Abstract
Sequentially along B cell differentiation, the different classes of membrane Ig heavy chains associate with the Igα/Igβ heterodimer within the B cell receptor (BCR). Whether each Ig class conveys specific signals adapted to the corresponding differentiation stage remains debated. We investigated the impact of the forced expression of an IgA-class receptor throughout murine B cell differentiation by knocking in the human Cα Ig gene in place of the Sμ region. Despite expression of a functional BCR, homozygous mutant mice showed a partial developmental blockade at the pro-B/pre-BI and large pre-BII cell stages, with decreased numbers of small pre-BII cells. Beyond this stage, peripheral B cell compartments of reduced size developed and allowed specific antibody responses, whereas mature cells showed constitutive activation and a strong commitment to plasma cell differentiation. Secreted IgA correctly assembled into polymers, associated with the murine J chain, and was transported into secretions. In heterozygous mutants, cells expressing the IgA allele competed poorly with those expressing IgM from the wild-type allele and were almost undetectable among peripheral B lymphocytes, notably in gut-associated lymphoid tissues. Our data indicate that the IgM BCR is more efficient in driving early B cell education and in mucosal site targeting, whereas the IgA BCR appears particularly suited to promoting activation and differentiation of effector plasma cells.
Keywords: B cell receptor, differentiation, immunoglobulin A
IgA is the most abundantly synthesized immunoglobulin in mammals and plays a key role at mucosal surfaces (1). IgA plasma cells differentiate from lymphocytes expressing membrane IgA (mIgA), with α heavy chains (HC) featuring a highly conserved membrane-anchoring domain and an intracellular tail of unknown function (2–5). IgM-to-IgA switching and IgA plasma cell differentiation are modulated by interactions with T cells and by multiple soluble factors (6).
Although all membrane Ig associate with the disulfide-linked Igα/Igβ (or CD79a/CD79b) complex to constitute the B cell receptor (BCR), signaling from the BCR was mostly studied for IgM (7, 8). The membrane μ HC has a dual role by both providing differentiation signals during early development and activation signals during peripheral antigen-dependent maturation. Replacement of μ by δ HC expression only results in delayed affinity maturation (9), as for mice lacking the secreted form of IgM (10). Surface γ1 HC (or μ HC with a γ1 membrane-anchoring/cytoplasmic domain) can also substitute for mIgM and support B cell development (11). The mIgG cytoplasmic tail increases extrafollicular antibody-secreting cell (ASC) formation in transgenics and was shown to mediate increased BCR signal due to specific recruitment of the Grb2 adaptor (12, 13). In addition, transfection experiments showed mIgG to be less sensitive than mIgM to CD22-mediated feedback (12, 14), a feature not observed in knock-in models in which Cμ was replaced by Cγ1 (15, 16). To date, little is known regarding a role for mIgA in conferring specific properties to memory mIgA+ cells by comparison with naïve mIgM+ cells. Cross-linking of mIgA raises intracellular calcium concentrations so that mIgA+ B cells residing in mucosa-associated lymphoid tissues (MALT) mediate IgA responses to local immunization (17, 18). Questions about a role of mIgA in B cell development arose from observations of the leakiness of the μMT mutation removing Cμ membrane exons and initially reported to block B cell development (19, 20). This mutation led to the accumulation of IgA ASCs in MALT, in the absence of mIgM/mIgD. It suggested that B cell progenitors can undergo class switch recombination (CSR), express mIgA, and then differentiate into ASCs (19, 20). In humans, early CSR to IgA has also been reported in B cell progenitors (21), but the efficiency and physiological relevance of this potential alternate pathway are unknown.
We thus intended to check whether, similarly to δ and γ1 HC, α HC would be able to substitute for μ and support lymphopoiesis, and whether it would modify B cell fate and homing. We generated mice in which the first gene downstream of joining genes (JH) is a knock-in human Cα1 (α1KI mice). Using a human Cα allowed us to tag the mutated allele and follow its expression. Because membrane-anchoring and cytoplasmic domains of human and mouse IgA are 85% identical (four conservative replacements and two mismatches), they are expected to provide similar signals in a context where association with transducing modules is known to rely on mIg transmembrane segments (22).
Homozygous mutants expressing α Ig HC instead of μ/δ showed how an α-class receptor can support the functions mediated by membrane μ HC, both in B cell progenitors and during antigen-driven B cell maturation in peripheral lymphoid organs.
Results
Expression of the α1KI IgH Allele.
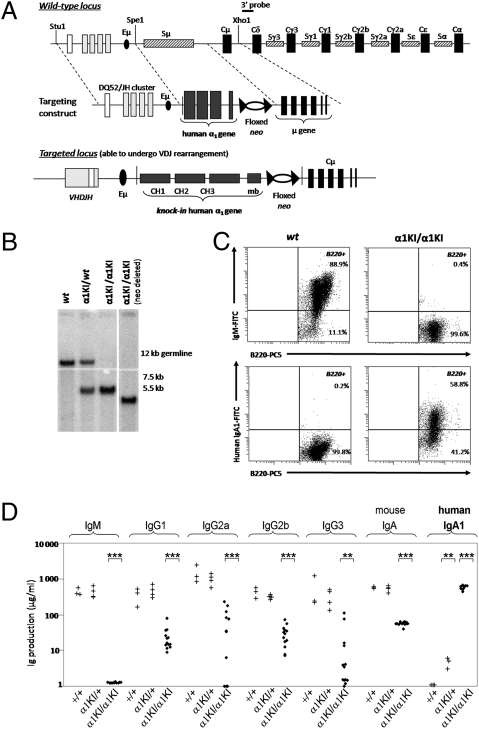
To force expression of mIgA throughout B cell differentiation, a gene encoding the secreted and membrane forms of human α1 HC was inserted into the Iμ-CH intron replacing Sμ (Fig. 1A). A neor cassette flanked by loxP sites was inserted downstream of the α gene and removed by mating with Cre transgenic mice (Fig. 1B). Northern blots comparing mutant to wild-type (WT) B cells confirmed that the amount of Ig HC transcripts was not altered by the α1KI mutation (Fig. S1A).
Fig. 1.
IgA1 expression instead of IgM in α1KI mice. (A) (Top) Structure of the targeted locus (not to scale) showing an unrearranged IgH locus and the extent of the deletion within the Iμ-Cμ intron. (Middle) Structure of the targeting vector in which Cα1 and a neor cassette flanked by loxP sites were inserted in place of Sμ. (Bottom) The α1KI locus is able to undergo V(D)J rearrangement after insertion of Cα1, whereas the neo gene can be deleted by cre-mediated recombination. (B) Southern blot analysis of tail DNA from representative WT, heterozygous, homozygous, and neo-deleted mutant mice. (C) Splenocytes from WT (Left) or α1KI/α1KI mice (Right) were labeled with PC5-conjugated anti-B220 and with either FITC-conjugated anti-IgM Abs showing the blockade of the IgM BCR expression in mutant animals (Top) or FITC-conjugated anti-human IgA1 Abs (Bottom). (D) Total endogenous Ig production was estimated by ELISA in sera from 6-week-old WT, α1KI/+, or α1KI/α1KI mice. Asterisks mark statistically significant differences with controls (Student’s t test, **P < 0.01; ***P < 0.001). The vertical axis is logarithmic; values are indicated as μg/mL.
Mutant mice were first analyzed by flow cytometry to test expression of α1 HC. In heterozygous α1KI/+ mice, mIgA1+ cells were barely detected in lymphoid organs (representing less than 1% of B220+ cells), thus indicating that during early maturation, cells expressing IgM from the WT allele outcompeted those cells expressing IgA1 from the α1KI allele. By contrast, homozygous mutant mice lacked surface IgM and IgD expression (Fig. S1B) and rather expressed mIgA1 on B220+ cells additionally displaying a strong reduction in number (Table 1, Fig. 1C, and Fig. S1C).
Table 1.
Relative values of the various lymphoid populations in bone marrow and spleen
| n | WT | α1KI/α1KI | |
| Bone marrow | |||
| B cells (B220+) | 4/8 | 30.75 ± 4.04% (6.41 ± 2.95) | 12.26 ± 1.89%*** (2.15 ± 0.66) |
| Gated on B cells (B220+) | |||
| Pro-B cells (CD117+B220+) | 3/7 | 7.87 ± 2.25% (0.39 ± 0.21) | 24.88 ± 3.26%* (0.44 ± 0.16) |
| Pro-B/pre-B cells (CD43+B220+) | 4/8 | 33.90 ± 4.57% (2.33 ± 1.24) | 77.75 ± 3.32%* (1.55 ± 0.38) |
| Pre-B cells (CD25+B220+) | 4/8 | 36.94 ± 3.85% (2.16 ± 0.8) | 11.13 ± 1.73%*** (0.3 ± 0.15**) |
| Immature B cells (B220lowIgMlow) | 3/7 | 11.40 ± 3.59% (0.48 ± 0.2) | ND |
| Recirculating B cells (B220highIgMhigh) | 3/7 | 4.95 ± 1.85% (0.17 ± 0.06) | ND |
| Spleen | |||
| Macrophages (F480+CD11b+CD19−) | 3/5 | 3.52 ± 0.50% (2.20 ± 0.57) | 6.44 ± 0.71%* (2.66 ± 0.47) |
| NK (DX5+CD3−CD19−) | 3/5 | 3.91 ± 0.12% (2.36 ± 0.40) | 4.50 ± 0.52% (1.76 ± 0.18) |
| NKT (DX5+CD3+CD19−) | 3/5 | 1.05 ± 0.05% (0.64 ± 0.11) | 2.47 ± 0.36%* (1.03 ± 0.20) |
| T cells (DX5−CD3+CD19−) | 3/5 | 39.81 ± 2.49% (23.77 ± 3.45) | 48.94 ± 3.71% (19.90 ± 2.65) |
| B220+ cells | 7/7 | 34.07 ± 0.87% (22.17 ± 2.32) | 7.29 ± 1.08%*** (2.16 ± 0.47***) |
| Plasma cells CD138+ K intra | 7/7 | 0.50 ± 0.07% (0.31 ± 0.03) | 1.06 ± 0.23%* (0.34 ± 0.08) |
| Gated on B220+ cells | |||
| Resting B cells (IgD+IgM+B220+) | 7/7 | 90.66 ± 0.86% (20.15 ± 2.14) | ND |
| Transitional B cells (AA4.1+B220+) | 3/3 | 12.37 ± 2.6% (2.98 ± 0.75) | 12.97 ± 1.78% (0.26 ± 0.13*) |
| FO B cells (CD23+CD21+B220+) | 7/7 | 67.27 ± 3.72% (15.12 ± 1.92) | 30.31 ± 4.74%*** (0.66 ± 0.21***) |
| MZ B cells (CD23−/low CD21+B220+) | 7/7 | 13.16 ± 2.67% (2.86 ± 0.62) | 7.71 ± 2.29% (0.16 ± 0.05***) |
| B1 cells (CD5lowB220+) | 3/3 | 8.71 ± 0.05% (1.32 ± 0.11) | 13.94 ± 2.82% (0.33 ± 0.07*) |
| Non-B cells expressing B220 (B220+LC−) | 7/7 | 6.14 ± 0.96% (1.36 ± 0.28) | 34.01 ± 4.16%*** (0.70 ± 0.14) |
| Total B lymphocytes (B220+LC+) | 7/7 | 93.86 ± 0.96% (20.81 ± 2.19) | 65.99 ± 4.156%*** (1.46 ± 0.38***) |
| κ+ cells | 7/7 | 90.54 ± 1.12% (20.07 ± 2.12) | 63.24 ± 4.03%*** (1.4 ± 0.36***) |
| λ+ cells | 7/7 | 5.10 ± 0.26% (1.13 ± 0.15) | 1.77 ± 0.22%*** (0.036 ± 0.009***) |
| CD19+ B cells (B220+CD19+) | 7/7 | 95.46 ± 0.26% (21.15 ± 2.2) | 64.96 ± 5.41%*** (1.44 ± 0.36***) |
| B220+mIgM+ cells | 7/7 | 93.17 ± 0.14% (20.65 ± 2.2) | ND |
| B220+mIgA+ cells | 7/7 | ND | 62.23 ± 5.00% (1.39 ± 0.39) |
Second column indicates numbers (n) of WT/mutant animals used. Third and fourth columns indicate percentages of cells (and absolute values in millions of cells in parentheses). ND, not detectable. Asterisks indicate statistically significant differences between values observed in the group of α1KI/α1KI mice and the control group: *P < 0.05; **P < 0.01; ***P < 0.001.
Heterozygous α1KI/+ mice had normal serum levels of all mouse Ig, including IgM, and a low level of circulating human IgA1 (5–10 μg/mL). Consistent with the absence of mIgM+ cells, serum IgM was undetectable in homozygous α1KI/α1KI mutants and was replaced by IgA1 (mean titer: 600 μg/mL); murine IgG and IgA production was not abolished in α1KI/α1KI mice, but showed a 10- to 100-fold reduction for all isotypes (Fig. 1D).
The α1KI mutation thus supported the development of a humoral immune system, albeit with reduced numbers of B lymphocytes.
B Cell Development Is Impaired in Homozygous Mutant Animals.
Early B cell development analyzed by flow cytometry showed no alteration in heterozygous mutant animals where it overwhelmingly relied on the WT IgH allele, yielding lymphocytes with conventional mouse mIg and finally resulting in very low production of serum human IgA1.
In α1KI/α1KI mice lacking any competition with WT μ HC, the α1KI allele supported early B cell differentiation but with a 3-fold reduction in the total number of bone marrow B-lineage cells and with phenotypic alterations (Table 1). Normal absolute values were observed for CD117+B220+ pro-B and CD43+B220+ pro-B/pre-BI/large pre-BII cells (with apparent expansion in relative percentages). By contrast, the CD25+B220+ pre-BII population underwent an 8-fold decrease in absolute value (a roughly 3-fold decrease in percentage) (Table 1 and Fig. S2A).
We used 24 h in vitro cultures of sorted B220+ bone marrow cells to improve staining for surface surrogate light chains (SLC) and showed that α1KI/α1KI mice had three times more abundant VpreB+ cells than WT mice (Fig. S2B). In both WT and mutant mice, these cells were large and included pro-B/preBI cells (negative for HC staining) and large pre-BII cells (coexpressing HC and surrogate LC). By contrast, the proportion of CD25+ VpreB− cells (small pre-BII cells in which expression of the pre-BCR is “diluted” after cell proliferation) was severely reduced (Fig. S2B). Pre-B cell staining showed the association of α HC and surrogate light chains within pre-BCR molecules (Fig. S2B).
A similar blockade at the transition from large pre-BII (BP1+) to small pre-BII (BP1−) was observed when sorted progenitor B cells were first cultured for 5 days in vitro with stromal cells and high doses of IL-7, and then switched for 2 days to low doses of IL-7 to stimulate pre-B cell differentiation (Fig. S2C).
In line with the early differentiation defect, numbers of peripheral mature B cells were severely reduced in the spleen (Table 1). Lymph nodes and Peyer’s patches also were small-sized, and the latter were reduced in number or even occasionally absent.
The reduction of spleen B cell numbers similarly affected the AA4.1+B220+ (transitional), CD23highCD21int (follicular), CD23−CD21high (marginal zone), and CD5+B220+ (B1) compartments (Table 1 and Fig. S3A). In α1KI/α1KI mice, B cell lymphopenia resulted in the apparent relative increase of a peculiar population of B220+ BCR-less cells (LC−). In absolute numbers, these unconventional B220+ cells were equally abundant in WT controls and carried a similar phenotype (only expressing CD19 on part of them and including mostly non-B cells) (Table 1 and Fig. S3B).
Whereas staining for different HC cannot allow comparison of BCR densities between WT and mutant mice, staining for LC clearly indicated a decreased BCR density in α1KI/α1KI mice (Fig. S3A).
In the peritoneum, α1KI/α1KI and WT mice also did not significantly differ for the proportions of B1 (Mac1+B220+) (47.7 ± 1.6% vs. 46.7 ± 7%) and B2 (Mac1− B220high) cells (31.2 ± 2.3% vs. 43.5 ± 7.9%) (Fig. S3C).
Functionality of the IgA1-Based Immune System in Homozygous α1KI Mice.
As mentioned above, Ig HC mRNA amounts from the α1KI allele were at a normal level and thus did not account for the decreased BCR density in mutant mice (Fig. S1A). Regarding membrane-anchored IgA1, immunoprecipitation with anti-mouse κ LC Abs followed by western blot with anti-CD79a/CD79b indicated association of the BCR with the Igα/Igβ heterodimer (Fig. S4A).
We next investigated whether the antibodies produced in α1KI/α1KI mice had a normal biochemical structure. We evidenced mostly monomeric IgA1 in serum (Fig. S4B) and polymers exported into secretions. IgA1 level reached 60 μg/mL in the jejunal fluid (55.8 ± 10.3 μg/mL), a level similar to that of secretory IgA in secretions of normal mice or humans. We analyzed a monoclonal IgA1 from an α1KI/α1KI hybridoma by SDS/PAGE under reducing conditions and found it was composed of normal-sized α1 Ig HC plus mouse LC (Fig. S4C). To test whether the chimeric IgA assembled correctly with endogenous mouse J chain, we performed immunoprecipitation of IgA from mice feces and intestinal contents with a monoclonal anti-human IgA1-specific antibody followed by immunodetection using an anti-mouse J-chain antiserum. In WT mice no signal was detected, whereas J-chain-positive chimeric IgA1 was present in jejunal and caecal content, as well as in feces of α1KI/α1KI animals (Fig. S4D).
Immunization of α1KI/α1KI mice with ovalbumin raised serum IgA1 antibodies, showing that functional mIgA1+ cells yielded antigen-specific ASCs. Low levels of class-switched antigen-specific mouse Ig (mostly IgG2a) were also detected (Fig. S4E). Immunizations with various bacterial or viral antigens confirmed that α1KI/α1KI mice responded to a repertoire of foreign antigens (staphylococcus, respiratory syncytial virus, and HIV gp120 were successfully used to obtain hybridomas). They also appeared to tolerate self-antigens normally, and both evaluations of anti-nuclear antibodies and rheumatoid factors were below the threshold of detection in 10 α1KI/α1KI mice assayed.
Altogether, α1KI/α1KI mice developed roughly normal humoral responses, in agreement with their normal longevity in a conventional environment challenging them with multiple pathogens.
Increased Plasma Cell Differentiation in α1KI/α1KI Mice.
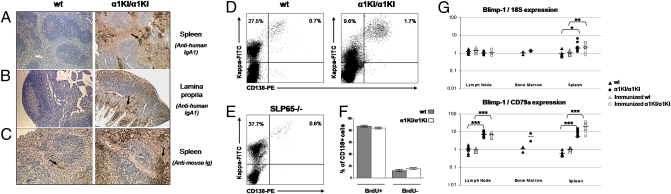
Because specific responses to immunization and the presence of follicular B cells in peripheral lymphoid organs indicated that mutant B lymphocytes could be activated, we wished to appraise their ability to differentiate into plasma cells. Tissues analyzed by immunohistochemistry for the presence of intracellular Ig showed much more abundant ASCs in lymphoid tissues of mutant animals than in WT mice, most of them producing IgA1. Plasma cells had a normal location in the splenic marginal zone and red pulp, in the enteric lamina propria along the intestinal crypts, and around rare Peyer’s patches (Fig. 2 A–C). Flow cytometry also showed an increased proportion of splenocytes engaged in ASC differentiation and brightly stained intracellularly for κ LC. These ASCs represented more than one seventh of all κ-expressing B-lineage cells in α1KI/α1KI mice, that is, 6-fold more than among WT B cells (Fig. 2D and Table 1). To check that this plasma cell accumulation would not be observed in any mouse model carrying a dysfunctional BCR, we compared α1KI/α1KI mice not only with WT but also with SLP65-deficient mice, but the latter rather showed increased numbers of mature B cells with no increase in plasma cell differentiation (Fig. 2E).
Fig. 2.
Global in vivo increase of the plasma cell compartment in mutant mice. (A and B) Staining of tissues from WT (Left) and α1KI/α1KI mice (Right) with hemalun and revelation of human IgA1 with HRP-conjugated Ig show the presence of plasma cells (arrows) producing IgA1 and accumulating in (A) the splenic marginal zone and red pulp or (B) around Peyer's patches and in the lamina propria along intestinal crypts of the jejunum. (Original magnification, 100×.) (C) Staining of all plasma cells with anti-mouse Ig (HC + LC) shows accumulation of plasma cells in the spleen of a mutant mouse by comparison with WT. (Original magnification, 200×.) (D) Staining for CD138 and intracellular κ LC shows ASCs as CD138+ cells brightly staining for κ. (E) Staining for CD138+ and intracellular κ LC+ ASCs in splenocytes from SLP65-deficient mice. (F) Absence of significant difference by cell flow cytometry between proportions of recently differentiated (CD138+BrdU+) versus long-lived plasma cells (CD138+BrdU−) from α1KI/α1KI and WT spleens (means from three and four animals fed with BrdU, respectively). (G) Expression of Blimp-1 transcript in lymphoid tissues by qPCR evaluates the amount of cells engaged in plasma cell differentiation among all of the cells present in tissue samples (reference to 18S RNA) or by comparison with the pool of B lymphocytes (CD79a as a reference transcript). Total RNA was prepared without prior immunization of (▲) WT and (•) α1KI/α1KI mice or 3 days after intraperitoneal immunization of (∆) WT and (○) α1KI/α1KI mice with BSA.
In vivo BrdU labeling showed a normal ratio of long-lived versus short-lived plasma cells in α1KI/α1KI mice, indicating not only accumulation but also increased ongoing differentiation of these cells (Fig. 2F).
A quantitative assessment of plasma cells versus B lymphocytes was additionally obtained by measuring the ratio of Blimp-1 to CD79a transcripts, which was 7- to 10-fold higher in mutant mouse lymph nodes, bone marrow, and spleen than in WT animals. Three days after intraperitoneal immunization, the plasma cell burst still increased in the spleen, with the Blimp-1:CD79a ratio being roughly 20-fold higher in α1KI/α1KI than in control mice (Fig. 2G).
α1KI/α1KI B Cells Are Constitutively Activated and Committed to CD138+ Differentiation.
Given the B cell lymphopenia and resulting increased proportion of B220+BCR− cells, we restricted the B lymphocyte phenotype analysis to true B220+LC+ cells (Table 1 and Fig. S3A). Mutant B lymphocytes included decreased numbers of cells expressing CD21, CD22, and CD23 and increased numbers of cells expressing the CD69 activation marker or having lost MHC class II expression (Fig. S5A).
B cells from α1KI/α1KI mice featured constitutive phosphorylation of multiple intracellular targets as compared to WT cells. Phosphorylation of the Akt and ERK kinases was further increased by BCR cross-linking (Fig. S5B).
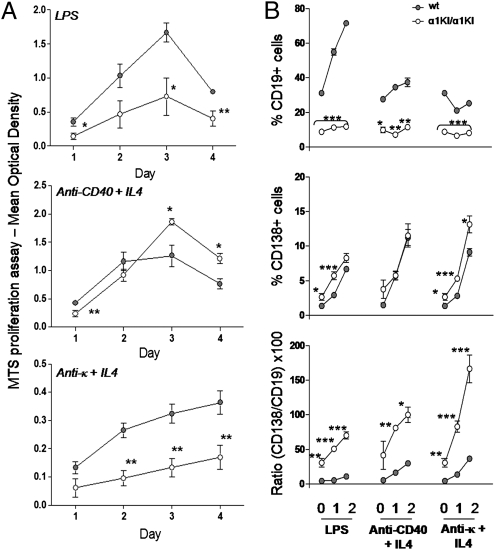
CD19+ α1KI/α1KI splenocytes proliferated less than CD19+ WT controls after lipopolysaccharide (LPS) stimulation and still more markedly after BCR cross-linking by anti-κ LC Abs (Fig. 3A). Among freshly isolated splenocytes, the ratio of CD138+ to CD19+ cells showed a bias toward plasma cell differentiation in mutant compared with WT mice. This bias increased after in vitro stimulation with LPS, anti-CD40 mAb plus IL4, or anti-κ Ab plus IL4 (Fig. 3B). Among such cultures, ongoing apoptosis was similar for WT and α1KI/α1KI cells and was higher for CD138+ cells than for CD19+ cells (48.7 ± 7.9% and 16.6 ± 6.7% of annexinV+ 7AAD− cells, respectively). These conditions thus measured newly formed plasma cells and not in vitro accumulated surviving plasma cells.
Fig. 3.
Mutant B cells are precommitted to plasma cell differentiation. (A) Proliferation of sorted CD43− splenocytes (standardized on the numbers of CD19+ B cells) from α1KI/α1KI mice compared to WT (gray) after 4 days in vitro stimulation by LPS (Top), anti-CD40 plus IL-4 (Middle), or anti-κ Abs plus IL-4 (Bottom). (B) Ratios of CD138- versus CD19-expressing cells compared between WT (gray) and α1KI/α1KI mice within splenocytes stimulated for 2 days by LPS, anti-CD40 plus IL-4, or anti-κ LC plus IL-4 (*P < 0.05; **P < 0.01; ***P < 0.001 significant difference).
Cells from α1KI/α1KI mice thus altogether appear as constitutively activated, with an increased tendency to yield CD138+ cells and a decreased proliferation in response to BCR cross-linking.
Heterozygous Cells Expressing mIgA1 from the Mutant Allele Poorly Compete with mIgM Cells, Even When CSR Is Blocked.
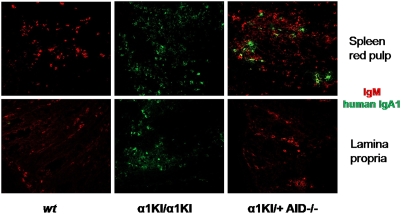
Because mIgA1+ cells were too rare in α1KI/+ mice for detection by flow cytometry whereas IgA1 was readily measurable in sera, we evaluated IgA1− ASCs by confocal microscopy. Competition between cells expressing only mIgA1 from the α1KI allele and cells expressing only mIgM from the WT allele was assessed in heterozygous α1KI/+ animals on an activation-induced deaminase (AID)-deficient background (to avoid competition with cells producing mouse IgA after CSR). In this background, the proportion of peripheral mIgA1+ cells remained undetectable, whereas some cells expressing IgA1 reached the plasma cell stage and accounted for 7.4% of spleen plasma cells (Fig. 4). Even for entry into MALT, mIgA1+ cells poorly competed with mIgM+ cells and no IgA1 ASCs were detected in the gut lamina propria of these α1KI/+ mice, contrasting with the situation in α1KI/α1KI mice (Fig. 4).
Fig. 4.
Confocal imaging of antibody-secreting cells in WT and mutant tissues. Evaluation of the number of ASCs secreting human IgA1 (green) versus IgM (red) by confocal microscopy in WT (Left), α1KI/α1KI (Center), or α1KI/+ heterozygous mice bred in an AID−/− background (Right). In the latter animals, counting of plasma cells in several microscopic fields showed that 24 out of 350 plasma cells (i.e., 7.4%) in the spleen were producing IgA1, whereas there was no IgA1-positive cell detectable in gut-associated lymphoid tissues. (Original magnification, 630×.)
Discussion
We wished to force expression of mIgA throughout B cell differentiation by replacing the Sμ region with a knock-in human Cα Ig gene. The use of a Cα gene from a different species unambiguously distinguished transgenic IgA expression from endogenous switching.
Mature α1KI/+ B cells showed a strong bias toward expression of the WT rather than the mutated allele. Cells expressing the knock-in α gene were only detectable within the splenic plasma cell pool and were virtually absent from the MALT, suggesting that in physiology, IgM+ cells are preferentially recruited to the MALT and locally undergo CSR to IgA under the influence of the mucosal microenvironment.
In α1KI/α1KI animals, the most dramatic B-lineage alterations included delayed pre-B differentiation contrasting with increased terminal plasma cell differentiation. The reduced size of the CD25+ small pre-BII cell compartment and of the total B220+ compartment in the bone marrow, together with the expanded proportion of pro-B/pre-BI and large pre-BII cells, indicated that the α-class pre-BCR was less efficient than a μ-class pre-BCR for turning off CD43 expression and signaling the completion of V(D)J rearrangement upon assembly of a functional pre-BCR or BCR complex. These features are reminiscent of those in mice with premature expression of membrane γ HC substituting for μ/δ (16).
Despite this partial early blockade reducing their number, B cells accumulated in all peripheral lymphoid organs of α1KI/α1KI mice including spleen follicles, marginal zone, lymph nodes, Peyer’s patches, and the peritoneum B1 compartment. Splenic marginal zone and follicular B cell numbers were affected in the same proportion, in contrast with the increased marginal zone differentiation reported in mice expressing an IgG BCR (15, 16). BCR density appeared lower in α1KI/α1KI than in WT animals, although mIgA1 was normally associated with the Igα/Igβ heterodimer. Expression of mIgA1 in peripheral B cells did not alter their ability to produce specific antibodies after immunization and even to undergo CSR at low levels in the absence of any Sμ repeat. This partial CSR defect was similar to that reported for the core Sμ or the complete Sμ deletions (23, 24). These data confirm that neither the pentameric repeats featuring the Sμ region nor expression of μ/δ are mandatory for CSR in the JH-C intron, in contrast to the Sγ1, whose deletion virtually abrogates CSR to Cγ1 (25).
Another feature of mIgA-driven B cell differentiation in α1KI/α1KI mice was the abundance of plasma cells, affecting to the same extent long-lived (accumulated) and short-lived (recently differentiated) plasma cells. Such a phenotype has not been reported as a general consequence of BCR dysfunction and was neither present in mice reconstituted with Syk-deficient B cells nor in Lyn-deficient mice (26, 27), and was not found either in the SLP65-deficient mice used in this study for comparison. More severe reported dysfunctions of the BCR resulted in a complete block at the pro-B stage with neither B cells nor plasma cells, as in the case of Igα or Igβ deficiency (28, 29). The α1KI/α1KI mice plasma cell expansion was remarkable in view of the reduced mature B cell number and might partly result from homeostatic accumulation, as reported both for mature B cells in response to reduced lymphopoiesis and for plasma cells which can be more preserved than mature B cells in lymphopenic animals (19, 20, 30). However, commitment to plasma cell differentiation was emphasized in vitro by the lower proliferation but higher differentiation into CD138+ cells of α1KI/α1KI cells activated by BCR cross-linking or mitogens, and in vivo by the increased spontaneous Blimp-1 expression in all lymphoid tissues or still more in spleen after immunization. In vivo, the commitment of B lymphocytes toward ASC differentiation also manifested in their more frequent expression of the CD69 activation marker and reduced expression of MHC class II, which is repressed by Blimp-1 and silenced early during ASC maturation. The presence of a significant number of IgA1 ASCs in spleen of α1KI/+ mice, where virtually no mIgA1+ B cells were detected, also showed this increased commitment.
Interestingly, normal mIgA+ cells purified from human blood include a high proportion of cells expressing CD38, a marker shared by immature cells and cells engaged to plasma cell differentiation (31). Whereas the IgA BCR has been shown to be subjected to CD22 inhibition as for IgM (32), other aspects may differ in signals provided both by classes of BCR and by impact on the behavior of B cells during primary or secondary responses to antigens.
Finally, we show that an IgA-class BCR can support all of the functions mediated by membrane IgM/IgD during B cell differentiation and immune responses but with reduced efficiency at early stages, although we cannot exclude that expression of a human heavy chain in mouse might by itself participate in altering B cell development. Once in a mature compartment, mIgA+ lymphocytes show a strong ability to be activated, to switch at a low level despite the absence of Sμ, and to terminally differentiate into plasma cells. The sum of these observations supports the concept that although associated with the same Igα/Igβ heterodimer, all membrane Ig HCs are not equivalent in terms of conveying specific signals emanating from their extracellular domains. Rather, cytoplasmic tails and/or extracellular C domains of the various classes of Ig are likely tailored to deliver signals fitting the maturation stage at which they are usually expressed. Such differences also likely account for the implications of different Ig classes in specialized immune responses and in physically distinct compartments of the immune system. In this view, membrane IgM may stand as an “affector BCR” shaping the preimmune repertoire, whereas both the IgG and IgA switched isotypes would constitute “effector BCR” shaping the immune repertoire and favoring the generation of ASCs (33). In human pathology, the frequency of IgG/IgA myeloma and plasmacytoma cases, compared to the paucity of IgM myeloma cases, fits adequately with these observations and suggests that the BCR by itself likely contributes to the plasma cell burst or to the accumulation of a long-lived plasma cell pool in such malignant proliferations.
Methods
Mice.
Experiments followed international guidelines. AID−/− mice were a gift of Dr. T. Honjo (Kyoto, Japan). EIIa-cre transgenics were a gift of Dr. H. Westphal (Bethesda, MD). SLP65-deficient mice were a gift of Dr. H. Jumaa (Freiburg, Germany).
Gene Targeting.
The α1KI construct included a 6-kb α1 genomic fragment (from a SacI site 200 bp upstream of CH1 to a BamHI site downstream of the last polyadenylation signal) flanked by an ∼5-kb long 5′ arm (a StuI-SpeI fragment located upstream of mouse Sμ) and an ∼5-kb long 3′ arm (the Cμ gene cloned as an XhoI fragment). A 1.3-kb ClaI-SalI neor gene flanked by loxP sites was also stuck in-between the α1 gene and the 3′ arm. E14 ES cells were transfected with linearized vector and selected using G418 (400 μg/mL). Recombinant clones were identified by Southern blot of EcoRI digests with an external 3′ probe (0.6-kb XhoI-XbaI fragment). Chimeras obtained from C57BL/6 blastocysts were mated with C57BL/6 females and their progeny was checked by Southern blot. Mutant α1KI/α1KI Neo mice were mated with EIIa-cre transgenic mice to yield the cre-deleted α1KI allele.
Cell Flow Cytometry.
Cells from 6- to 8-week-old mice were stained with Abs conjugated to either FITC, PE, Alexa 633, allophycocyanin, phycoerythrin-cyanin 7 (PC7), or phycoerythrin-cyanin 5 (PC5). Abs are listed in SI Methods. For surface staining, cells were labeled in the presence of rat anti-mouse CD16/CD32 (BD Biosciences) to block Fc receptor binding. For intracellular staining, cells were fixed in PBS, 4% paraformaldehyde and permeabilized in PBS, 0.1% saponin (Sigma-Aldrich). Cells were analyzed on a Beckman Coulter FC500 apparatus.
Ig Analysis in Biologic Samples and Autoantibody Screening.
ELISAs for mouse Ig were done using plates coated and revealed with 1 μg/mL isotype-specific goat Abs (Southern Biotechnologies). ELISA for human IgA was with 1 μg/mL goat anti-IgA F(ab′)2 Abs for coating and with alkaline phosphatase-conjugated goat anti-IgA Abs (Dako). Mouse sera were assayed at 1:100 and 1:1000 dilutions.
Search for anti-nuclear Abs was done by classical indirect immunofluorescence on Hep2 cells (Biomedical Diagnostics) on microscope slides first incubated with mouse sera at a 1:20 dilution and further incubated with a FITC-labeled anti-mouse κ-chain antiserum (Southern Biotechnologies). Rheumatoid factors were quantified with the N-latex RF kit II (Behring) using a Behring Nephelometer II analyzer. Assays for anti-nuclear Abs and rheumatoid factors were strongly positive with control sera from autoimmune MRL mice.
Immunohistochemistry.
Paraffin-embedded samples were cut in 4-μm thick slices and counterstained with Mayer’s hemalun solution. To stain ASCs and specifically check human IgA1, tissue sections were incubated with either unconjugated goat anti-mouse Ig (heavy + light chains) or rabbit anti-human IgA (Dako). Slides were then washed and incubated with HRP-conjugated secondary Abs specific either for goat or rabbit Ig (Dako). HRP staining was done with diaminobenzidine and hydrogen peroxide.
Proliferation and Activation Assays.
Total splenocytes were stimulated for 2–4 days with 20 μg/mL LPS from Salmonella typhimurium (Sigma) or with 5 μg/mL anti-CD40 (R&D Systems) and 40 ng/mL murine IL-4 (PeproTech) or with 10 μg/mL goat anti-mouse κ (Southern Biotechnologies) and 40 ng/mL murine IL-4 in DMEM supplemented with 10% heat-inactivated FCS. Cells were analyzed each day for staining by rat anti-CD138 or -CD19, with Annexin V and 7AAD to evaluate apoptosis and exclude dead cells (BD Pharmingen).
Proliferation of sorted CD43− splenocytes (on standardized numbers of CD19+ B cells) was measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H (MTS) tetrazolium nonradioactive cell proliferation assay (Promega) in triplicate and expressed as the mean optical density (OD) ± SEM.
BrdU Labeling.
Mice were given drinking water containing 1 mg/mL BrdU (Sigma) for 3 weeks. Splenocytes were stained with rat anti-mouse CD138-PE, made permeable, and stained with FITC-labeled anti-BrdU (BD Biosciences). Newly formed (short-lived) plasma cells stained positive for BrdU, whereas long-lived plasma cells remained BrdU negative.
Real-Time PCR.
Real-time PCR was performed in duplicate using 20 ng of cDNA for each sample. Relative amounts of transcripts were determined using Taqman assays specific for Blimp-1 (Mm00476128_m1), CD79a (Mm00432423_m1), and 18S rRNA (Hs99999901_s1) on an ABI PRISM 7700 cycler (Applied Biosystems).
Confocal Microscopy.
Tissue cryosections of 8 μm or magnetically sorted CD19+ splenocytes (Miltenyi Biotech) were fixed with methanol and permeabilized in 0.15% Triton X-100. Unspecific and FcR binding was blocked with PBS/3% BSA and rat anti-mouse CD16/CD32 (BD Biosciences). Ig staining used Alexa 488-goat anti-human IgA or Alexa 546-rat anti-mouse IgM (1B4B1). Slides were observed on an LSM 510 confocal microscope (Carl Zeiss).
Statistical Analysis.
Results are expressed as mean ± SEM, and overall differences between variables were evaluated by a two-tailed unpaired Student’s t test.
Supplementary Material
Acknowledgments
The expertise of Micaël Bardel, Claire Carrion, and Christelle Oblet are greatly acknowledged. We thank Salvatore Valitutti and Loïc Dupré for critical reading of the manuscript. This work was supported by grants from Agence Nationale de la Recherche, Ligue Nationale Contre le Cancer, and Région Limousin. S.D. was supported by a fellowship from Région Limousin and by Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912393107/DCSupplemental.
References
- 1.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan JG, Lefranc MP, Rabbitts TH. Mechanisms of divergence and convergence of the human immunoglobulin α 1 and α 2 constant region gene sequences. Cell. 1984;36:681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- 4.Word CJ, Mushinski JF, Tucker PW. The murine immunoglobulin α gene expresses multiple transcripts from a unique membrane exon. EMBO J. 1983;2:887–898. doi: 10.1002/j.1460-2075.1983.tb01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogné M, Preud’homme JL. Gene deletions force nonsecretory α-chain disease plasma cells to produce membrane-form α-chain only. J Immunol. 1990;145:2455–2458. [PubMed] [Google Scholar]
- 6.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 8.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 9.Lutz C, et al. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797–801. doi: 10.1038/31716. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci USA. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogue SL, Goodnow CC. Gene dose-dependent maturation and receptor editing of B cells expressing immunoglobulin (Ig)G1 or IgM/IgG1 tail antigen receptors. J Exp Med. 2000;191:1031–1044. doi: 10.1084/jem.191.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol. 2002;3:182–188. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 13.Engels N, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. 2009;10:1018–1025. doi: 10.1038/ni.1764. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi C, Adachi T, Wienands J, Tsubata T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298:2392–2395. doi: 10.1126/science.1076963. [DOI] [PubMed] [Google Scholar]
- 15.Horikawa K, et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waisman A, et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig α/β. J Exp Med. 2007;204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leduc I, Drouet M, Bodinier MC, Helal A, Cogné M. Membrane isoforms of human immunoglobulins of the A1 and A2 isotypes: Structural and functional study. Immunology. 1997;90:330–336. doi: 10.1111/j.1365-2567.1997.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandtzaeg P, et al. Regional specialization in the mucosal immune system: What happens in the microcompartments? Immunol Today. 1999;20:141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- 19.Macpherson AJ, et al. IgA production without μ or δ chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 20.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the μMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Milili M, Fougereau M, Guglielmi P, Schiff C. Early occurrence of immunoglobulin isotype switching in human fetal liver. Mol Immunol. 1991;28:753–761. doi: 10.1016/0161-5890(91)90118-4. [DOI] [PubMed] [Google Scholar]
- 22.Neuberger MS, et al. The mouse B-cell antigen receptor: Definition and assembly of the core receptor of the five immunoglobulin isotypes. Immunol Rev. 1993;132:147–161. doi: 10.1111/j.1600-065x.1993.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 23.Luby TM, Schrader CE, Stavnezer J, Selsing E. The μ switch region tandem repeats are important, but not required, for antibody class switch recombination. J Exp Med. 2001;193:159–168. doi: 10.1084/jem.193.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khamlichi AA, et al. Immunoglobulin class-switch recombination in mice devoid of any S μ tandem repeat. Blood. 2004;103:3828–3836. doi: 10.1182/blood-2003-10-3470. [DOI] [PubMed] [Google Scholar]
- 25.Shinkura R, et al. The influence of transcriptional orientation on endogenous switch region function. Nat Immunol. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- 26.Turner M, et al. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186:2013–2021. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 28.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig β. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 29.Minegishi Y, et al. Mutations in Igα (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999;104:1115–1121. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agenès F, Freitas AA. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J Exp Med. 1999;189:319–330. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irsch J, et al. Switch recombination in normal IgA1+ B lymphocytes. Proc Natl Acad Sci USA. 1994;91:1323–1327. doi: 10.1073/pnas.91.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato M, Adachi T, Tsubata T. Augmentation of signaling through BCR containing IgE but not that containing IgA due to lack of CD22-mediated signal regulation. J Immunol. 2007;178:2901–2907. doi: 10.4049/jimmunol.178.5.2901. [DOI] [PubMed] [Google Scholar]
- 33.Manser T. Effector BCRs: Inside information on IgG. Nat Immunol. 2002;3:114–116. doi: 10.1038/ni0202-114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.