Abstract
We have shown previously that cancer/testis (CT) antigen, CT45, is expressed in various epithelial cancers at a frequency of <5% to ∼35%. In this study, the protein expression of CT45 was examined in non-Hodgkin B-cell lymphomas and classical Hodgkin lymphoma by immunohistochemical analysis. Serological response to CT45 was also evaluated by ELISA using CT45 recombinant protein and sera from patients with Hodgkin lymphoma. None of the 80 low-grade B-cell lymphomas, including chronic lymphocytic leukemia/small lymphocytic lymphoma, follicular lymphoma, and mantle cell lymphoma, expressed CT45. In comparison, CT45 was expressed in 28 of 126 (22%) diffuse large B-cell lymphomas (DLBCL). A remarkably high percentage (42/72, 58%) of classical Hodgkin lymphoma contained CT45-positive Reed–Sternberg cells. Nodular sclerosis and mixed-cellularity subtypes had similar frequency of CT45 expression, but most EBV-positive cases were CT45 negative. Gray-zone lymphoma (cases with features of both DLBCL and classical Hodgkin lymphoma) also showed frequent (64%) CT45 expression. Evaluation of reactive lymphoid tissues showed scattered CT45-positive lymphocytes in a single case of florid follicular hyperplasia, raising the possibility that this case was an evolving malignancy. Despite frequent CT45 expression, only 1 of 67 Hodgkin lymphoma patients had detectable anti-CT45 antibodies in the serum, suggesting that the immune response to CT45 may be suppressed. In conclusion, classical Hodgkin lymphoma has the highest frequency of CT45 expression among all malignancies tested to date, the frequency of CT45 expression in DLBCL is similar to that seen in epithelial cancers, and low-grade non-Hodgkin B-cell lymphomas do not express CT45.
Keywords: cancer vaccine target, immunotherapy, tumor immunology
Cancer/testis (CT) antigens are defined as protein antigens with restricted expression in adult testicular germ cells and in a proportion of a wide variety of human cancers in a lineage-unrelated fashion (1 –3). Because of their restricted pattern of expression, CT antigens often are recognized by the immune system of cancer patients. This antigenicity raised the possibility of their use as therapeutic cancer vaccine targets. The prototype examples of CT antigens, MAGE-A (4) and NY-ESO-1 (5), were among the first human tumor antigens shown to elicit a spontaneous cytotoxic T-cell response in cancer patients (4, 6). Cancer vaccine trials with these two antigens have shown them to be capable of inducing humoral and cell-mediated immune responses in some patients, and examples of clinical responses have also been documented (7 –9).
To identify new CT antigen genes, we previously used massively parallel signature sequencing to identify genes with cancer/testis-restricted mRNA expression patterns. This approach resulted in the discovery of more than 20 CT or “CT-like” genes, including CT45 (10). Similar to MAGE-A and NY-ESO-1, CT45 is a multigene family on the telomeric end of chromosome X, at Xq26.3, with six almost identical gene copies in direct tandem repeats within a 125-kb region. CT45 encodes a putative protein of 189 amino acids with two nuclear localization signals, but no other functional domain has been identified. Using a mouse monoclonal anti-CT45 antibody, we recently have confirmed CT45 as a nuclear protein with cancer/testis restricted expression. We have identified aberrant CT45 protein expression in melanoma and in epithelial cancers of ovary, lung, breast, uterus, bladder, and other sites, with the ovarian cancer exhibiting the highest rate of positivity (37%) (11).
The expression of CT antigens in cancer has been attributed to epigenetic activation, as evidenced by the induction of CT expression in cell lines following hypomethylation and histone deacetylation (12 –14). However, for reasons that are unclear, different tumor types vary significantly in the frequency of CT antigen expression. Melanoma and ovarian cancer, for instance, are “CT-rich” tumors, with 20–50% of tumors expressing MAGE-A and NY-ESO-1. In comparison, carcinomas of colon and kidney, as well as hematological malignancies, are “CT-poor” tumors: Less than 2% of leukemia and lymphoma have been shown to be positive for MAGE-A or NY-ESO-1 mRNA (2, 3, 15, 16).
Although non-Hodgkin lymphomas are reported to be rarely positive for CT antigens, only limited data have been published regarding CT expression in classical Hodgkin lymphoma (cHL) (17 –19). Chambost et al. (18) evaluated mRNA expression of the MAGE-A gene family (MAGE-A1, -A2, -A3, -A4, and –A12) in 18 cases of cHL and found five cases (28%) expressing MAGE-A4 but none expressing the other MAGE-A transcripts. Furthermore, using a broad-spectrum anti-MAGE-A antibody (clone 57B) (20), they found MAGE-A protein expression in only 21% (11/53) of the cHL cases. Evaluating the expression of the SSX gene family, another CT antigen family on chromosome X (14), Colleoni et al. (17) similarly showed 16% (5/32) of the cases to express SSX1, SSX2, or SSX4. Thus, the overall frequency of CT antigen expression in cHL is between 10% and 30%, comparable to the frequency of CT expression observed in diffuse large B-cell lymphoma (DLBCL) and in some epithelial malignancies, such as lung cancer (15–25%) and breast cancer (<10%) (11).
Given these findings, it was unexpected when K i-A10, a Reed–Sternberg (RS) cell-specific monoclonal antibody which was generated a decade ago by immunizing mice with lysates of the cHL cell line L428 (21) and which reacted with RS cells and variants in more than 50% of the cHL cases was found recently to recognize CT45 (19). To explore further the expression of CT45 in cHL and in other B-cell malignancies, we used an anti-CT45 mouse monoclonal antibody that we recently generated and tested it against a wide variety of non-Hodgkin lymphomas as well as cHL. In addition, we investigated the spontaneous immunogenicity of CT45 in these patients.
Results
CT45 Expression in Non-Hodgkin Lymphoma.
All 80 of the low-grade B-cell non-Hodgkin lymphomas examined were CT45-negative, including 26 chronic lymphocytic lymphoma/small lymphocytic lymphomas, 25 mantle cell lymphomas, and 29 follicular lymphomas (FLs). In contrast, 28 of 126 DLBCLs (22%) showed CT45 expression. Of the 28 positive cases, 19 (68%) showed diffuse strong reactivity, exhibiting moderate (++) to strong (+++) nuclear staining in >60% of the tumor cells (Fig. 1A). In comparison, the other nine cases showed much weaker, often focal, staining, with <10% positive cells seen in eight of the nine cases that were not diffusely positive; the one remaining case showed weak staining in 30–50% of cells (Fig. 1B). Of the 19 CT45 strongly positive DLBCLs, 5 were of germinal-center (GC) phenotype, 11 were non-GC, and 3 were null phenotype. However, the overall percentages of GC, non-GC, and null DLBCLs that showed strong CT45 positivity (10%, 19%, and 18%, respectively) or any CT45 positivity (19%, 26%, and 18%, respectively) were similar. Of the other markers tested, CT45 was positive in 25% (27/108) of BCL2-positive DLBCLs but only in 1 of 16 (6%) of BCL2-negative cases (P = 0.116). No significant difference in CT45 expression was seen between the p53-positive and -negative cases (20% vs. 23%). These results are summarized in Table 1.
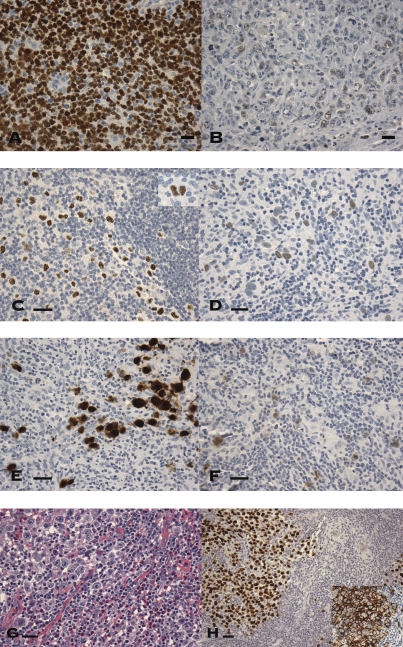
Fig. 1.
Expression of CT proteins in various types of lymphoma (A–H). (A and B) CT45 expression in diffuse large B-cell lymphoma. (A) Two thirds of the positive cases showed diffuse strong nuclear staining. (B) The remaining cases showed weaker, and often focal, staining pattern. Nonneoplastic small lymphocytes are negative. (C–F) Expression of CT45 in cHL lymphoma, in comparison with MAGE-A and NY-ESO-1. (C) Most CT45-positive Hodgkin lymphomas showed diffuse nuclear staining of RS cells and variants in moderate to strong intensity (Inset shows an RS cell). (D) Weaker staining was seen in the remaining cases. (E) Expression of MAGE-A was detected in only 1 of 25 cases examined, showing a mixed nuclear and cytoplasmic expression pattern. (F) Similarly, expression of NY-ESO-1 was seen in only one case, as a weak to moderate cytoplasmic staining. (G and H) Expression of CT45 in gray zone lymphoma. (G) Large anaplastic tumor cells with prominent nucleoli are seen in a background of small lymphocytes and abundant eosinophils, similar to that seen in cHL. (H) The tumor cells were diffusely strongly positive for CT45 as well as for B-cell surface marker CD20 (Inset). (Scale bars, 100 μm.)
Table 1.
Expression of CT45 in non-Hodgkin B-cell lymphoma
| Lymphoma type | # CT45 positive (++/+++)* | # CT45 negative | % positive | P value |
| CLL/SLL | 0 | 26 | 0 | |
| MCL | 0 | 25 | 0 | |
| FL | 0 | 29 | 0 | |
| DLBCL | 28 (19) | 98 | 22 | |
| GC | 10 (5) | 42 | 19 | |
| Non-GC | 15 (11) | 42 | 26 | 0.598 |
| Null | 3 (3) | 14 | 18 | |
| BCL2 positive | 27 (19) | 81 | 25 | |
| BCL2 negative | 1 (0) | 16 | 6 | 0.116 |
| p53 positive | 5 (3) | 20 | 20 | |
| P63 negative | 23 (16) | 77 | 23 | 1.000 |
CLL/SLLs, chronic lymphocytic leukemia/small lymphocytic lymphoma; DLBCL, diffuse large B cell lymphoma; GC, non-GC and null, lymphomas with germinal center, nongerminal center, and null phenotypes; MCL, mantle cell lymphoma; FL, follicular lymphoma.
Numbers in parentheses indicate number of cases with moderate to strong CT45 positivity.
CT45 Expression in Classical Hodgkin Lymphoma.
Table 2 summarizes the expression data in cHL. The RS cells in the cHL cases were all CD30 positive and either negative or weakly focal positive for CD20. For CT45, 42 of 72 (58%) tumors showed CT45 expression in the nuclei of RS cells and variants but not in any of the surrounding nonneoplastic inflammatory cells. Unlike the commonly observed heterogeneous expression pattern of CT antigens in most carcinomas, 28 of the 42 positive cases (67%) showed moderate (++) to strong (+++) diffuse staining of the RS cells and variants (Fig. 1C); the remaining cases showed variable staining, mostly weak to moderate in intensity, usually in <20% of cells (Fig. 1D). The CT45-positive cases included 29 nodular-sclerosis, 7 mixed-cellularity, 3 lymphocyte-depleted, and 2 lymphocyte-rich subtypes, and the frequency of CT45 expression was similar in the nodular sclerosis (29/52, 56%) and mixed cellularity (7/14, 50%) subtypes; all 3 lymphocyte-depleted and 2 lymphocyte-rich cases were CT45 positive. CD15-positive cases were more frequently CT45 positive (69% vs. 33%, P = 0.012), and a positive correlation was seen between CT45 expression and CD15 expression, with 68% (47/69) of cases showing concordant expression (35 cases) or concordant nonexpression (12 cases) of both antigens.
Table 2.
Expression of CT45 in Hodgkin lymphoma
| cHL type | # CT45 positive | # CT45 negative | % positive | P value |
| cHL | 42 (28)* | 30 | 58 | |
| NS | 29 | 23 | 56 | |
| MC | 7 | 7 | 50 | |
| LD | 3 | 0 | 100 | 0.306 |
| LR | 2 | 0 | 100 | |
| NOS | 1 | 0 | 100 | |
| CD15 positive | 35 | 16 | 69 | |
| CD15 negative | 6 | 12 | 33 | 0.012 |
| EBER positive | 1 | 7 | 14 | |
| EBER negative | 18 | 15 | 55 | 0.050 |
*cHL, classical Hodgkin lymphoma; NS, nodular sclerosis; MC, mixed cellularity; LD, lymphocyte depleted; LR, lymphocyte rich; NOS, not otherwise specified; EBER, EBER in situ hybridization.
Number in parentheses indicates number of cases with moderate to strong CT45 positivity.
In situ hybridization for EBV using an EBER probe was performed in 41 cases. Most of the EBER-positive cases were CT45 negative (7/8, 88%), whereas EBER- negative cases were equally distributed with respect to their CT45 status (18 CT45 positive, 15 CT45 negative). This difference was statistically significant (P = 0.050).
Expression of Other CT Antigens in Classical Hodgkin Lymphoma.
The expression frequency of CT45 in cHL was compared with the expression of two other prototype CT antigens, MAGE-A and NY-ESO-1. For detecting MAGE-A expression, a broad-reactive anti-MAGE-A antibody (6C1) that recognizes MAGE-A1, ---A2, -A3, -A4, -A6, -A10, and -A12 was used (20). Using a tissue microarray (TMA) consisting of 25 cases of cHL, only 1 case was found to be positive for MAGE-A (Fig. 1E), and a different case was positive for NY-ESO-1 (Fig. 1F). In contrast, 11 of 25 cases were positive for CT45, including both the MAGE-A– and NY-ESO-1–positive cases.
CT45 Expression in Gray Zone Lymphoma.
Eleven cases of gray zone lymphoma were analyzed for CT45 expression. These cases showed overlapping morphologic and immunophenotypic features between cHL and DCLBL, including the presence, at least focally, of confluent growth of tumor cells resembling RS cells. However, despite the morphologic similitude, the tumor cells of this subtype of gray zone lymphoma generally express CD45, and CD20 also is typically strongly expressed (Fig. 1 G and H Inset). Seven of the 11 cases (64%) were CT45 positive, and 5 of them showed strong staining of almost all neoplastic cells (Fig. 1H); the remaining 2 cases showed weak focal staining of <10% of the neoplastic cells.
CT45 Expression in Normal and Reactive Lymphoid Tissues.
Although our previous study has shown all nontesticular adult tissues, including lymphoid tissues, to be CT45 negative, the possible expression of CT45 in normal and reactive lymphoid tissues was evaluated further in light of the high frequency of CT45 expression in cHL. Tonsil, spleen, thymus, bone marrow, and all normal nonreactive lymph nodes, those were confirmed to be CT45 negative. Of thirty reactive lymph nodes, those showing predominately sinus histiocytosis and/or paracortical hyperplasia showed no evidence of CT45 expression. In contrast, three reactive lymph nodes with follicular hyperplasia contained scattered CT45-positive cells. In two cases the positive cells were present as rare cells (<20 cells on one section) in the follicular center and in the mantle zone, showing weak to moderate nuclear expression. In the third case, a case of florid follicular hyperplasia involving an axillary lymph node, numerous CT45 strongly positive lymphocytes were seen scattered in the follicle centers as well as in the interfollicular areas (Fig. 2). Morphologically, this case had many “geographically” shaped large germinal centers, and no RS cells were identified. The protein expression of NY-ESO-1 in this case was evaluated immunohistochemically, and no evidence of NY-ESO-1 expression was detected. CD30 staining showed scattered CD30-positive cells, more abundantly present than CT45-positive cells, but did not appear to stain the same population of cells.
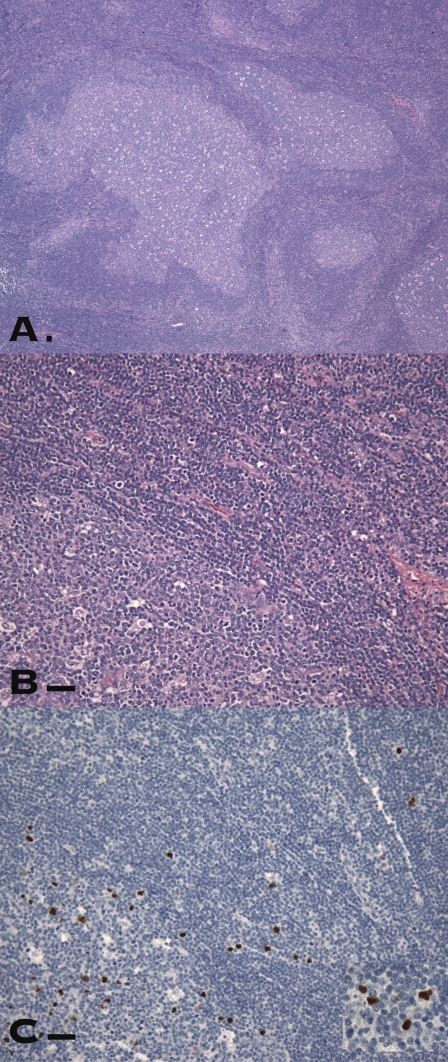
Fig. 2.
CT45-positive cells in a lymph node with florid follicular hyperplasia. (A) Large, irregularly shaped follicles were prominent. (B) Scattered intermediate to large lymphoid cells with vesicular nuclei were noted in the follicles and in the interfollicular areas. (C and Inset) Some of these cells probably correspond to CT45-positive cells. (Scale bars, 100 μm.)
Serological Response to CT45 and Other Tumor Antigens in Hodgkin Lymphoma Patients.
To evaluate the possible humoral immune response to CT45, 253 sera samples from 67 cHL patients were tested by ELISA against recombinant CT45 protein, other CT antigens, and p53. At least two sera from every patient were tested, including one taken at the time of diagnosis. In only one case did two sera from the same patient show an anti-CT45 antibody at a titer of 1:150. No sera samples reacted with NY-ESO-1, MAGE-A1, MAGE-A3, or p53.
Discussion
Hematological malignancies, both leukemia and lymphoma, have been shown to express CT antigens at low frequencies (15, 16, 18), the only exception being myeloma, which frequently expresses CT antigens, particularly in the later stages of disease (18, 22). Most of these studies, however, did not classify the types of lymphoma analyzed, and whether the CT expression pattern would vary in different types of lymphomas is not clear. Xie et al. (15) examined the mRNA expression of nine CT antigens (MAGE-A3, MAGE-A4, NY-ESO-1, CT7, SSX1, SSX2, SSX4, SCP1, and HOM-TES-85) by RT-PCR and found that 10 of 10 follicular lymphomas and six of seven chronic lymphocytic leukemias were negative for all CT antigens, the only exception being a case of chronic lymphocytic leukemia that expressed SCP1 but no other CT antigens. In comparison, these authors found DLBCLs expressed various CT antigens at a frequency of <10% (SSX2) to 25% (SCP1). Similar to these findings, we found all low-grade lymphomas, including small lymphocytic lymphoma, follicular lymphoma, and mantle cell lymphoma, to be negative for CT45 expression. In contrast, DLBCL showed strong CT45 positivity in 15% (19/126) of the cases (and in 22% if cases with weaker focal expression were included). This finding is consistent with the previous observation that CT antigens tend to be activated in high-grade neoplasms and less frequently in their low-grade counterparts (2), and the observed frequency of 10–20% in this subset of lymphoma also falls into the expected range for CT antigen expression.
In contrast, the 50–60% frequency of CT45 expression in cHL is clearly surprising and is higher than that previously observed in the “CT-rich” ovarian carcinomas (37%) (11) and melanomas. This exceptionally high frequency was similar to that observed by Heidebrecht et al. (19) and raised the possibility that, unlike the aberrant epigenetic activation of CT45 gene in most tumor types, CT45 might represent a differentiation antigen of a cell type from which the neoplastic RS cell emerges. Given this possibility, we evaluated resting as well as reactive lymphoid tissues for CT45 expression. CT45-positive cells were found in significant numbers only in a case of florid follicular hyperplasia with progressively transformed germinal centers. This finding differs from the study by Heidebrecht et al. (19) in which the authors found no evidence of CT45 expression in the reactive lymphoid tissues that they examined, including lymph nodes and tonsils from cases of infectious mononucleosis that showed “immunoblasts and Hodgkin-like B blasts.”
At least three possibilities can be contemplated to explain the expression of CT45 in the reactive lymph node. First, as B cells undergo rapid proliferation following strong exogenous antigen stimulation, many genes are transcriptionally activated and, there could be a phase of global hypomethylation that would allow CT antigens to be transiently and perhaps aberrantly expressed. However, reactive lymph nodes have not been shown to express other CT antigens, and our failure to detect expression of NY-ESO-1, a CT antigen known to be activated by hypomethylation, also would argue against this hypothesis. The second possibility is that CT45 might be a physiological, nonneoplastic marker for a small subset of activated B cells that occur in follicular hyperplasia. This scenario would suggest that CT45-positive Hodgkin lymphomas, as well as some CT45-positive gray zone lymphomas and DLBCLs, represent a clonal expansion of B cells that are arrested at this particular state. This hypothesis is attractive in that it also would explain the observation that CT45 expression in cHL often is diffuse (>60% of neoplastic cells) and strong in intensity, unlike the common heterogeneous and focal expression pattern of other CT antigens in carcinomas (2, 11). Arguing against this possibility, however, is the rarity with which CT45-positive cells were observed in the reactive lymphoid tissues, absent in most lymph node and tonsil samples evaluated by us and by others (19). Also, although EBV has been associated with about 50% of the cHL, almost all EBV-positive cHLs in our study were CT45 negative, and examination of EBV-associated lymphadenopathy (19) also showed no evidence of CT45 expression in these activated B cells. The third possibility is that this case of florid follicular hyperplasia indeed represents an emerging cHL, and CT45 positivity is a marker of the transformed B cells that may or may not have achieved a fully malignant phenotype. Unfortunately, no further clinical follow-up was available for this patient, and the fate of the lymphadenopathy in this case was unknown. Analysis of the same lymph node for CD30 expression showed many more CD30-positive cells than CT45-positive cells and no evidence of coexpression of these two markers. This last hypothesis, therefore, remains to be explored further.
Pertinent to this notion that CT45 might be related to the pathogenesis of at least a subset of cHL is the finding of frequent CT45 expression in gray zone lymphoma. In our series of 11 cases, 5 cases showed strong diffuse CT45 expression. This frequency is similar to that in cHL and is significantly higher than the frequency seen in DLBCL, indicating that gray zone lymphoma more closely resembles cHL, at least in the cases included in this study. Given the generally aggressive clinical course of gray zone lymphoma and the prominent expression of CT45, it would be logical to consider CT45 as a potential immunotherapeutic target for this entity and for treatment-refractory cHL. To evaluate this possibility and to explore the spontaneous immunogenicity of CT45, we analyzed a large series of serum samples from patients who had cHL, but convincing anti-CT45 antibody response was only found in two sera from a single patient. Given the >50% frequency of CT45 antigen expression in these patients, this finding indicates that either CT45 is of low immunogenicity or that the anti-CT45 immune responses in these patients have been suppressed. It is well documented that tumor-infiltrating lymphocytes (TILs) in cHL, distinctive from anti-tumor TILs found in solid tumors such as melanoma, are dominated by Th2 cells and FOXP3-positive regulatory T (Treg) cells (9, 23, 24), whereas cytotoxic T cells are underrepresented. Moreover, it was shown recently that the nature of the TILs in cHL is of prognostic significance, and patients with a high ratio of Treg cells to Th2 cells would have a significantly shortened disease-free survival (24). This finding, in conjunction with our failure to identify any antibody response to MAGE-A, NY-ESO-1, and p53 in these patients, led us to believe that specific antitumor responses are profoundly suppressed in cHL patients as a result of the Treg-rich microenvironment, and this suppression would explain the lack of anti-CT45 response in the presence of abundant CT45 antigen expression. If this notion is correct, a cancer-vaccine approach, possibly in conjunction with strategies to “take the brake off” the anti-tumor immunity, such as anti-CTLA4 antibody (25), might enhance anti-CT45 effector responses and be clinically beneficial.
Materials and Methods
Tissues and Serum Samples.
Normal and tumor tissues used for this study were obtained from Department of Pathology and Laboratory Medicine at New York Presbyterian Hospital-Weill Cornell Medical Center following approval by the Institutional Review Boards (IRB). Except for DLBCL, TMA for all other non-Hodgkin lymphoma and for reactive lymph nodes were composed of three 1.5-mm tissue cores per case. For the DLBCLs, two types of TMA were employed: one consisting of three 1.5-mm cores per case (76 cases) and the other consisting of three 0.6-mm cores per case (50 cases). Because of the focal nature of the neoplastic RS cells in cHL, whole sections were used initially; however, evaluation of an array of 25 cHL cases consisting of three 1.5-mm cores produced similar findings and thus were included in the study. Gray zone lymphomas were analyzed using whole sections. All cases analyzed showed overlapping morphologic, and immunophenotypic features between cHLs and DCLBL, including the presence, at least focally, of confluent growth of tumor cells resembling RS cells. Immunohistochemically, the tumor cells in these cases generally expressed CD45 while strongly expressing CD20.
Sera samples used for ELISA testing were obtained from cHL patients at Medizinische Klinik I, Saarland University Medical School, Homburg, Germany. Serum samples were collected at the time of initial diagnosis and at various treatment points afterward, following an IRB-approved protocol. These cases are different from the tissue samples used for immunophenotypic analysis.
Antibodies Against CT45 and Other Markers.
LX-CT45-10, an anti-CT45 mouse monoclonal antibody produced against recombinant CT45, was used as previously described (11). A mouse monoclonal antibody 6C1 (20) was used for detection of MAGE-A family genes. NY-ESO-1 was detected by an affinity-purified rabbit polyclonal antibody produced against recombinant NY-ESO-1 protein. All other antibodies were obtained commercially.
In addition to CT antigens, all cHL were analyzed for expression of CD20, CD15, and CD30, and all non-Hodgkin lymphomas were evaluated for the expression of CD10, BCL6, MUM1/IFR4, p53, and BCL2. Lymphomas that were CD10 positive or CD10 negative/BCL6 positive/MUM1 negative were classified as GC phenotype, whereas lymphomas that were CD10 negative/BCL6 negative or CD10 negative/BCL6 positive/MUM1 positive were considered to be of the non-GC phenotype. Cases that did not conform to these phenotypes were classified as null phenotype (26).
Immunohistochemical Analysis.
Immunohistochemical analysis was performed using formalin-fixed paraffin-embedded tissues and on tissue microarrays composed of routinely processed (formalin-fixed and, rarely, B5-fixed) tissues. Five-micrometer tissue sections on coated slides were deparaffinized, rehydrated, and treated with 0.03% H2O2 to block the endogenous peroxidase activity. For CD15 (BD Transduction Laboratories), CD10 (Novocastra-Vision Biosystems), p53 (BioGenex), CD20, CD30, BCL2, BCL6, and MUM1/IFR4 (Dako Cytomation), the tissue and TMA sections were subjected to previously optimized antigen retrieval and detection as determined by the Immunopathology Laboratory/Weill Cornell Pathology Research Laboratory and counterstained with hematoxylin. All these immunostaining studies were performed on the Bond Max Autostainer (Leica). Antigen retrieval for CT45 was performed by microwaving in 10 mM citrate buffer, pH 6.0, for 15 min. The sections then were incubated with the CT45 monoclonal antibody for 1 h at room temperature. This incubation was followed by incubation with the DAKO Envision+ horseradish peroxidase mouse detection system (Dako Cytomation) and colorimetric development with diaminobenzidine (Leica-Vision Biosystems). The slides were counterstained with hematoxylin and evaluated. Because CT antigens are known to be heterogeneously expressed in cancer, often in small subsets of tumor cells, any case with any cells showing detectable nuclear staining was scored as CT45-positive, and the percentage of positive cells was estimated and recorded. Tumors with >50% positive cells were considered “diffuse,” whereas those with <50% were considered “focal.” Positive immunoreactivity also was scored from + to +++ based on the intensity of the nuclear staining. Negative controls used in the study included tissue cores from CT45-negative tissues (spleen, tonsil, kidney, liver, and placenta) on the TMAs and IgG control antibodies.
ELISA.
Analysis of serum samples by ELISA was performed using the method described in (27). Briefly, recombinant CT45 protein and control protein antigens LAGE-1, MAGE-1, MAGE-3, and p53 were adsorbed at 1 μg/mL to low-volume 96-well plates (Corning). After blocking with PBS containing 5% nonfat milk, serum samples were tested over a range of serial 4-fold dilutions from 1:100 to 1:6,400. After incubation, plates were washed with PBS containing 0.2% Tween and rinsed with PBS. Plasma IgG bound to antigens was detected with alkaline-phosphatase–conjugated specific monoclonal antibodies (Southern Biotech). After addition of ATTOPHOS substrate (Fisher Scientific), absorbance was measured using a fluorescence reader Cytofluor Series 4000 (PerSeptive Biosystems). A reciprocal titer was calculated for each serum sample as the maximal dilution still significantly reacting to a specific antigen. Specificity was determined by comparing seroreactivity among various antigens tested. In each assay, sera of patients with known presence or absence of specific reactivity were used as controls. Titers were considered significant if >100 and if confirmed in repeat experiments.
EBV in Situ Hybridization.
The EBV status of the cHL cases was determined by in situ hybridization using an EBER probe performed according to the manufacturer’s instructions (Vision Biosystems).
Statistics.
The frequencies of CT45 expression in various subtypes of cHL and in B-cell lymphomas with various phenotypes were compared statistically using SPSS software and Fisher’s exact test. A difference with p-value of ≤0.05 was considered statistical significant.
Footnotes
The authors declare no conflict of interest.
References
- 1.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 2.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 4.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 5.Chen YT, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jäger E, et al. Monitoring CD8 T cell responses to NY-ESO-1: Correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis ID, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jäger E, et al. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: Correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Marchand M, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, et al. Identification of cancer/testis-antigen genes by massively parallel signature sequencing. Proc Natl Acad Sci USA. 2005;102:7940–7945. doi: 10.1073/pnas.0502583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YT, et al. Cancer/testis antigen CT45: Analysis of mRNA and protein expression in human cancer. Int J Cancer. 2009;124:2893–2898. doi: 10.1002/ijc.24296. [DOI] [PubMed] [Google Scholar]
- 12.De Smet C, Lurquin C, Lethé B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Smet C, Loriot A, Boon T. Promoter-dependent mechanism leading to selective hypomethylation within the 5′ region of gene MAGE-A1 in tumor cells. Mol Cell Biol. 2004;24:4781–4790. doi: 10.1128/MCB.24.11.4781-4790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Güre AO, Wei IJ, Old LJ, Chen YT. The SSX gene family: Characterization of 9 complete genes. Int J Cancer. 2002;101:448–453. doi: 10.1002/ijc.10634. [DOI] [PubMed] [Google Scholar]
- 15.Xie X, et al. Differential expression of cancer testis genes in histological subtypes of non-Hodgkin’s lymphomas. Clin Cancer Res. 2003;9:167–173. [PubMed] [Google Scholar]
- 16.Lim SH, Austin S, Owen-Jones E, Robinson L. Expression of testicular genes in haematological malignancies. Br J Cancer. 1999;81:1162–1164. doi: 10.1038/sj.bjc.6690824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colleoni GW, et al. Expression of SSX genes in the neoplastic cells of Hodgkin’s lymphoma. Hum Pathol. 2002;33:496–502. doi: 10.1053/hupa.2002.124909. [DOI] [PubMed] [Google Scholar]
- 18.Chambost H, et al. Expression of gene MAGE-A4 in Reed-Sternberg cells. Blood. 2000;95:3530–3533. [PubMed] [Google Scholar]
- 19.Heidebrecht HJ, et al. Characterization and expression of CT45 in Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:4804–4811. doi: 10.1158/1078-0432.CCR-06-0186. [DOI] [PubMed] [Google Scholar]
- 20.Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer. 2000;86:749–751. doi: 10.1002/(sici)1097-0215(20000601)86:5<749::aid-ijc24>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph P, et al. Ki-A10, a germ cell nuclear antigen retained in a subset of germ cell-derived tumors. Am J Pathol. 1999;154:795–803. doi: 10.1016/S0002-9440(10)65326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungbluth AA, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 23.Poppema S, Potters M, Emmens R, Visser L, van den Berg A. Immune reactions in classical Hodgkin’s lymphoma. Semin Hematol. 1999;36:253–259. [PubMed] [Google Scholar]
- 24.Schreck S, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–39. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 25.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans CP, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 27.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]