMV-specific IFNγ-positive or IFNγ/TNFα double positive CD8 T cells. In addition to increasing the magnitude of the virus-specific CD4 T cell response, IL-10R blockade also enhanced CD4 T cell functional capacity. Following restimulation ex vivo, a higher proportion of virus-specific CD4 T cells from mice initially treated with IL-10R blocking antibody produced both TNFα and IL-2 compared to isotype treatment (Fig. 5B). In addition to the increased frequency of cytokine producing CD4 T cells, the level of IFNγ, TNFα, and IL-2 produced per cell was increased (based on geometric mean fluorescence intensity; Fig. 5C), indicating that IL-10 blockade increased the long-term functional quantity and the quality of the CD4 T cell memory compartment after viral infection.
Fig. 5.
IL-10R antibody blockade increases the quantity and quality of CD4 T cell memory responses. (A) WT C57BL/6 mice were treated with isotype or anti-IL-10R blocking antibody on days 0 and 5 after LCMV-Arm infection and virus-specific CD4 T cell responses quantified on day 45 after infection. Numbers in each flow plot represent the frequency and bar graphs the number of IFNγ-producing memory LCMV-GP61–80 specific CD4 T cells. (B) Flow plots illustrate TNFα and IL-2 expression by IFNγ+ LCMV-GP61–80 specific CD4 T cells on day 45 after LCMV-Arm infection. Cells in the lower left quadrant are IFNγ SP. The bar graph indicates the percent of LCMV-GP61–80 specific CD4 T cells that simultaneously produce IFNγ, TNFα, and IL-2. (C) Geometric mean fluorescence intensity (GMFI) of IFNγ, TNFα, and IL-2 production by LCMV-GP61–80 specific CD4 T cells in isotype and anti-IL-10R treated mice. Bar graphs indicate the average ± SD of four to five mice per group and two independent experiments. *P < 0.05.
Discussion
How the multitude of regulatory factors individually and in conjunction augment immune responses to infection in vivo remains unclear. Herein, we demonstrate that the immunosuppressive cytokine IL-10 directly limits the quantity and the quality of CD4 T cell responses following an acutely cleared LCMV infection. IL-10 expression is rapidly increased following an acute virus infection and initially is similar to that observed in persistent LCMV-Cl 13 infection. However, IL-10 levels rapidly decrease following LCMV-Arm infection in conjunction with virus clearance. In contrast, during persistent LCMV infection, following a dip on days 2–5, IL-10 levels again rise and remain high. It is still unclear whether decreasing IL-10 expression during LCMV-Arm infection facilitates sustained antiviral T cell responses or whether the control of virus replication decreases the signals that sustain IL-10 production. However, a similar relationship was recently observed during HIV infection wherein the level of HIV replication directly correlated with IL-10 expression (27), indicating a conserved relationship between the extent of virus replication and IL-10 expression. Thus, it seems likely that in response to the level of virus replication undefined immune “sensors” respond by inducing the expression of the negative regulatory factor IL-10. As infection is resolved, expression of IL-10 decreases, facilitating immune-mediated viral clearance, whereas progressively enhanced levels of virus replication trigger increased/sustained levels of IL-10 expression, leading to immune exhaustion and viral persistence. It is interesting that IL-10 levels initially rise and then decrease in a similar fashion following either LCMV-Arm or -Cl 13 infection. The reason for the resurgence in IL-10 production is unclear, but could result from increased infection levels or the onset of new IL-10 producing cell types. Temporally, the increase in IL-10 expression at day 5 following LCMV-Cl 13 infection coincides with the onset of robust antiviral T cell responses (10). Thus, the resurgence in IL-10 may be a result of factors produced by virus-specific T cells or be due to the interactions between virus-specific T cells and other IL-10 producing cells. It will ultimately be important to define these immune “sensors” and the factors and mechanisms that regulate IL-10 expression following an acute and a persistent virus infection.
In the absence of the suppression mediated by IL-10, virus-specific CD4 T cells may become more susceptible to positive signals (e.g., IFNγ) (32) without negative restriction resulting in an increased number of effector cells. The finding that early antibody blockade of IL-10 generates enhanced memory CD4 T cell responses suggests that the effect of IL-10 is early following viral infection. In addition to directly affecting virus-specific CD4 T cells, IL-10 may also enhance CD4 T cell responses indirectly by targeting and affecting the stimulatory capacity of other cell types. The direct impact of IL-10 on CD4 T cells is further substantiated using transgenic virus-specific CD4 T cells in which there is an early increase in the amount of IL-10R−/− SMARTA cells compared to WT IL-10R+/+ SMARTA cells that is then sustained at the same ratio into the memory phase. Conversely, the effect does not appear due to increased T cell contraction following LCMV-Arm clearance (considering that the 3:1 ratio observed in the effector phase is sustained in the memory phase), homeostatic proliferation, IL-7R expression, or the expression of the anti-apoptotic factor Bcl-2. Accelerated virus clearance is likely not the reason for the enhanced CD4 T cell responses in IL-10 KO mice considering IL-10R−/− SMARTA cells similarly expand more than WT IL-10R+/+ SMARTA cells following cotransfer into WT mice wherein both populations are exposed to the same levels of virus replication. Instead, the initial increase in the amount of virus-specific CD4 T cells in the absence of direct IL-10 signaling leads a greater memory response. The same is not observed for virus-specific CD8 T cells. Although there is an initial increase in the number of virus-specific CD8 T cells following infection in IL-10−/− mice compared to WT mice, the levels rapidly equalize as the cells contract and transition into memory. Importantly, we now demonstrate that IL-10 deferentially regulates CD4 and CD8 T cell responses to virus infection.
There is a critical need for effective vaccine strategies to prevent viral infections. Although there has clearly been some success to prevent infection with persisting viruses (i.e., hepatitis B virus, papilloma virus), vaccination as a whole has been unsuccessful in preventing persistent viral infections, including HIV, HCV, EBV and CMV. Current evidence suggests that numerically larger and multifunctional CD4 and CD8 T cell responses are associated with enhanced control of HIV and HCV replication and prevention of HCV persistence (33–37), suggesting that the efficacy of vaccination is dependent on the size and particularly the quality of the T cell responses it elicits. Thus, adjuvants and vaccines that quantitatively and qualitatively enhance immunity might be able to overcome the immune threshold that dictates viral clearance or persistence. The immune system invokes stimulatory and suppressive mechanisms following pathogen encounter to generate an effective immune response, while simultaneously limiting immunopathology and autoimmunity. We now demonstrate that up-regulation of IL-10 impacts the generation of T cell immunity even during an acutely cleared (i.e., “optimal”) immune response and that blockade of IL-10 increases CD4 T cell immunity. This may be particularly important in the design of adjuvants for vaccination. The importance of the CD4 T cell response in preventing HCV persistence (37–41) and sustaining antiviral immunity to control persistent viral infection (42–46) suggests vaccines that specifically enhance CD4 T cell memory may be beneficial. CD4 T cells are critical orchestrators of multiple facets of the ensuing immune response. Most notably, CD4 T cell help is required for the programming of CD8 T cell memory to sustain CD8 T cell responses in the face of prolonged virus encounter and are important to stimulate B cell differentiation and the production of antibodies that further prevent and limit viral replication. Thus, once the factors that impact different components of the immune response are identified, it may be possible to institute vaccines with multiple adjuvants that specifically enhance (or decrease) defined immune reactions to eliminate a particular pathogen although limiting undesired immune responses. Such “designer” vaccines will be dependent on a better understanding of how distinct factors regulate individual immune components. Our data now provide evidence that IL-10 blockade may be useful in situations where robust CD4 T cell responses, in conjunction with other cells of the immune system, are required to prevent or clear viral infection. Ultimately, resolution of the factors that both stimulate and inhibit optimal T cell responses will lead to the development of better adjuvants and vaccines to elicit T cell responses and potentially prevent infections currently resistant to vaccination.
Materials and Methods
Mice and Virus.
C57BL/6 mice were from the Rodent Breeding Colony at the Scripps Research Institute or purchased from the Jackson Laboratory. IL-10 knockout, congenic Thy1.1+ C57BL/6, and congenic Ly5.1+ C57BL/6 mice were initially obtained from the Jackson Laboratory. The LCMV-GP61–80-specific CD4+ TcR transgenic (SMARTA) mice have been described previously (28). IL-10R2 (CRF2-4) knockout mice were kindly provided by Dr. Paul Allen at Washington University, St. Louis, MO. All mice were housed under specific pathogen-free conditions. Mouse handling conformed to the requirements of the National Institutes of Health, The Scripps Research Institute Animal Research Committee, and the University of California, Los Angeles Animal Research Committee guidelines. Mice were infected i.v. with 2 × 106 plaque forming units (PFU) of LCMV-Arm or LCMV-Cl 13. Virus stocks were prepared and viral titers were quantified as described (47).
IL-10 ELISA.
IL-10 protein was detected in the serum using the Quanitikine Mouse IL-10 Immunoassay Kit (R & D Systems). Serum was diluted fourfold using the kit reagents and used directly in the assay. To establish the change in IL-10 amounts following infection, the average IL-10 ELISA value from uninfected mice was subtracted from individual infected mice. The lower limit of detection was 15.6 pg/mL based on serial dilution of known concentrations of IL-10 protein (provided with the kit).
T Cell Isolation and Transfer.
CD4 T cells were purified from the spleens of naïve SMARTA mice by negative selection (StemCell Technologies). One thousand purified WT (Ly5.1+) and IL-10R KO (Thy1.2+) SMARTA cells were then cotransferred i.v. into Ly5.1−, Ly5.2+, Thy1.1+, and Thy1.2− C57BL/6 mice. Mice were infected 1 day after cell transfer.
Intracellular Cytokine Analysis and Flow Cytometry.
Splenocytes were stimulated for 5 h with 5 μg/mL of the MHC class II restricted LCMV-GP61–80 or 2 μg/mL of the MHC class I restricted LCMV-NP396–404, GP33–41, or GP276–286 peptide (all >99% pure; Synpep) in the presence of 50 U/mL recombinant murine IL-2 (R&D Systems) and 1 mg/mL brefeldin A (Sigma). Cells were stained for surface expression of CD4 (PE, APC, or Pacific Blue; clone RM4-5), CD8 (Pacific Blue; clone 53–6.7), IL-7Rα (APC; clone SB/199), Ly5.1 (APC or APC-Cy7; clone A20), or Thy1.2 (PE or PerCP; clone 30-H12). Cells were fixed, permeabilized, and stained with antibodies to TNFα (FITC; clone MP6-XT22), IFNγ (PE or APC; clone XMG1.2), IL-2 (PE or APC; clone JES6-5H4), and Bcl-2 (PE, 3F11). Flow cytometric analysis was performed using the Digital LSR II (Becton Dickinson), and data were analyzed using FlowJo Software (Treestar). The absolute number of virus-specific T cells was determined by multiplying the frequency of IFNγ+ cells by the total number of cells in the spleen.
To measure homeostatic proliferation, mice were treated on day 38 after LCMV-Arm infection with an i.p. injection of 0.8 mg BrdU (Sigma) followed by continuous administration of BrdU in their drinking water (0.8 mg/mL) for 7 days. The water and BrdU were changed daily. BrdU analysis was performed on day 45 postinfection (1 day after the cessation of BrdU treatment). Splenocytes were stained for BrdU incorporation using the manufacturer’s protocol (BD Pharmingen).
In Vivo IL-10R Specific Antibody Treatment.
C57BL/6 mice received 500 μg/mouse/injection i.p. of anti-IL-10R1 specific antibody (clone 1B1.3a, provided by Schering-Plough) or 500 μg/mouse/injection i.p. of rat IgG1 isotype control antibody [clone KM1.GL113 (anti-E. coli β-galactosidase), provided by Schering-Plough] on day 0 and day 5 after infection.
Statistical Analysis.
Student’s t tests and Mann-Whitney rank-sum tests were performed using SigmaStat 2.0 software (Systat Software Inc.).
Acknowledgments
This is Publication 20375 from the Department of Immunology and Microbial Science, The Scripps Research Institute, La Jolla, CA. Our work was supported by National Institutes of Health Grants AI077012, AI082975, and AI085043 (to D.G.B.), AI009484, and AI045927 (to M.B.A.O).
Footnotes
The authors declare no conflict of interest.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 9.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 10.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 16.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–569. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 17.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 19.Carbonneil C, Donkova-Petrini V, Aouba A, Weiss L. Defective dendritic cell function in HIV-infected patients receiving effective highly active antiretroviral therapy: Neutralization of IL-10 production and depletion of CD4+CD25+ T cells restore high levels of HIV-specific CD4+ T cell responses induced by dendritic cells generated in the presence of IFN-alpha. J Immunol. 2004;172:7832–7840. doi: 10.4049/jimmunol.172.12.7832. [DOI] [PubMed] [Google Scholar]
- 20.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 21.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 22.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174:5382–5389. doi: 10.4049/jimmunol.174.9.5382. [DOI] [PubMed] [Google Scholar]
- 23.Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: Pre- and posttreatment with interferon alfa. Hepatology. 1996;24:6–9. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 24.Clerici M, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landay AL, et al. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: Effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996;173:1085–1091. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- 26.Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor—a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Brockman MA, et al. IL-10 is upregulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: Effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmire JK, Benning N, Whitton JL. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 32.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 33.Folgori A, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 34.Folgori A, et al. Acute Hepatitis C Italian Study Group. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg ES, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Thimme R, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlach JT, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 39.Day CL, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grakoui A, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 41.Urbani S, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 42.Battegay M, et al. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fröhlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 45.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: Immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]