Abstract
Functional neuroimaging studies have converged on a core network of brain regions that supports speech production, but the sublexical processing stages performed by the different parts of this network remain unclear. Using an fMRI adaptation paradigm and quantitative analysis of patterns of activation rather than contrast subtractions alone, we were able to identify a set of neural substrates predominantly engaged in phonemic, syllabic, and supra-syllabic levels of processing during speech. Phoneme-level processes were found in the left SMA, pallidum, posterior superior temporal gyrus, and superior lateral cerebellum. Syllable-level processes were found in left ventral premotor cortex, and supra-syllabic processes related to phonological chunking were found in the right superior lateral cerebellum. Active regions that were not sensitive to sublexical manipulations included primary motor and auditory cortical areas, and medial cerebellum. These results offer a quantitative technique for localizing sublexical neural processes that are difficult to dissociate using non-invasive imaging techniques and provide the beginnings of a “brain map” for language output.
Keywords: fMRI, speech, premotor cortex, SMA
Introduction
Many studies in recent years have investigated the brain network involved in speech production and have identified a set of regions supporting processes related to reading, retrieving, and articulating words. These regions include primary motor, somatosensory, and auditory cortical areas, medial and lateral premotor areas, the inferior frontal gyrus, superior temporal gyrus , anterior insula, and subcortical regions including the medial and lateral cerebellum, basal ganglia, and thalamus (Chein et al., 2002; Indefrey and Levelt, 2004; Price, 2000; Turkeltaub et al., 2002; Vigneau et al., 2006). Nevertheless, the details of the functional-anatomical relationships of the neural processes related to preparation and execution of articulatory programs remain unclear. One problem is that there is still some disagreement in the literature regarding the units that drive articulation (e.g. phonemes vs syllables or even entire words and short phrases). Another problem is that high spatial resolution non-invasive imaging techniques such as fMRI have low temporal resolution (on the order of seconds), which makes it hard to disentangle processes happening over hundreds of milliseconds.
Despite the uncertainty over the units that drive articulation, previous neuroimaging studies have presented evidence that manipulating syllabic and phonemic content of stimuli modulates the activity of certain regions from the speech network. Among the areas modulated by syllable-level processes are the pre-supplementary motor area (pre-SMA), anterior insula/frontal operculum (FO), and the cerebellum (Bohland and Guenther, 2006). Regions that have been associated with different aspects of phoneme-level processes include the inferior frontal sulcus (Bohland and Guenther, 2006), the posterior parts of the inferior frontal gyrus (Riecker et al., 2008), the posterior superior temporal gyrus (Hickok, 2000), and the superior cerebellum (Chen and Desmond, 2005a). However, most of these studies relied on techniques such as manipulating syllable complexities (which typically involves simultaneous manipulation of phonemic content, thereby creating a confound), or on syllable frequency effects, which, although clearly demonstrated in psycholinguistic experiments (Carreiras and Perea, 2004; Cholin et al., 2006; Laganaro and Alario, 2006), have proven difficult to capture in fMRI studies (Carreiras et al., 2006; Riecker et al., 2008).
Neurologically plausible computational models of speech provide further insight into the potential neural substrates of sublexical processes. The DIVA (Directions Into Velocities of Articulators) model, which is a neuroanatomically and mathematically defined model of speech motor control (Guenther et al., 2006), is one such model that has been developed in our lab since 1992. In this model, production of a syllable starts with the activation of a set of cells in the left ventral premotor cortex (vPMC) that correspond to that syllable. Projections from vPMC to somatosensory and auditory areas encode sensory expectations for the syllable, and projections from vPMC to the primary motor cortex (both directly and via the anterior medial cerebellum) generate the appropriate motor commands for the syllable (Guenther et al., 2006). The model thus predicts that cells in the left hemisphere ventral premotor cortex utilize syllabic units, whereas cells in the primary motor and sensory areas are indifferent to phonemic, syllabic, or supra-syllabic identity of the words being spoken.
In the present study we took some of the more common speech units believed to drive articulation – phonemes, syllables, and supra-syllabic sequences – and looked within the speech network for regions that are particulalry tuned to implement processes related to those units. Psycholinguistic research has highlighted the role played by phoneme and syllable sized units in speech production (Houde and Jordan, 1998). Phonemes are often involved in slips of the tongue (e.g. uttering “heft lemisphere”, for “left hemisphere”; see the classic studies by (Fromkin, 1971; Garrett, 1975; Shattuck-Hufnagel, 1983). This suggests that phonemes are retrieved individually at some stage in the speech production process. Similarly, Houde and Jordan (1998) showed that when participants are presented with perturbed auditory feedback of their ongoing vowel pronunciation, they change the way they produce the vowel to compensate for the perturbation and this change can carry over to syllables other than the perturbed syllable. They interpreted their results to suggest that the phoneme has a distinct neural representation. Syllables have also been shown to be functional units of speech production in a number of studies (Laganaro and Alario, 2006; Levelt and Wheeldon, 1994). Nevertheless, it is still largely unclear how these sublexical processes are implemented at the neural level and combined to drive the final speech motor programs during articulation.
In order to investigate the levels of phonological processing that the different brain regions of the speech production network subserve, we employed an imaging protocol that takes advantage of the observed decrease of hemodynamic response to repeated stimuli, a phenomenon known as fMRI repetition suppression (fMRI-RS), also known as fMRI adaptation, habituation, or repetition priming (Grill-Spector et al., 2006; Grill-Spector and Malach, 2001; Henson, 2003). Many studies have suggested that the reduction of signal following repetition is stimulus-specific and occurs in regions that are a subset of those regions that were most active during the initial stimulus presentation, providing evidence for the functional-anatomic selectivity of the fMRI signal attenuation (Buckner et al., 1998; Grill-Spector and Malach, 2001; Rice et al., 2007). Moreover, fMRI-RS signal attenuation has been shown to be related to the actual processing of the stimuli and not to effects such as reduced processing load resulting from familiarity with the stimulus (Xu et al., 2007). Finally, fMRI-RS effects are not confined to perceptual processes; previous studies have used successfully fMRI-RS paradigms to look at the functional organization of motor and speech processes (Buckner et al., 2000; Dehaene-Lambertz et al., 2006; Gabrieli et al., 1996; Gold et al., 2005; Kilner et al., 2009; Lingnau et al., 2009; Majdandzic et al., 2009).
In the present experiment we constructed blocks of stimuli (pseudowords) that differed in the repetition rate of phonemes, syllables, or entire pseudowords (supra-syllabic sequences). Pseudowords were used instead of words because of our focus on sublexical processes leading to articulation rather than lexical retrieval or semantic access. We hypothesized that if there are regions that are particularly tuned to one or another of the sublexical processes, then these areas should exhibit differential adaptation patterns depending on which sublexical stimulus features are being repeated. Additionally, we investigated representations at the supra-syllabic sequence level, defined here as whole pseudowords (with the implicit understanding that these representations relate to the formation of sequences of phonemes and/or syllables in real multi-syllabic words or short phrases). We also looked for phonologically insensitive regions, defined as active regions whose processing does not depend on phonemic, syllabic, or suprasyllabic content.
Materials and Methods
Participants
Twenty two right-handed native French speakers with normal or corrected to normal vision and no reported history of neurological, language, or hearing disorders took part in the study. Written informed consent was obtained from all participants and the study protocol was approved by the CCPPRB-Marseille-1 ethics committee and Boston University Institutional Review Board. Two participants were unable to complete the experiment and two others displayed unacceptable head motion (more than 2mm) and were excluded from the study. The final analysis was performed on 18 participants (11 men, 7 women; age range 18-30 years).
Paradigm design
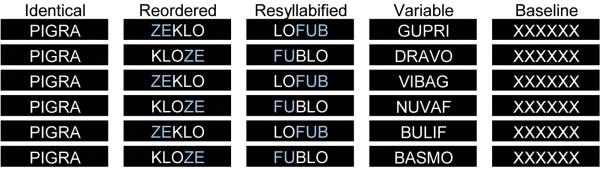
Participants lay inside an MRI scanner and read aloud bi-syllabic pseudowords displayed orthographically one by one on a screen placed at the back of the scanner. The task involved four different conditions (Identical, Reordered, Re-syllabified, and Variable) and a baseline condition (Figure 1). Stimuli were presented in blocks of six trials, each block corresponding to one of the four experimental conditions or the baseline condition. In the Identical condition the same pseudoword was repeated (e.g., FU.BLO, FU.BLO, FU.BLO, FU.BLO, FU.BLO, FU.BLO). In the Reordered condition syllables were rearranged while preserving the syllable consonant-vowel order (e.g., ZE.KLO, KLO.ZE, ZE.KLO, KLO.ZE, ZE.KLO, KLO.ZE). In the Resyllabified condition pseudowords were constructed by re-syllabification with the same set of phonemes (e.g., LI.MUF, MU.FLI, LI.MUF, MU.FLI, LI.MUF, MU.FLI). Finally, the Variable condition involved reading six different phonetically unrelated pseudowords (e.g. GU.PRI, DRA.VO, VI.BAG, NU.VAF, BU.LIF, BAS.MO). Note that the syllable boundaries indicated here were not in the actual stimuli, which were presented to the subject as a single pseudoword with no breaks indicated. Participants had no indication of where syllables should break other than their implicit native knowledge of the language. The baseline condition required silent viewing of the string ‘XXXXX’ presented six times.
Figure 1.
The four experimental conditions and baseline condition of the fMRI study. Each black box corresponds to a single presentation of a pseudoword displayed on the screen in white on a black background. There are no color indications of syllabic structure in the experimental setup; different colors are used here only to highlight the syllabic boundaries (according to French language rules) and the differences between conditions. In the Identical condition, the same word is repeated six times within a block of trials. In the Reordered condition, two words formed by reordering the same two syllables are repeated three times each. In the Resyllabified condition, two words formed using the same phonemes but different syllabification are repeated three times. In the Variable condition, six unique words with unique syllables are produced. The Baseline condition consisted of silent viewing of a string of X’s.
Stimuli (individual pseudowords) were presented on the screen for 1200 ms, followed by a blank screen for 300 ms. The duration of a block was 9 seconds. Each block was followed by a variable resting time of 3-5 seconds during which subjects viewed a white fixation cross in the center of a dark screen. Participants were instructed to begin speaking as soon as they saw a stimulus appear on the screen. The experiment consisted of 3 runs, each with 35 blocks, 7 from each of the 5 conditions, ordered pseudo-randomly. There were a total of 21 blocks per condition for each participant. Each run was approximately 8 minutes long.
Constraints on material selection (in particular using different consonants within each pseudoword, and following resyllabification rules) necessitated multiple presentations of some blocks to the participants. In the Variable condition, a given block was presented between one and four times. The same block was never used more than twice in the same run. In the other conditions (Identical, Reorder, and Resyllabify) a given block was presented at most twice and never in the same run (a complete list of the stimuli in each block is provided in the Supplementary Materials).
Stimuli
All stimuli were phonetically legal French bisyllabic pseudowords. Pseudowords were preferred to words because of our focus on sublexical processes rather than lexical access or semantic processes. Neither the pseudowords nor the syllables that composed them are listed as French orthographic words in the database Lexique (New et al., 2001). The syllables do exist in French, as they are present in other multi-syllabic words. Syllable frequency was calculated using log transformed token counts in occurrences per million based on the Lexique database. This frequency measure did not differ across the four conditions (F[3-188] = .98, p = .41). The pseudowords consisted of sequences of consonant (C) and vowel (V) phoneme combinations forming common syllabic frames (CV, CCV for the Identical and Reorder conditions and CV, CCV, CVC for the Resyllabify and Variable conditions). All pseudowords had only one legal breakpoint into syllables of the French language (e.g. in the Re-syllabified condition FU.BLO and LO.FUB are both legal but FUB.LO or LOF.UB are not legal syllabifications). The stimuli had the same number of phonemes. No phoneme or syllable appeared more than once within any pseudoword. A straightforward orthographic transcription was constructed for each item. There was a variable number of letters in these transcriptions (between 5 and 7); whereas most French phonemes can be transcribed with one letter, some are naturally transcribed with two letters.
fMRI data acquisition
Imaging was performed on a 3-Tesla MRI whole body scanner (Bruker Medspec). Participants lay supine on the scanner bed with foam padding applied between the participant’s head and the coil to help constrain head movement. Before entering the scanner, participants practiced reading the pseudowords with minimal articulatory movements and avoiding head motion. The pseudowords used for these practice sessions were not reused during the actual experiment. Inside the scanner, stimuli were projected centrally, white on a black background, at the back of the magnet bore. Participants viewed the projected stimuli through a head-coil mounted mirror placed comfortably in front of their eyes. A high resolution anatomical volume was acquired for each participant (T1 weighted MP-RAGE sequence, TR=11.9 ms, TE = 5.6 ms, flip angle = 30°, voxel size = 0.898 × 1 × 1.42 mm). Functional images were collected using 32 axial slices covering the whole brain (slice thickness = 3 mm; slice gap = 1 mm; field of view = 192 mm2; matrix size 64 × 64; TR 2133.3 ms; TE = 30 ms; flip angle = 79.5°). The slices were oriented parallel to a plane through the anterior and posterior commissures. The continuous sampling design was chosen over sparse sampling design due to the need to induce fMRI adaptation effects through rapid repetition of the presented stimuli.
Voxel-wise data analysis
All fMRI data were preprocessed and analyzed using the SPM2 software package provided by the Wellcome Department of Imaging Neuroscience, University College London, UK (Friston et al., 1995). The first six scans of each run contained no stimulus information and were discarded to allow for signal stabilization. The remaining functional images were corrected for slice acquisition timing in reference to the middle temporal slice (interlaced acquisition). To correct for motion, a rigid body transformation was applied to each frame in order to realign images within and across runs. Images were realigned with respect to the first functional image of the run temporally closest to the anatomical image acquisition. After realignment, translation and rotation movement parameters were examined for each participant individually, and those participants who exhibited head movements of more than 2mm in any direction were discarded from further analysis (only two such participants were found). Subsequently, images were coregistered with the T1- weighted anatomical dataset (Collignon et al., 1995), and spatially normalized into standard stereotaxic space using the EPI template provided by the Montreal Neurological Institute (Evans et al., 1993; Mazziotta et al., 2001). Finally, all normalized images were spatially smoothed with a 12 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. This smoothing was applied only during the voxel-wise data analysis and not during the subsequent ROI analysis (see below).
Group statistics were assessed using fixed and mixed effects procedures. In fixed-effect analysis, contrast-of-interest images were generated for each participant by comparing the relevant condition parameter estimates on a voxel-by-voxel basis. Five conditions were modeled: Identical, Reordered, Re-syllabified, Variable, and Baseline. Estimates for these analyses were obtained using a general linear model where conditions are treated as fixed effects. The first stimulus of each block was modeled separately from the rest of the block since the first presentation would induce the same response in all conditions. The response at each epoch was convolved with a canonical hemodynamic response function and data were high-pass filtered with a standard filter cutoff frequency of 128s. To avoid artifacts due to possible signal fluctuations, signal normalization was performed by acquiring the mean signal value for each volume from the preprocessed data and introducing these values to our model as an additional regressor for each session. Group effects on all 18 participants were then assessed by treating the participants as random effects and performing one-sample t-tests across the individual contrast images. Effects related to a particular analysis were considered significant if they passed a false discovery rate (FDR), PFDR, < .05. Each individual condition was contrasted with baseline to assess relative activation (Identical - baseline, Reordered - baseline, Re-syllabified - baseline, and Variable - baseline). Additionally, all speaking conditions were combined and contrasted against baseline to determine the average effect across all conditions (Collapsed - baseline).
The contrast maps are shown in terms of effect size. The ‘Automated Anatomical Labeling’ (ALL) atlas (Tzourio-Mazoyer et al., 2002) was used to identify labels for activated peaks. The results were projected onto a cortical surface representation of the canonical SPM brain. The surface rendering was obtained using the FreeSurfer software (http://surfer.nmr.mgh.harvard.edu).
Region-wise data analysis
In addition to the voxel-wise analysis, a region of interest (ROI) analysis was performed to improve statistical power and provide a more accurate mapping of function to anatomical region (Nieto-Castanon et al., 2003). Regions included in the analysis were chosen from among those typically active in speech production studies (Alario et al., 2006; Bohland and Guenther, 2006; Chein et al., 2002; Guenther et al., 2006; Indefrey and Levelt, 2004; Price, 2000; Riecker et al., 2008; Tourville et al., 2008; Turkeltaub et al., 2002). These regions included ventral parts of primary motor and somatosensory areas, medial premotor areas, ventral premotor cortex, the inferior frontal gyrus, superior temporal gyrus, anterior insular cortex, the cerebellum, and the basal ganglia. Functionally heterogenic regions such as the inferior frontal gyrus (IFG) that showed variable peak loci in the different speech production studies were subdivided into smaller regions in order to capture the potential differences in activations within the same region. The inclusion of the ventral portions of the primary motor and somatosensory regions is based on the evidence that the motor and sensory states of the articulators are represented mainly in the ventral portions of pre- and post-central gyri (see Guenther et al., 2006, Appendix A for a detailed review). The remaining regions were divided according to anatomical markers into anterior/posterior and superior/inferior portions (see Table 1 for a complete list of ROIs).
Table 1.
Regions of interest (ROIs) included in the statistical analysis
| ROI acronym | ROI name |
|---|---|
| Rolandic cortex | |
| vMC | Ventral motor cortex |
| vPMC | Ventral premotor cortex |
| SMA | Supplementary motor area |
| pre-SMA | Pre-supplementary motor area |
| vSC | Ventral somatosensory cortex |
| Frontal Cortex | |
| aIFs | Anterior inferior frontal sulcus |
| pIFs | Posterior inferior frontal sulcus |
| vIFt | Ventral inferior frontal triangularis |
| dIFt | Dorsal inferior frontal triangularis |
| vIFo | Ventral inferior frontal opercularis |
| dIFo | Dorsal inferior frontal opercularis |
| FO | Frontal operculum |
| Temporal Cortex | |
| aSTg | Anterior superior temporal gyrus |
| pSTg | Posterior superior temporal gyrus |
| adSTs | Anterior dorsal superior temporal sulcus |
| pdSTs | Posterior dorsal superior temporal sulcus |
| Hg | Heschl’s gyrus |
| PP | Planum porale |
| PT | Planum temporale |
| PO | Parietal operculum |
| pCO | Posterior central operculum |
| Insular cortex | |
| aINS | Anterior insula |
| Parietal | |
| aSMg | Anterior supramarginal gyrus |
| Cerebellum | |
| amCB | Anteror medial cerebellum |
| spmCB | Superior posterior medial cerebellum |
| splCB | Superior posterior lateral cerebellum |
| alCB | Anteror lateral cerebellum |
| ipmCB | Inferor posterior medial cerebellum |
| Subcortical nuceli | |
| Caud | Caudate |
| Put | Putamen |
| Pal | Pallidum |
| Tha | Thalamus |
ROIs were selected based on consistent findings from previous speech production studies, the present results, and on the DIVA model predictions. The table provides the ROI abbreviated label and its expanded name.
Parcellation of cortical, subcortical, and cerebellar ROIs from structural MRI scans was performed using the Freesurfer software package. Subcortical ROIs were determined with the FreeSurfer subcortical training set (Fischl et al., 2002). Cortical and cerebellar ROI definitions were determined by training FreeSurfer cortical (Fischl et al., 2004) and subcortical (Fischl et al., 2002) classifiers based on a functional-neuroanatomical atlas tailored for speech studies (Tourville and Guenther, 2003). The cortical ROI boundaries are a modified version of the parcellation system defined by the Center for Morphometric Analysis (CMA) at Massachusetts General Hospital (Caviness et al., 1996). Following spatial realignment, functional data were subjected to a rigid body transform and coregistered with the structural data set. The BOLD response averaged across all voxels within each ROI mask was then extracted. Regional noise temporal correlations were removed by whitening a fit of the estimated noise spectrum within each ROI. Average regional responses for each event were modeled using a canonical hemodynamic response function and the same contrasts as described in the voxel-wise analysis were evaluated. The ROI tools were also used to test for lateralization in particular ROIs. The effect sizes estimated for each subject in the left and right hemisphere for a particular ROI were entered into a paired t-test. Lateralization was considered significant for p < 0.05. Results from the ROI analysis were also used to evaluate the activity patterns described below.
Across-condition activity patterns
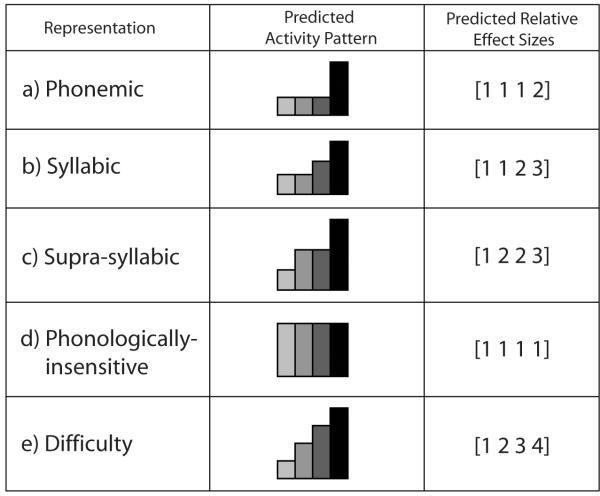
The stimuli and task were chosen to result in different patterns of adaptation across conditions depending on the primary phonological representation (if any) processed by a particular brain region. To assess this quantitatively, we first constructed models of activity across the four conditions for each representation type (Figure 2). In regions where neurons are sensitive to phonemic identity independent of syllabic identity, we expect the first three speaking conditions (all of which utilize the same number of phonemes) to show the same amount of activity; the fourth condition, which contains many more phonemes than the first three conditions, should show less adaptation or, equivalently, higher activity (specifically, effects size) (pattern a, quantified as an expected pattern of [1 1 1 2]). Regions in which processing is dominated by syllable-level units will have equal activity in the first two conditions, higher activity in the third condition, and even higher activity in the fourth condition (pattern b, quantified as [1 1 2 3]). Regions processing supra-syllabic sequences should exhibit activity pattern c ([1 2 2 3]) since in condition one the same pseudoword is repeated, in conditions two and three two different pseudowords are being repeated, and in condition four, six different pseudowords are produced. Regions that are insensitive to any of the investigated phonological representations (phonemes, syllables, and supra-syllabic sequences) are not expected to show a significant difference across conditions (pattern d in Figure 2) since all four conditions were very similar in nature apart from the phonological variations we introduced at the phonemic and syllabic level. Thus the phonologically insensitive regions were expected to exhibit a pattern of activity across the four conditions of [1 1 1 1]. Note that the numbers 1 through 4 simply indicate the rank order of activity; thus [1 1 1 2] and [2 2 2 3] denote the same pattern of activity. One limitation of this design is that if a region processes both phonemic and syllabic units (a combination of patterns a and b), the current design will only allow us to identify the syllable-level processes. Similarly, if a region processes both suprasyllabic sequences and individual phonemes, our design will only allow us to identify the existence of supra-syllabic sequence level processes..
Figure 2.
Predicted activation patterns for putative functional representations. Depending on the pattern of activity across the four experimental conditions, the speech regions can be classified into four groups: a) phonemic, b) syllabic, c) supra-syllabic, d)phonologically-insensitive, and e) task difficulty (see Materials and Methods for details). The bar plots in the center column represent the relative level of signal change across the four conditions (conditions 1 through 4 from left to right). On the right, each model condition activity is assigned an effect size value from 1- 4 representing the relative size of activation in that condition.
Finally, as a form of control, we constructed an activity pattern that represented the concept of “task difficulty” i.e. regions that are not associated with any particular phonological unit in our study. Although difficulty is a somewhat subjective concept, the current conditions lent themselves to a straightforward estimate of difficulty level as follows. Increasing the elements that repeated within a condition (phonemes, syllables, and pseudowords) was considered to decrease the difficulty of the condition. Thus the Identical condition was viewed as the simplest and the Variable as the most difficult. Similarly, the third condition is more difficult than the second due to the resyllabification requirement. This leads to predicted activity pattern e in Figure 2 for a region whose activity is sensitive to task difficulty but not to any one specific sublexical unit. The Difficulty pattern may also result from a combination of processes related to all units - phonemic, syllabic, and suprasyllabic – and thus a match to this pattern does not automatically imply that a region is not involved in phonological processes.
Model comparison framework: theory
For any given set of a priori models {M1 ... Mm}, where each model Mi can be characterized by a set of inter-related hypotheses {Hi1...Hin}, we define pij as the estimated p-value (false positive level under null hypothesis Hij) of a chosen statistical test assessing the j-th hypothesis of the i-th model. For each model Mi we then define a simple measure of fit as:
This measure represents the p-value of a standard conjunction test for multiple hypotheses. We then define the model-level p-value Pi from the expected distribution of λi values under any alternative model:
This distribution is computed numerically using Monte Carlo simulations of the alternative models Mk (k≠i). For each simulation of an alternative model Mk the resulting value of the fit measure λi is computed, and the conditional cumulative distribution function prob(·) of the resulting λi values is empirically estimated by accumulation over multiple simulations. The resulting model-level p-values Pi can be interpreted in the usual way as the false positive level of a test for each model under the null hypothesis of an alternative model.
Model comparison framework: application
This model comparison framework was used to characterize each region of interest across-condition pattern of BOLD responses. Five models were used (phonologically-insensitive, phonemic, syllabic, supra-syllabic, and difficulty). Each model was characterized by six hypotheses, defining the presence and expected directionality of each pairwise contrast comparing the BOLD response between each pair of baseline conditions (among four baseline conditions). Individual hypothesis tests consisted of second-level (across-subjects) t-tests (n=18). The hypothesis-level p-values pij were defined as:
pij = 2 · min(p, 1 – p) for any hypothesis Hij with expected pattern A=B
pij = p for any hypothesis Hij with expected pattern A<B
pij = 1 – p for any hypothesis Hij with expected pattern A>B
where the value p represents the p-value of a one-sided t-test comparing the conditions A and B. Monte Carlo simulations for the empirical estimation of the expected distribution of model-fit λ values were then computed using Gaussian random noise with means defined by each model’s expected across-condition activity pattern. Noise means were scaled by a factor equal to the 95-th percentile of the observed between-condition BOLD differences across all regions. Noise variances were matched to the observed between-subject variance in BOLD responses averaged across all regions and conditions. Empirical conditional cumulative distributions for each model-level test were computed from a total of 5000 Monte Carlo simulations, and used to derive model-level p-values from the data model-fits. The resulting model-level p-values Pi were reported for each region. Only region/model pairs with p-values below .05 are considered significant.
Results
Pseudoword production network
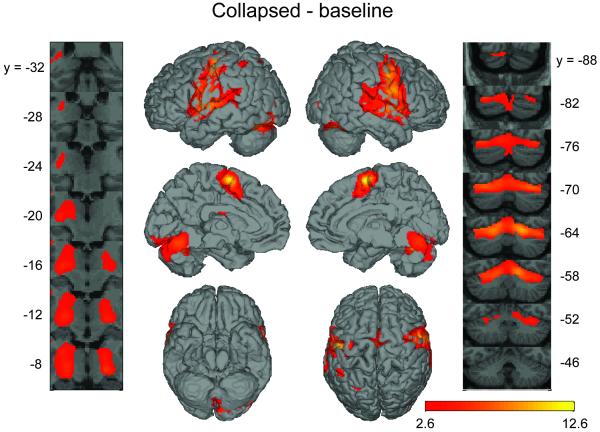
Reading pseudowords compared to the baseline condition (Collapsed - baseline) activated a network of regions commonly seen in a number of previous neuroimaging studies of speech production (Alario et al., 2006; Bohland and Guenther, 2006; Chein et al., 2002; Guenther et al., 2006; Indefrey and Levelt, 2004; Price, 2000; Riecker et al., 2008; Tourville et al., 2008; Turkeltaub et al., 2002). The voxel-wise activity analysis (Figure 3 and Table 2) showed peak activations in bilateral pre- and post-central gyri, bilateral superior temporal gyrus (STg), the medial premotor cortex (near the border of SMA and pre-SMA), and left insula near the opercular portion of the inferior frontal gyrus. Subcortical activations included bilateral putamen and right pallidum. There was also cerebellar activity in Lobule VI bilaterally, in Crus I on the left and Crus II on the right. The location of activity was similar in each individual speaking condition compared to baseline, though relatively minor differences existed (see Table 2).
Figure 3.
Significant voxel-wise activity in the collapsed – baseline contrast. Color scale represents t statistics plotted on surface-rendered canonical SPM brain. The statistical image was thresholded at PFDR < .05. Left hemisphere is represented by the top two cortical images on the left side and right hemisphere is represented by the top two cortical images on the right side. The figure also includes coronal slice series through the thalamus and basal ganglia (image on the extreme left) and the cerebellum (image on the extreme right) regions. In the slice series, the left hemisphere is shown at the left of the image. The MNI y-axis level of each coronal image is indicated by the adjacent coordinate.
Table 2.
Peak responses for each individual speaking condition and the collapsed data all compared to baseline
| Identical |
Reordered |
Re-syllabified |
Variable |
Collapsed |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Peak voxel (MNI) | Peak voxel (MNI) | Peak voxel (MNI) | Peak voxel MNI | Peak voxel MNI | ||||||||||||||||
| x | y | z | T | x | y | z | T | x | y | z | T | x | y | z | T | x | y | z | T | ||
| Rolandic cortex | |||||||||||||||||||||
| Left | Cingulum Mid | 0 | 8 | 44 | 6.53 | ||||||||||||||||
| Postcentral | −46 | −18 | 42 | 11.4 | −46 | −16 | 42 | 11.3 | −42 | −18 | 42 | 12.10 | −42 | −18 | 42 | 14.5 | −44 | −16 | 42 | 12.6 | |
| Postcentral | −56 | −14 | 26 | 8.42 | −56 | −14 | 24 | 8.55 | −56 | −14 | 24 | 8.71 | −52 | −12 | 50 | 10.2 | −56 | −14 | 24 | 8.9 | |
| Precentral | −52 | −8 | 32 | 8.88 | −50 | −8 | 32 | 9.32 | −52 | 2 | 20 | 8.1 | −50 | −8 | 30 | 9.36 | |||||
| Supp Motor Area | 0 | −2 | 62 | 9.09 | 0 | −2 | 62 | 11.79 | −2 | 0 | 60 | 14.8 | 0 | −2 | 62 | 12.3 | |||||
| Right | Postcentral | 48 | −10 | 36 | 9.63 | 50 | −10 | 36 | 10.4 | 48 | −10 | 36 | 10.47 | 48 | −10 | 36 | 10.8 | 48 | −10 | 36 | 10.7 |
| Postcentral | 64 | 0 | 20 | 8.01 | 66 | −2 | 22 | 8.25 | 66 | 2 | 22 | 8.58 | |||||||||
| Precentral | 66 | 6 | 22 | 9.36 | 58 | −2 | 42 | 9.76 | 54 | 0 | 40 | 9.02 | 50 | −2 | 44 | 9.71 | 52 | 0 | 42 | 9.62 | |
| Supp Motor Area | 2 | −2 | 62 | 11.4 | |||||||||||||||||
| Frontal Cortex | |||||||||||||||||||||
| Left | Frontal Inf Tri | −36 | 20 | 10 | 4.45 | −42 | 20 | 6 | 3.39 | ||||||||||||
| Right | Frontal Inf Tri | 44 | 38 | 26 | 3.33 | ||||||||||||||||
| Frontal Inf Oper | 46 | 12 | 6 | 7.29 | 48 | 14 | 2 | 7.65 | |||||||||||||
| Temporal Cortex | |||||||||||||||||||||
| Left | Temp Sup | −52 | 2 | 0 | 10.1 | −54 | 0 | −2 | 8.5 | −54 | 4 | 2 | 9.21 | −54 | 2 | −2 | 9.72 | −54 | 2 | 0 | 9.66 |
| Temp Sup | −54 | −32 | 20 | 6.74 | −62 | −12 | 6 | 7.97 | −66 | −10 | 4 | 7.67 | −60 | −8 | 0 | 7.88 | −60 | −8 | 2 | 8.3 | |
| Temp Sup | −56 | −40 | 22 | 5.74 | −54 | −40 | 20 | 4.95 | −52 | −42 | 20 | 3.82 | −56 | −40 | 20 | 5.57 | |||||
| Temp Sup | −44 | −34 | 10 | 6.66 | −44 | −38 | 10 | 5.26 | −46 | −38 | 8 | 4.54 | −44 | −34 | 8 | 6.05 | |||||
| Right | Temp Sup | 64 | −2 | 2 | 8.58 | 50 | −26 | 6 | 7.36 | 68 | −4 | 0 | 8.29 | 66 | −4 | 0 | 7.93 | 66 | −2 | 0 | 8.14 |
| Temp Sup | 56 | −30 | 6 | 8.19 | 66 | −2 | 0 | 6.89 | 56 | −28 | 6 | 4.23 | 54 | −28 | 6 | 6.98 | |||||
| Temp Sup | 66 | −24 | 12 | 4.71 | 64 | −22 | 14 | 6.5 | |||||||||||||
| Temp Sup | 70 | −18 | −4 | 5.15 | 70 | −16 | −4 | 5.15 | 72 | −18 | 6 | 4.04 | 70 | −18 | −4 | 5.04 | |||||
| Temp Pole Sup | 58 | 6 | −6 | 8.49 | 58 | 4 | −4 | 6.75 | 58 | 6 | −4 | 8.01 | 58 | 8 | −4 | 7.17 | 58 | 4 | −4 | 7.81 | |
| Parietal Cortex | |||||||||||||||||||||
| Left | Parietal Sup | −24 | −66 | 50 | 3.16 | −26 | −68 | 54 | 5.12 | −26 | −66 | 52 | 3.78 | ||||||||
| Parietal Inf | −48 | −50 | 58 | 3.93 | −40 | −52 | 46 | 5.45 | −26 | −66 | 44 | 5.82 | −50 | −48 | 58 | 4.27 | |||||
| Supramarginal | −50 | −44 | 34 | 3.89 | |||||||||||||||||
| Right | Supramarginal | 64 | −34 | 32 | 5.71 | 66 | −32 | 44 | 3.23 | 66 | −34 | 28 | 2.85 | ||||||||
| Insular cortex | |||||||||||||||||||||
| Left | Insula | −34 | 18 | 10 | 3.83 | −32 | 2 | 10 | 6.22 | −36 | 24 | 6 | 6.47 | −38 | 20 | 8 | 4.93 | ||||
| Right | Insula | 46 | 12 | 6 | 7.29 | 50 | 12 | −2 | 7.04 | 48 | 14 | 2 | 7.65 | ||||||||
| Cerebellum | |||||||||||||||||||||
| Left | Cereb L6 (splCB) | −14 | −64 | −18 | 8.65 | −20 | −64 | −26 | 7.91 | −22 | −64 | −24 | 7.74 | −22 | −64 | −22 | 8.49 | −20 | −64 | −24 | 8.28 |
| Cereb Crus I (splCB) |
−32 | −70 | −24 | 7.04 | −28 | −86 | −18 | 8.51 | −34 | −82 | −18 | 6.03 | |||||||||
| Right | Cereb L6 (splCB) | 20 | −64 | −22 | 9.28 | 18 | −62 | −22 | 9.13 | 16 | −62 | −20 | 9.06 | 20 | −62 | −22 | 10.2 | 18 | −62 | −22 | 9.67 |
| Cereb Crus I (splCB) |
42 | −68 | −26 | 4.77 | 42 | −64 | −28 | 6.56 | |||||||||||||
| Cereb Crus II | 2 | −78 | −36 | 4.45 | 2 | −80 | −34 | 4.04 | 2 | −80 | −36 | 4.07 | |||||||||
| Subcortical nuceli | |||||||||||||||||||||
| Left | Put | −26 | 2 | 8 | 7.54 | −32 | 2 | 10 | −32 | 6 | 8 | 6.74 | −22 | 2 | 6 | 6.32 | |||||
| Pallidum | −24 | −10 | −2 | 6.51 | −28 | −18 | 0 | 5.01 | −26 | −16 | 0 | 4.35 | −26 | −16 | 0 | 5.08 | |||||
| Pallidum | −18 | −2 | 2 | 5.46 | −18 | 0 | 6 | 7.47 | |||||||||||||
| Thalamus | −14 | −20 | 2 | 4.31 | −16 | −6 | −6 | ||||||||||||||
| Caudate | −18 | −12 | 20 | 3.36 | |||||||||||||||||
| Right | Put | 28 | 12 | 8 | 4.74 | 22 | 0 | 12 | 4.23 | 22 | −2 | 12 | 4.75 | ||||||||
| Pallidum | 22 | −8 | −2 | 6.83 | 22 | −10 | −4 | 4.73 | 22 | −8 | −4 | 4.67 | 20 | −8 | −4 | 4.24 | 20 | −8 | −4 | 5.77 | |
| Caudate | 20 | −6 | 8 | 6.05 | 20 | −6 | 12 | 4.82 | 18 | 0 | 14 | 3.66 | |||||||||
| Visual areas | |||||||||||||||||||||
| Left | Calcarine | −8 | −98 | −6 | 5.21 | −12 | −98 | −4 | 5.45 | −12 | −96 | −4 | 9.45 | −10 | −98 | −4 | 6.86 | ||||
| Occip Sup | −20 | −64 | −24 | 8.73 | |||||||||||||||||
| Occip Mid | −40 | −84 | −4 | 4.08 | −42 | −84 | −2 | 5.44 | −46 | −74 | −18 | 6.27 | |||||||||
| Occip Inf | −48 | −74 | −14 | 6.2 | |||||||||||||||||
| Lingual | −8 | −96 | −14 | 4.55 | −8 | −94 | −16 | 4.76 | −10 | −94 | −14 | 5.77 | |||||||||
| Right | Occip Inf | 28 | −88 | −4 | 5.59 | −18 | −94 | −4 | 6.25 | ||||||||||||
| Occip Inf | 26 | −94 | 0 | 3.3 | 30 | −86 | −8 | 5.57 | 26 | −90 | −4 | 3.88 | |||||||||
| Occip Mid | 42 | −68 | −26 | 4.77 | 30 | −96 | 2 | 3.34 | 36 | −94 | 4 | 3.42 | |||||||||
| Calcarine | 14 | −76 | 8 | 3.41 | |||||||||||||||||
Peak responses are based on voxel-wise analysis (PFDR < .05). Peak locations are given in MNI coordinates and listed with the associated AAL atlas anatomical label.
The results of region-of-interest (ROI) analysis largely paralleled those of the voxel-wise analysis (Table 3). The Collapsed – baseline contrast showed bilateral activations in the ventral motor cortex (vMC) and ventral somatosensory cortex (vSC), the supplementary motor area (SMA), the ventral premotor cortex (vPMC), ventral portions of the inferior frontal opercularis (vIFo), the frontal operculum (FO), superior temporal gyrui and sulci, Heschl’s gyrus (Hg), the anterior medial cerebellum (amCB) and lateral cerebellum (splCB, including Crus I and Lobule VI), and the basal ganglia (putamen and pallidum). Lateralized activations were observed in right pre-SMA, right ventral inferior frontal triangularis (vIFt), left anterior insula (aINS), and left anterior supramarginal gyrus (aSMg). A few areas were active in one or more of the individual speaking conditions contrasted with baseline but not in the Collapsed – baseline contrast. These regions and the respective baseline contrasts in which they were active were as follows: left pre-SMA (Variable), left anterior inferior frontal sulcus (aIFs, Variable), left dIFo (Variable), right dIFo (Repeated) bilateral parietal operculum (PO, Repeated), right aINS (Repeated), and right aSMg (Repeated).
Table 3.
ROI results for each individual speaking condition and the collapsed data (all speaking conditions combined) all compared to baseline
| ROI acronym | Speaking condition vs baseline |
|||||
|---|---|---|---|---|---|---|
| Identical | Reordered | Re-syllabified | Variable | Collapsed | ||
| Rolandic cortex | ||||||
| Left | vMC | X | X | X | X | X |
| vPMC | X | X | X | X | X | |
| SMA | X | X | X | X | X | |
| pre-SMA | X | |||||
| vSC | X | X | X | X | X | |
| Right | vMC | X | X | X | X | X |
| vPMC | X | X | X | X | X | |
| SMA | X | X | X | X | X | |
| pre-SMA | X | X | X | X | X | |
| vSC | X | X | X | X | X | |
| Frontal Cortex | ||||||
| Left | aIFs | X | ||||
| vIFo | X | X | X | X | X | |
| dIFo | X | |||||
| FO | X | X | X | X | ||
| Right | vIFt | X | X | X | ||
| vIFo | X | X | X | X | X | |
| dIFo | X | |||||
| FO | X | X | X | X | ||
| Temporal Cortex | ||||||
| Left | aSTg | X | X | X | X | X |
| pSTg | X | X | X | X | X | |
| pdSTs | X | X | X | X | X | |
| Hg | X | X | X | X | X | |
| PP | X | X | X | X | X | |
| PT | X | X | X | X | X | |
| PO | X | |||||
| pCO | X | X | X | X | X | |
| Right | aSTg | X | X | X | X | X |
| pSTg | X | X | X | X | X | |
| pdSTs | X | X | X | X | X | |
| Hg | X | X | X | X | X | |
| PP | X | X | X | X | X | |
| PT | X | X | X | X | X | |
| PO | X | |||||
| pCO | X | X | X | |||
| Insular cortex | ||||||
| Left | aINS | X | X | |||
| Right | aINS | X | ||||
| Parietal cortex | ||||||
| Left | aSMg | X | X | X | X | |
| Right | aSMg | X | ||||
| Cerebellum | ||||||
| Left | amCB | X | X | X | X | |
| spmCB | X | X | X | X | X | |
| splCB | X | X | X | X | X | |
| Right | amCB | X | X | X | X | X |
| spmCB | X | X | X | X | X | |
| splCB | X | X | X | X | X | |
| Subcortical nuceli | ||||||
| Left | Put | X | X | X | ||
| Pal | X | X | X | X | X | |
| Right | Put | X | X | |||
| Pal | X | X | X | X | X | |
An X denotes that the ROI was significantly active (PFDR < .05) for that contrast. Only positive activations are reported. [Abbreviations: vMC = ventral motor cortex; vPMC = ventral premotor cortex; SMA = supplementary motor area; pre-SMA = pre-supplementary motor area; vSC = ventral somatosensory cortex; aIFs = anterior inferior frontal sulcus; vIFo = ventral inferior frontal opercularis; dIFo = dorsal inferior frontal opercularis; FO = frontal operculum; vIFt = ventral inferior frontal triangularis; aSTg = anterior superior temporal gyrus; pSTg = posterior superior temporal gyrus; pdSTs = posterior dorsal superior temporal sulcus; Hg = Heschl’s gyrus; PP = planum polare; PT = planum tempolare; PO = parietal operculum; pCO = posterior central operculum; aINS = anterior insula; aSMg = anterior supramarginal gyrus; amCB = anterior medial cerebellum; spmCB = superior posterior medial cerebellum; splCB = superior posterior lateral cerebellum; Put = putamen; Pal = pallidum]
The remaining regions included in our ROI analysis did not show significant activations in any of the baseline contrasts. These regions included bilateral posterior inferior frontal sulcus (pIFs), dorsal inferior frontal triangularis (dIFt), anterior lateral cerebellum (alCB), inferior posterior medial cerebellum (ipmCB), caudate (Caud), and thalamus (Tha) as well as right anterior inferior frontal sulcus (aIFs) and left ventral frontal triangularis (vIFt).
Across-condition activity comparisons
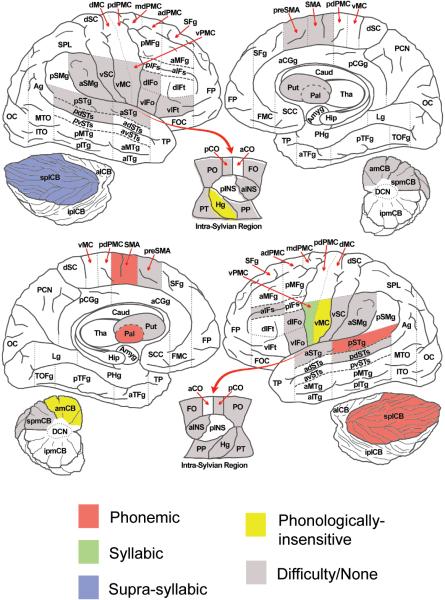
The activity of ROIs that were significant in at least one of the speech vs. baseline contrasts was compared to the model patterns shown in Figure 2. Fourty eight regions met this criterion (with ROIs that were bilaterally active counting twice in this count). Of these, eleven regions were found to significantly match at least one of the five predicted patterns (P < 0.05; Figures 4 and 5 show the observed activity pattern for each of these ROIs and the best matching predicted pattern). Regions exhibiting across-conditions activity similar to the phonemic predicted pattern were left lateralized and included SMA, pallidum, posterior STg (pSTg), and the superior lateral cerebellum. The syllabic pattern was matched only by the left ventral premotor cortex (vPMC). Activity related to supra-syllabic sequences was observed only in the right superior lateral cerebellum (splCB). Three regions showed activity unaffected by the phonological content of the stimuli (i.e., they exhibited the phonologically-insensitive pattern of activity): left vMC, left amCB, and right Hg.
Figure 4.
Predicted activity patterns (left column) and the actual patterns of ROIs that match one of the predicted patterns with a corrected p value of less than 0.05. [Abbreviations: vMC = ventral motor cortex; vPMC = ventral premotor cortex; SMA = supplementary motor area; dIFo = dorsal inferior frontal opercularis; pSTg = posterior superior temporal gyrus; Hg = Heschl’s gyrus; amCB = anterior medial cerebellum; splCB = superior posterior lateral cerebellum; spmCB = superior posterior medial cerebellum; Pal = pallidum]
Figure 5.
Brain map of ROIs and their best predicted activity pattern match. Color indicates activity pattern found in the ROI. Gray ROIs were active during at least one of the speech – baseline contrasts but did not meet the criteria for a match to one of the predicted patterns (see main text and Table 1 for further information, including region abbreviation definitions).
Activity patterns for the remaining thirty seven ROIs that did not significantly match any of the predicted patterns are provided in the Supplementary Materials.
Discussion
The results of the present study distinguish a number of speech regions preferentially engaged in processes related to phonemes, syllables, and supra-syllabic sequences during speech production. Additional regions engaged in processes that are insensitive to these phonological representations were also identified. The quantitative methods applied here rely on the effectiveness of fMRI repetition suppression (fMRI-RS) to produce stimulus specific signal attenuation in the regions that are engaged with processing the phonological units that are the focus of the current study. Previous fMRI-RS studies have demonstrated that signal attenuation effects are specific to the repeated stimuli and even the individual features within those stimuli (Buckner et al., 1998; Grill Spector et al., 2001; Rice 2007). Moreover, evidence from multiple previous studies shows that the fMRI-RS phenomenon is not restricted to repeated perceptual processing of identical stimuli but rather to repeated engagement of neural resources in processing specialized information, whether that is a perceptual analysis or a higher cognitive task such as word retrieval, semantic encoding, sentence processing, or motor planning and execution (Buckner et al., 2000; Dehaene-Lambertz, 2006; Gabrieli et al., 1996; Gold et al, 2005; Kilner et al., 2009; Lingnau et al., 2009; Majdandzic et al., 2009; Xu et al, 2007).
We applied fMRI-RS in combination with a quanitative analysis of across condition activity pattern evaluation and definition of ROIs using an anatomical brain parcellation process tailored to speech studies. In this manner we were able to single out speech regions that are predominantly engaged in processes related to phonemes, syllables, and supra-syllabic sequences. However, the current methods do not allow us to probe regions that subserve multiple phonological processes in a complex fashion or a group or regions with different functions that fall within one ROI. Thus, our discussion focuses on those regions whose activity was dominated by processes related to one or another of the units that are the focus of our study without presuming that the rest of the speech regions are not involved in such processes.
SMA - basal ganglia circuit
Regions matching the phonemic pattern included two areas involved in a medial premotor cortico-basal-ganglia-thalamo-cortical loop: the left SMA and the left globus pallidus (one of the output structures of the basal ganglia). While the SMA is consistently shown active in speech studies involving a variety of tasks, conclusive evidence of the organization of SMA in speech is still largely lacking. Based on what is known about this area, specifically that it is very strongly involved in motor control, we hypothesize that the SMA-BG loop is involved in the initiation of phoneme-level motor programs (see also Guenther et al., 2006). More specifically, we hypothesize that there is a separate representation in the SMA for each phoneme, irrespective of where in the speech sequence the phoneme occurs. The existence of cells in the SMA that activate for a specific action (e.g. ‘push’ or ‘pull’) regardless of the sequence position in which this motor action occurs have been shown in single cell recordings in primates (Shima and Tanji, 2000). Furthermore, we hypothesize that the left SMA is involved in the initiation of the subsequent phoneme-level motor program with the help of the basal ganglia, which inhibits competing phonemic motor programs and disinhibits the upcoming phoneme in the utterance. Some evidence for this interpretation of the present results comes from previous motor and speech studies. First, non-human electrophysiological studies indicate that the SMA plays an important role in the initiation of motor programs (Hoshi and Tanji, 2004; Shima and Tanji, 2000). Second, single-neuron activity measurements in the human SMA showed that activity in this region peaks once during sequence preparation and a second time after a GO signal (Amador and Fried, 2004). Third, in the lesion literature, the most commonly observed deficit immediately after SMA lesion, i.e. before any reorganization has taken place, is complete mutism or substantial reduction of spontaneous speech (Krainik et al., 2003). Furthermore, neuroimaging studies of speech have previously implicated SMA in the initiation of speech motor programs. When contrasting trials where a response has to be produced (Go trials) with those requiring no actual response (NoGo trials), the SMA is active for Go but not for NoGo trials in which speakers only prepare the utterance without overtly producing it (Alario et al., 2006; Bohland and Guenther, 2006). One controversy worth noting here is that in a mata-analysis of fMRI speech studies Indefrey and Levelt (2004) had identified the right and not the left SMA to be involved in phonetic encoding and articulation. One explaination for this controversy may be that their criterion for the areas involved in articulatory planning was less robust than for the other speech production categories due to the difficulty to identify regions involved in articulatory processes using their method of analysis. This is further evident from the fact that the phonetic encoding and articulation classification produced a number of unexpected regions for this category. We therefore believe that the Indefrey and Levelt (2004) meta analysis does not address definitively the question of what regions are involved in articulatory processes. While the present results do not argue against the involvement of right SMA in speech production (indeed the right SMA was active in both the voxel-wise and ROI-based analyses) they present evidence that left SMA is involved in phoneme-level articulatory processes. The right SMA did not match any of the activation patterns that we had constructed but it could be involved in articulation in a more complex mix of processes that our study design cannot detail. Thus we restrict our discussion to the left SMA-BG circuit.
The basal ganglia on the other hand is believed to be involved in the inhibition of undesired movements (both sequential and general) while simultaneously removing inhibition focally from the currently desired movements (Mink, 1996). The latter theory also proposes that the movements themselves are generated by cortical and cerebellar areas. In speech, the basal ganglia have been previously hypothesized to be involved in aspects of cortical initiation of phonological representations (Booth et al., 2007). Anatomically, the globus pallidus receives fast excitatory input from the subthalamic neucleus (STN) which in turn receives direct projections from motor and premotor areas including SMA (Mink, 1996), and slower, more focused projections from the striatum (caudate and putamen), which receive inputs from multiple cortical areas. Taken together, the above evidence suggests that the SMA and the basal ganglia are involved in initiating motor programs during speech production, with SMA involved in the phoneme-specific movement initiation and the BG most likely involved in the inhibition of competing movements. Our results additionally suggest that the speech motor programs initiated by this SMA-BG loop are at a phonemic level. However, rather than affecting articulation directly, we hypothesize that these phoneme-level motor programs act as components within larger syllabic and supra-syllabic sequence motor programs to drive articulatory movements during speech.
Alternatively, the cross-condition activity differences in the SMA-BG circuit might be related to the additional time required to sequence new pseudowords in the Variable condition as compared to the time necessary to repeat the same pseudoword. Both SMA and BG have been shown to be differentially active in relation to changing syllable repetition rate (Riecker et al., 2005). However, while SMA increases its activity when syllable production rate increases, activity in BG decreases. In the present study both SMA and BG activity changed in the same direction. Furthermore, participants were instructed to begin the production of each pseudoword only after it appeared on the screen, and they are trained to do that outside the scanner, thereby greatly reducing variations in production rate across conditions. Finally, since each condition gradually increased the complexity of stimuli, if the SMA-BG activation was related to speech production rate, we would have expected a more gradual increase in activity pattern across the four conditions and thus a match to the Difficulty pattern rather than the phonemic pattern.
Lateral premotor areas
The ventral premotor cortex (vPMC) was bilaterally active for the speech vs nonspeech comparison in both voxel-wise and ROI analyses. The left vPMC across-condition activity pattern analysis further indicated activity related to syllable-level processes. It is possible that in addition to the syllable-level processes, left vPMC is also involved in phoneme-level processes. Due to the fact that the syllabic pattern can also occur in a region containing both syllable and phoneme-level representations, we are not able to determine whether phonemes are also being processed in left vPMC in addition to syllables. It is however noteworthy that this region had a virtually perfect match to the syllabic pattern both qualitatively and quantitatively (p<0.001).
The vPMC region has been previously implicated in a number of studies of speech processes related to articulation. Apraxia of speech, a disorder characterized by impaired or missing motor programs for speech production, is frequently associated with damage to left ventral premotor areas or posterior portions of the inferior frontal gyrus (Hillis et al., 2004; Robin et al., 2007). Additionally, intraoperative brain stimulation of the vPMC disrupts speech articulation (Duffau et al., 2003) and transcranial magnetic stimulation (TMS) applied to the vPMC markedly diminishes the number of correct syllables produced during overt speech (Tandon et al., 2003). Neuroimaging studies have also provided some clues for the involvement of the vPMC in speech production processes. Bilateral premotor cortex, together with superior cerebellum and SMA, is active during the phonological encoding phase of a task involving subvocal reading of consonant strings, suggesting that these areas contribute to the translation of visually presented consonant strings into articulatory motor programs (Chen and Desmond, 2005b). However, it is unclear from the results of this study what level of motor programs are being subserved in the vPMC during natural speech production since the stimuli in this study did not have syllabic or word-level structure. Alario et al. (2006) showed that activity in vPMC increased as participants produce longer words/pseudowords composed of more syllables. Activity in this premotor area also increased for speech sequences composed of different syllables as opposed to repeating the same syllable (Bohland and Guenther, 2006). However these studies simultaneously manipulated phonemic content, syllabic structure, and sequence complexity, making it hard to determine definitively in what way the vPMC region is involved in articulation.
The present study extends these findings by providing evidence that the engagement of left vPMC in articulation involves syllable-level processes (although phoneme-level processes may also be mediated by this region). As previously mentioned during discussion of SMA, implicating left vPMC in syllable-level processes does not imply that the final stage articulatory movements are solely guided by this sublexical unit; instead it appears from our results that phoneme-level information from SMA and syllable-level information from ventral premotor cortex converge on primary motor cortical cells that are indifferent to phonemic content..
The involvement of left vPMC in syllable level processing was explicitly predicted by the DIVA model (Guenther et al., 2006), which posits that the left ventral premotor cortex contains a speech sound map whose cells represent syllabic motor programs. An important issue for future study is how the medial premotor circuit, the lateral premotor circuit, primary motor cortex, and the superior cerebellum (see Cerebellum section) interact to transform phonemic, syllabic, and sequence-level processes into the finely orchestrated movements of the lips, jaw, tongue, and other articulators that underlie speech. These interactions are likely responsible for the process of coarticulation, in which articulator movements corresponding to neighboring phonemes in a speech sequence are overlapped in order to achieve highly optimized articulator movements for frequently occurring phoneme strings. Based on the current results we hypothesize that the medial premotor circuit, in association with the BG, is involved in initiating motor programs (corresponding to phonemes), whereas the lateral premotor areas are involved in “stitching” these subprograms into an optimized syllabic motor program, with the cerebellum involved in the refinement of the final movements. Stated in terms of the gestural view of speech production (Browman and Goldstein, 1989; Saltzman and Munhall, 1989), one might view the SMA representation as corresponding to phoneme-specific articulatory gestures such as lip protrusion during production of the vowel “oo”, whereas ventral premotor cortex is involved in generating the “gestural score” that specifies the timing and overlap of these individual gestures. Further investigation is needed to verify these interpretations.
Superior lateral cerebellum
Lateral portions of the superior cerebellar cortex (Crus I and Lobule VI) were active for speech compared to silent viewing of meaningless strings in our voxel-wise analysis and our ROI analysis. The activity pattern analysis further detailed the possible contributions of sub-regions of the cerebellum during overt speech. More specifically, phoneme-level activity was present in the left superior posterior lateral cerebellum (splCB) while right splCB showed activity related to the suprasyllabic sequence structure of either phonemes or syllables. These regions were distinguished from the anteromedial portion of the cerebellum whose activity appeared unmodulated by phonemic or syllabic content (see Phonologically insensitive areas below).
It remains unclear what processing is performed by the superior lateral cerebellum during speech, though clues can be found in the neuroimaging literature. Bohland and Guenther (2006) found bilateral superior lateral cerebellum (Crus I and Lobule VI) to be more active for the production of syllable sequences composed of different syllables as compared to repeating the same syllable multiple times. However activity in this region was not sensitive to the complexity level of the syllables within the sequences (Bohland and Guenther, 2006), indicating a role in speech sound sequencing without regard to the complexity of the items in the sequence. In a rhyming task of orthographically presented pairs of words and pseudowords performed by Fulbright et al. (1999), superior lateral cerebellum was more active in the pseudoword rhyming condition compared to word rhyming, suggesting that more familiar sequences of phonemes pose less demand on the superior cerebellum during phonological assembly. Chen and Desmond (2005a) found concomitant bilateral activity in Lobule VI/Crus I of the cerebellum and the ventral premotor cortex for a motoric rehearsal task involving sub-vocal reading of consonants. In a subsequent study they showed that the right superior lateral cerebellum (together with SMA and bilateral precentral regions) was active specifically during the encoding phase of the task (Chen and Desmond, 2005b). The authors interpret their results to indicate that the superior lateral cerebellum is involved in the rapid conversion of visual to phonological code during letter reading. Our results support this hypothesis and further indicate that function in the superior cerebellum is hemisphere-specific, with left splCB being involved with phoneme level codes while right splCB activity is influenced by the supra-syllabic context in which individual phonemes or syllables occur. A straightforward interpretation of right splCB function consistent with our results as well as those just summarized is that it is involved in breaking down orthographic stimuli (visually presented words or pseudowords) into phonological chunks before passing these chunks onto the premotor areas for articulatory encoding. The phonemic pattern found in left superior lateral cerebellum is less amenable to straightforward interpretation at this time.
Temporal lobe
Bilateral superior temporal gyrus was active for the speech vs non-speech comparison in both our voxel-wise and ROI analyses but only the left posterior superior temporal gyrus (pSTG) matched one of the investigated activity patterns, corresponding to phoneme-level processes. The pSTG region has been implicated in both speech perception and speech production. Since our participants were hearing their responses it is possible that activity in this region was affected by either perception (i.e. self-monitoring) or production processes or both. It has been previously suggested that pSTg is implicated in phonemic-level processes employed during speech production even when there is no verbal auditory input (Hickok, 2000). Furthermore, the left pSTG has been implicated in reproduction conduction aphasia, a disorder characterized by good auditory comprehension and fluent speech production but frequent phonemic errors in production during repetition, oral reading, spontaneous speech, and naming tasks (Damasio, 1992; Goodglass, 1992), with reproduction conduction aphasia reflecting a primary deficit in phonological encoding (Shallice and Warrington, 1977). Although the classic explanation for conduction aphasia is disconnection syndrome (due to arcuate fasciculus damage), more recent evidence has suggested that conduction aphasia is caused by cortical lesions in the pSTG area resulting in damage to the computational systems important for phonemic processes in speech (Hickok et al., 2000). Finally, the left lateralization of the phonemic pattern in pSTG is in line with studies showing left pSTG-focused activity during the phonological encoding stage of a picture naming task (Levelt et al., 1998) as well as lesion studies indicating aphasic symptoms with left but not right hemisphere STG damage.
Phonologically insensitive areas
Regions whose activity matched the phonologically insensitive pattern included left hemisphere ventral motor cortex (vMC), left anterior medial cerebellum (amCB), and right primary auditory cortex (Hg). The activity pattern of left vMC has a relatively straightforward interpretation: the hemodynamic response of this region is likely related to the musculature of the vocal tract independent of phoneme or syllable identities. It does not exhibit activation differences across conditions since approximately the same set of muscles is utilized in the different conditions. Similarly, the primary auditory cortex (Hg) processes information in a tonotopic manner, and the stimuli in the different conditions involved approximately the same set of audible frequencies. Lesions in the anterior medial cerebellum have been shown to result in speech dysarthria characterized by reduced articulatory precision, and the same region has been shown active during silent lip and tongue movements (Urban et al., 2003). In the DIVA model, the amCB and vMC are hypothesized to encode feedforward motor commands issued to the articulators during speech in a phonologically insensitive manner (Guenther et al., 2006), consistent with the current experimental results.
Although amCB, vMC, and Hg are known to be bilaterally active during speech, only left amCB and vMC and right Hg produced significant matches to the phonologically insensitive pattern. Furthermore, according to the DIVA model there are only relatively minor differences in the nature of processing in these primary cortical regions across the two hemispheres, as opposed to the lateralized functions of higher-level premotor and linguistic regions of the brain (Guenther et al., 2006). This raises the question of why the phonologically insensitive pattern was only found in one hemisphere for each of these areas. Our statistical analysis of right vMC, right amCB, and left Hg indicated that, although not statistically significant according to our 0.05 false discovery rate, the best matching pattern in all of these regions was the phonologically insensitive pattern. The same was true for bilateral ventral somatosensory cortex (vSC), an area also expected to be insensitive to phonological content based on the DIVA model (see Supplementary Materials). The lack of statistical significance of these matches may be due in part to unintended differences in the stimulus materials across conditions that were not fully controlled for, e.g. the specific muscles used to produce the pseudowords in the various conditions. This highlights the fact that the pattern matches indicated in Figures 4 and 5 are a conservative estimate based on a strict statistical analysis, and thus some areas shown in gray in Figure 5 may in fact represent information within one of the representation types studied here. We expect future studies based on the current design to help clarify the units utilized in these gray areas, thus providing an increasingly detailed map of sublexical processes in the speech and language areas of the brain.
It is worth noting that phonologically insensitive areas in our study may be differentially active for other aspects of speech not tested in our study, such as rhythm or prosody. The rhythmic and prosodic contours of the speech stimuli were highly consistent across conditions, and thus an area sensitive to rhythm or prosody would not be expected to show differential activity across conditions in our study.
Task Difficulty
Only the left dorsal inferior frontal gyrus pars opercularis (dIFo) and right superior posterior medial cerebellum (spmCB) had a significant fit to the Difficulty pattern, which was used as a control. This result shows that while the activity in these areas was somehow related to the sublexical variations in the stimuli, it is not specific to any one of these sublexical units per se but rather is more correlated with increased neural demands due to increasing task difficulty. Including a task difficulty control pattern was important as many of the same regions that are active in speech tasks, most notably left prefrontal cortical areas, are also active for a variety of cognitive control processes that are not related to speech per se, such as task switching, response conflict, task novelty, and working memory (for review see (Duncan and Owen, 2000). The task difficulty pattern may also result from a combination of processes related to all units - phonemic, syllabic, and suprasyllabic – and thus a match to this pattern does not automatically imply that a region is not involved in phonological processes.
Regions with other activity patterns
There were also a number of regions that showed significant activity for at least one of the baseline contrasts in the ROI analysis but which did not match any of the investigated patterns; these regions are shown in gray on Fig. 5. Some of these regions may be involved in aspects of speech that were not systematically varied in our paradigm (e.g., syllable frame complexity), or they may be related to speech processes that were not invoked by our paradigm at all. For example, the inferior frontal sulcus, which showed differential activity in our Variable condition, has been previously implicated in working memory related processes such as supporting representations of the upcoming utterance (Bohland and Guenther, 2006) and non-articulatory maintenance of phonological informaiton (Gruber, 2001). Both of these studies used a delay period in the task design, while in the present study participants produced the stimuli as soon as they saw them on the screen. Thus, although this region is important for certain speech processes (particularly those with a working memory component), it is not heavily involved in single word reading and thus is not likely to match any of the tested patterns.
Other regions may be involved in processes related to more than one of the investigated phonological units, thus showing no clear match to any one pattern of activity. For example, previous studies have shown that moving posteriorly from anterior IFG to posterior IFG to vPMC, the processes subserved by those regions change in a gradient manner from a mix of semantic and phonological ones, to increasingly restricted to pure phonological ones (Gold et al., 2005). This may explain why vPMC matched a predicted pattern while IFG did not despite the fact that we subdivided this region functionally into four regions shown to activate differentially in speech studies. Similarly, there is clear evidence in the literature that inferior parietal areas are part of the verbal working memory system and participate in translating sensory codes (such as letters or syllables) to motor codes (Hickok and Poeppel, 2007). However, the inferior parietal area has been shown to exhibit a more complex role in speech production involving a mix of processes across multiple language domains (Gold et al, 2005). This study showed that inferior parietal cortex is involved in all three tasks that they investigated (semantic, phonological, and letter) and adaptation effects in this area were significant in all three of them.
Finally, some outstanding issues exist due to stimulus differences that we were unable to fully control for given limitations in the types of syllables allowable in French. One such issue is the use of CVC and CCV syllables in the ReSyllabified and Variable conditions but only CCV syllables in the Identical and Reorder conditions. The addition of CVC-type syllabic structures was necessary for resyllabification purposes and to construct a sufficient number of non-repeating stimuli. It is unclear whether differences in neural processing load for CCV and CVC syllables are strong enough to cause statistically significant BOLD signal variations, but this issue should be considered in future studies. In the present study, such differences are unlikely to have caused a false match due to the stringency of the statistical tests used to evaluate matches between predicted patterns and measured activation patterns.
Conclusion
This study aimed to determine the levels of processing (phonemic, syllabic, suprasyllabic, or phonologically insensitive) carried out by various cortical and subcortical regions involved in speech production using an fMRI repetition suppression paradigm designed to elicit different patterns of activation across experimental conditions depending on which (if any) sublexical representation is utilized in an area. The phonologically insensitive pattern was found in left primary motor cortex, right primary auditory cortex, and left anterior medial cerebellum, suggesting that processing in these regions is not sensitive to phonemic, syllabic, or pseudoword (supra-syllabic) content of spoken utterances. In the left ventral premotor cortex, responses were sensitive to syllabic variations. This result supports the prediction made by the DIVA model of speech production (Guenther et al., 2006) that syllable-level motor programs are represented in left ventral premotor cortical areas. This interpretation is also in keeping with reports from the lesion literature of apraxia of speech with left ventral premotor cortex damage. Phonemic processes involved the largest number of areas, including the left posterior superior temporal gyrus, left SMA and pallidum, and the superior lateral cerebellum bilaterally. Finally, only the right superior lateral cerebellum appeared to represent information in a suprasyllabic form. Taken together, these findings suggest that the medial and lateral premotor regions of the left hemisphere process phonemes and syllables, respectively, and projections from these areas to primary motor cortex transform these sublexical representations into a set of motor commands to the speech articulators.
Supplementary Material
Figure 1. Across-condition activity patterns for the ROIs that were included in our analysis but that did not meet both criteria of inclusion – being active in at least one of the baseline contrasts and matching one of the predicted patterns with a corrected p value of less than .05. P values are given for the best-matching pattern (P1=phonologically-insensitive, P2=phonemic, P3=syllabic, P4=supra-syllabic, and P5=difficulty).
Table 1. A complete list of the stimuli used in the present experiment. Each row in the Repeated, Reordered, and Re-syllabified conditions corresponds to an example of the stimuli presented in one block. For clarity stimuli are presented in pairs although in the experiment each stimulus was presented individually. In the Variable condition one row represents one full block of stimuli. Each boldface entry represents the beginning of a new stimulus list; each list is reused from the beginning a maximum of twice in one run for the first three conditions, and four times for the last condition. The blocks in the experiment were distributed pseudo-randomly as explained in the main text.
Table 2. Goodness of match (denoted by corrected p values) of all ROIs included in our analysis to each of the five predicted patterns. [SM=sensory-motor].
Acknowledgments
This work was supported by the National Institute on Deafness and other Communication Disorders (NIDCD grant R01 DC07683, Frank Guenther PI). Imaging was performed at the fMRI center of the Timone Hospital in Marseille (France), with support from the Centre National de la Recherche Scientifique (CNRS). The authors would like to thank Jason Bohland, Simon Overduin, and Muriel Roth for their assistance with this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Amador N, Fried I. Single-neuron activity in the human supplementary motor area underlying preparation for action. J Neurosurg. 2004;100:250–259. doi: 10.3171/jns.2004.100.2.0250. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Research. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman CP, Goldstein L. Articulatory gestures as phonologiecal units. Phonology. 1989;6:201–251. [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;2000:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Michelli A, Price CJ. Effects of word and syllable frequency on activation during lexical decision and reading aloud. Human Brain Mapping. 2006;27:963–972. doi: 10.1002/hbm.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Perea M. Naming pseudowords in Spanish:Effects of syllable frequency. Brain Lang. 2004;90:393–400. doi: 10.1016/j.bandl.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy D. MRI-based topographic parcellation of human neocortex: an anatomically specified method with estimate of reliability. J Cogn Neurosci. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Cholin J, Levelt WJ, Schiller NO. Effects of syllable frequency in speech production. Cognition. 2006;99:205–235. doi: 10.1016/j.cognition.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. In: Bizais YY, Barillot C, Di Paola R, editors. Automated multi-modality image registration based on information theory; Proc. Information Processing in Medical Imaging; Dordrecht, The Netherlands, Kluwer Academic Publishers. 1995.pp. 263–274. [Google Scholar]
- Damasio AR. Aphasia. New Engl J Med. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Anton J-L, Campagne A, Ciuciu P, Dehaene G, Denghien I, Jobert A, LeBihan D, Sigman M, et al. Functional seggregation of cortical language areas by sentence repetition. Human Brain Mapping. 2006;27 doi: 10.1002/hbm.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopez M, Sichez J-P, Effenterre RV. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. Neuroimage. 2003;20:1903–1914. doi: 10.1016/s1053-8119(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen A. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI Volumes. Proceedings of the IEEE Nuclear Science Symposium on Medical Imaging. 1993;3:1813–1817. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Fromkin VA. The non-anomalous of anomalous utternaces. Language. 1971;47:27–52. [Google Scholar]
- Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, Shaywitz SE, Frost SJ, Skudlarski PR, Constable T, Lacadie CM, et al. The cerebellum’s role in reading: a functional MR imaging study. AJNR Am J Neuroradiol. 1999;20:1925–1930. [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Psychological Science. Vol. 7. 1996. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes; pp. 278–283. [Google Scholar]
- Garrett MF. The analysis of sentence production. In: Bower G, editor. Psychology of learning and motivation. Academic Press; New York: 1975. [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from fMRI adaptation. Cereb Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Diagnosis of conduscion aphasia. In: Kohn SE, editor. Conduction Aphasia. Lawrence Erlbaum Associates; Hillsdale: 1992. pp. 39–49. [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus specific effects. Trends Cogn Sci. 2006;10:93–94. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Gruber O. Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cerebral Cortex. 2001;11:1047–1055. doi: 10.1093/cercor/11.11.1047. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Hickok G, Erhard P, Kassubek J, Helms-Tillery A, Naeve-Velguth S, Strupp JP, Strick PL, Ugurbil K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neuroscience Letters. 2000;287:156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Rerviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. J Neurophysiol. 2004;92:3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neuroscience. 2009;29:10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainik A, Lehericy S, Duffau H, Capelle L, Chainay H, Cornu P, Cohen L, Boch A-L, Mangin J-F, Le Bihan D, Marsault C. Postoperative speech disorder after medial frontal surgery. Neurology. 2003;60:587–594. doi: 10.1212/01.wnl.0000048206.07837.59. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Alario FX. On the locus of the syllable frequency effect in language production. Journal of Memory and Language. 2006;55:178–196. [Google Scholar]
- Levelt WJ, Wheeldon LR. Do speakers have access to a mental syllabary? Cognition. 1994;50:239–269. doi: 10.1016/0010-0277(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Praamstra P, Meyer AS, Helenius P, Salmelin R. An MEG study of picture naming. J Cog Neurosci. 1998;10:553–567. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- Lingnau A, Gesierich B, Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. PNAS. 2009;106:9925–9930. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdandzic J, Bekkering H, van Schie HT, Toni I. Movement-specific repetition suppression in ventral and dorsal premotor cortex during action observation. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp049. Advance Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, et al. A four-dimensional probabilistic atlas of the human brain. J Am Med Inform Assoc. 2001;8:401–430. doi: 10.1136/jamia.2001.0080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- New B, Pallier C, Ferrand L, Matos R. Une base de données lexicales du français contemporain sur internet: LEXIQUE. Psychologique. 2001;101:447–462. [Google Scholar]
- Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage. 2003;19:1303–1316. doi: 10.1016/s1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice NJ, Valyear KF, Goodale MA, Milner AD, Culham JC. Orientation sensitivity to graspable objects: an fMRI adaptation study. Neuroimage. 2007;36:T87–T93. doi: 10.1016/j.neuroimage.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Groschel K, Grodd W, Ackerman H. The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. NeuroImage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang. 2008 doi: 10.1016/j.bandl.2008.01.008. In press. [DOI] [PubMed] [Google Scholar]
- Robin D, Jacks A, Ramage AE. The neural substrates of apraxia of speech as uncovered by brain imaging: a critical review. In: Ingham RJ, editor. Neuroimaging in communication sciences and disorders. Plural Publishing; New York: 2007. pp. 129–154. [Google Scholar]
- Saltzman EL, Munhall KG. A dynamical approach to gestural patterning in speech production. Ecological Psychology. 1989;1:333–382. [Google Scholar]
- Shallice T, Warrington E. Auditory-verbal short-term memory impairment and conduction aphasia. Brain Lang. 1977;4:479–491. doi: 10.1016/0093-934x(77)90040-2. [DOI] [PubMed] [Google Scholar]
- Shattuck-Hufnagel S. Sublexical units and suprasegmental structure in speech production planning. In: MacNeilage PF, editor. The production of speech. Springer; New York: 1983. pp. 109–136. [Google Scholar]
- Shima K, Tanji J. Neuronal activity in the supplementary and presupplementary motor areas for temporal organization of multiple movements. J Neurophysiol. 2000;84:2148–2160. doi: 10.1152/jn.2000.84.4.2148. [DOI] [PubMed] [Google Scholar]
- Tandon N, Narayana S, Lancaster JL, Brown S, Dodd S, Vollmer DG, Ingham R, Ingham J, Liotti M, Fox P. CNS resident award: Role of the lateral premotor cortex in articulation. Clinical Neurosurgery. 2003;50:341–349. [PubMed] [Google Scholar]
- Tourville JA, Guenther FH. A cortical and cerebellar parcellation system for speech studies. Boston University; Boston, MA: 2003. Boston University Technical Report CAS/CNS-03-022. [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39:1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urban PP, Marx J, Hunsche S, Gawehn J, Vucurevic G, Wicht S, Massinger C, Stoeter P, Hopf HC. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch Neurol. 2003;60:965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Xu Y, Turk-Browne NB, Chun MM. Dissociating task performance from fMRI repetition attenuation in ventral visual cortex. Journal of Neuroscience. 2007;27:5985–5981. doi: 10.1523/JNEUROSCI.5527-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Across-condition activity patterns for the ROIs that were included in our analysis but that did not meet both criteria of inclusion – being active in at least one of the baseline contrasts and matching one of the predicted patterns with a corrected p value of less than .05. P values are given for the best-matching pattern (P1=phonologically-insensitive, P2=phonemic, P3=syllabic, P4=supra-syllabic, and P5=difficulty).
Table 1. A complete list of the stimuli used in the present experiment. Each row in the Repeated, Reordered, and Re-syllabified conditions corresponds to an example of the stimuli presented in one block. For clarity stimuli are presented in pairs although in the experiment each stimulus was presented individually. In the Variable condition one row represents one full block of stimuli. Each boldface entry represents the beginning of a new stimulus list; each list is reused from the beginning a maximum of twice in one run for the first three conditions, and four times for the last condition. The blocks in the experiment were distributed pseudo-randomly as explained in the main text.
Table 2. Goodness of match (denoted by corrected p values) of all ROIs included in our analysis to each of the five predicted patterns. [SM=sensory-motor].