SUMMARY
Low birth weight is associated with an increased risk for development of hypertension. Our laboratory utilizes a model of reduced uterine perfusion in the pregnant rat that results in intrauterine growth restricted (IUGR) offspring that develop hypertension at a pre-pubertal age. Although hypertension develops in both pre-pubertal male and female IUGR offspring, only male IUGR offspring remain hypertensive after puberty. We previously reported that bilateral renal denervation abolishes hypertension in adult male IUGR offspring indicating an important role for the renal nerves in the maintenance of established IUGR-induced hypertension. We also reported that ACE inhibition abolishes hypertension in adult male IUGR offspring. However, activation of the renin angiotensin system does not occur in male IUGR offspring until after puberty, or after development of established IUGR-induced hypertension. Therefore, the mechanisms involved in the development of IUGR-induced hypertension may differ from those involved in the maintenance of established IUGR-induced hypertension. Thus, the purpose of this study was to determine whether the renal nerves play a causative role in the early development of IUGR-induced hypertension in pre-pubertal IUGR offspring.
IUGR and control offspring were subjected to bilateral renal denervation or sham denervation at 4 weeks of age. Mean arterial pressure was determined at 6 weeks of age in conscious chronically instrumented animals. Adequacy of renal denervation was verified by renal norepinephrine content.
Whereas renal denervation had no effect on mean arterial pressure in control offspring (103±2 versus 102±3 mmHg; sham versus denervated), it reduced blood pressure in growth restricted offspring (114±3 versus 104±1 mmHg; P<0.01, sham versus denervated). Renal norepinephrine content was significantly reduced in denervated animals relative to sham operated.
Thus, these data indicate a role for the renal nerves in the etiology of IUGR-induced hypertension and suggest the renal nerves may participate in the early development of hypertension in IUGR offspring, in addition, to established hypertension observed in adult male IUGR offspring.
Keywords: Intrauterine growth restriction, Low birth weight, Blood Pressure, Renal nerves
INTRODUCTION
Hypertension includes both a genetic and an environmental component. Recent epidemiological studies report an inverse relationship between low birth weight (LBW) and hypertension (1–3), suggesting that environmental influences leading to hypertension may begin during fetal development (4–5). Although LBW is not a pre-requisite for raised blood pressure (6, 7), it does serve as a marker for intrauterine insult (8). LBW, defined as a birth weight below the 10th percentile for a given gestational age (6, 7), is due to fetal, placental and/or maternal factors (10) that limit nutrient and oxygen supply to the fetus resulting in intrauterine growth restriction (IUGR) and small-for-gestational-age newborns (10, 11). Animal models of IUGR support the association between LBW and hypertension (12–16). In addition, animal models of IUGR, induced by either maternal nutrition deficiency during pregnancy (14, 16, 17) or placental insufficiency (12, 15, 18), demonstrate that initiation of an adverse fetal environment during a critical period of fetal development affects organ development. The kidney is an example of one organ affected by an adverse fetal environment, as reductions in nephron number are associated with hypertension in several animal models of IUGR (14–17). Because of the importance of the kidneys in the long-term control of blood pressure (19), adverse conditions in utero may result in alterations in renal function that lead to hypertension in adult life. However, the exact mechanisms linking LBW, abnormal renal function and hypertension are unknown.
As IUGR within the Western world is more likely due to placental insufficiency than maternal undernutrition (20), our studies utilize a unique model of IUGR induced by reduced uterine perfusion. In this model, reduced uterine perfusion initiated at day 14 of gestation in the rat results in IUGR offspring predisposed to the development of hypertension (12). Furthermore, in this model of IUGR, bilateral renal denervation in adult animals completely abolishes established hypertension in adult male IUGR offspring (21). This finding is consistent with reports in both human subjects (22, 23) and experimental animals (24, 25) suggesting that activation of the sympathetic nervous system (SNS) may play an important role in the pathogenesis of hypertension induced by IUGR. It is also relevant that increased renal sympathetic activity is a common finding in human primary hypertension (26–28). Although renal denervation at 10 weeks of age clearly abolishes the established hypertension in this model of IUGR, it is not clear whether the renal nerves play a critical role in the early onset of hypertension. The mechanisms that initiate hypertension in this model of IUGR may differ from mechanisms involved in the maintenance of established hypertension. Sex differences are observed in this model of IUGR hypertension as pre-pubertal male and female IUGR offspring develop hypertension, but only adult male IUGR offspring remain hypertensive (12). Hypertension in adult male IUGR offspring involves activation of the intra-renal renin angiotensin system (RAS) (29). However, activation of the RAS does not occur until after puberty in male IUGR offspring suggesting other factor involvement in the development of hypertension in IUGR offspring. Thus, the purpose of this study was to determine whether the renal nerves play a contributory role in the early development of IUGR-induced hypertension.
METHODS
Animals
All experimental procedures were in accordance with National Institutes of Health guidelines with approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. Rats were housed in a temperature-controlled room (23°C) with a 12:12 hour light/dark cycle with food and water available ad libitum. Timed pregnant Sprague Dawley rats were purchased from Harlan Inc. (Indianapolis, IN). At day 14 of gestation, rats destined for reduced uterine perfusion were clipped as described below. All dams delivered at full term and were size matched at birth with equal distribution of male and female offspring per dam. Litters were culled to 8 pups per dam to ensure equal access to nutrients for offspring of every litter. Offspring were weaned at 3 weeks of age. At 4 weeks of age, male control and male IUGR offspring were randomly assigned to undergo either bilateral renal denervation (RDNX) or sham denervation. Only male IUGR offspring were used in this study as only male offspring of reduced uterine perfusion dams develop hypertension that persists into adulthood (9). Mean arterial pressure (MAP) was measured 2 weeks after RNDX, at 6 weeks of age. Male offspring from 5 control pregnant rat and 8 reduced uterine perfusion pregnant rat were randomly assigned into 4 groups: control sham (n=8), control RDNX (n=7), IUGR sham (n=9), and IUGR RDNX (n=8).
Reduced Uterine Perfusion in the Pregnant Rat
IUGR was induced by reducing placental perfusion. In short, rats were anesthetized with isoflurane and silver clips were slipped around the lower abdominal aorta and on both branches of the ovarian arteries at day 14 of gestation (12).
Measure of Arterial Pressure in Conscious Rats
During isoflurane anesthesia, rats were surgically instrumented with a carotid arterial catheter (PE 50 tubing) for blood pressure monitoring. Following a recovery period of two days, rats acclimated to restraint were placed in modified restraining cages with arterial pressure monitored with a pressure transducer connected to a data acquisition kit (DATAQ Instruments, Inc., Akron, OH) and computer for continuous recording. After a one-hour equilibration period, 2-twenty minute pressure determinations were obtained. All pressure measurements were made during mid morning hours.
Bilateral Renal Denervation
Bilateral renal denervation was achieved by stripping visible nerves and connective tissue from the renal arteries and veins and subsequent painting of these vessels with 10% phenol in alcohol (21). Sham-operated rats underwent a similar incision, but renal nerves were left intact.
Renal Norepinephrine Content
Completeness of renal denervation was determined by measurement of renal norepinephrine content by electrochemical detection (21).
Measure of plasma renin activity
Renin activity in plasma collected following decapitation was measured by radioimmunoassay (RIA) (30).
Statistical Analyses
Graph pad PRISM version 4 was used for all statistical analyses. For comparison made between groups, ANOVA, with adjustments for multiple comparisons was used. A value of P<0.05 was considered statistically significant.
RESULTS
Birth and Body weight
Weight at birth was significantly reduced in offspring from reduced uterine perfusion dams as compared to offspring from control dams (Table I). However, as summarized in Table I, body weight did not differ between IUGR and control offspring by 6 weeks of age. In addition, body weight at 6 weeks of age did not differ upon comparison of intact offspring to denervated offspring from either control or reduced uterine perfusion pregnant rats. Therefore, bilateral renal denervation had no effect on weight gain (Table I). Kidney weight (data not shown) and kidney weight normalized to body weight (Table 1) also did not differ upon comparison of control to IUGR offspring at 6 weeks of age, regardless of whether animals underwent sham or bilateral renal denervation
Table I.
Comparison of body weight and kidney weight between Control and IUGR offspring.
| Birth |
6 weeks of age |
||||
|---|---|---|---|---|---|
| Sham |
Denervated |
||||
| Body weight (grams) |
Body weight (grams) |
Kidney weight /Body weight × 100 (grams) |
Body weight (grams) |
Kidney weight /Body weight × 100 (grams) |
|
| Control | 6.7 0.2 | 157 6 | 0.68 .03 | 148 5 | 0.63 .04 |
| IUGR | 5.9 0.2* | 147 2 | 0.70 .03 | 152 6 | 0.64 .04 |
P<0.05 vs. Control. All data are expressed as mean ± SEM.
Effect of Bilateral Renal Denervation on Mean Arterial Pressure
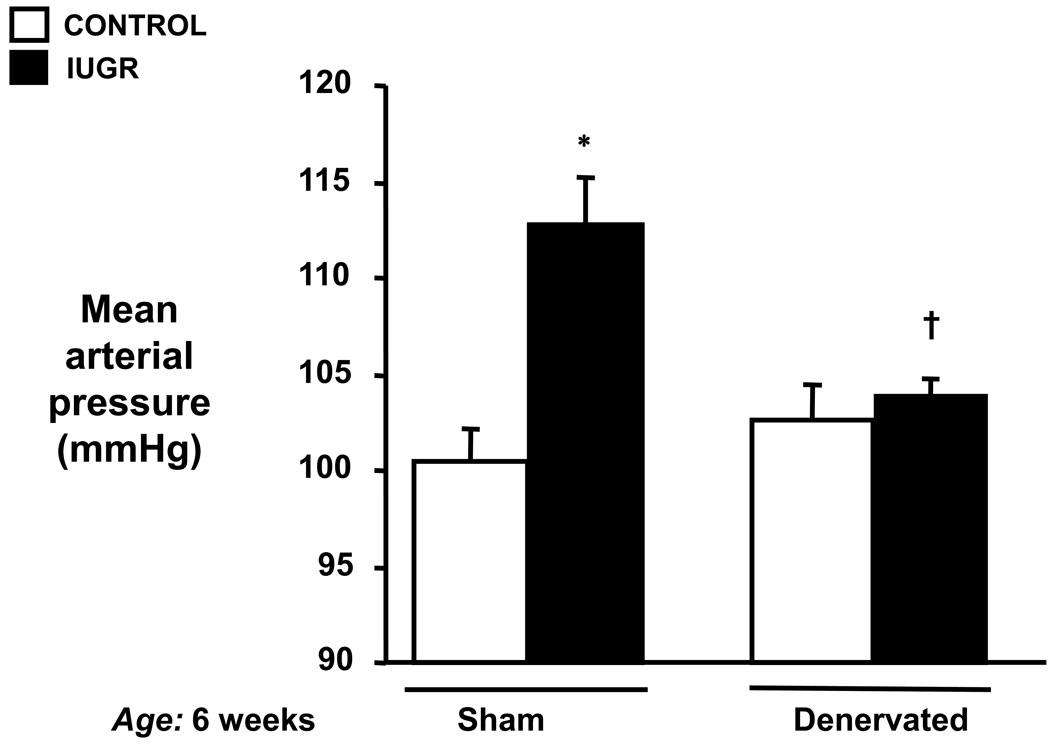
As illustrated in Figure 1, MAP was significantly elevated in intact IUGR offspring as compared to intact control offspring at 6 weeks of age. MAP was significantly lower in denervated IUGR offspring as compared to their intact counterparts (104±1 vs. 113±2 mmHg, IUGR RDNX vs. IUGR sham, respectively). Further, there were no significant differences in MAP between denervated IUGR offspring and either sham control or denervated control offspring (101±2 vs. 103±2 mmHg; control sham vs. control RDNX, respectively). Thus, early bilateral renal denervation abolished hypertension at pre-pubertal age.
Figure 1.
Effect of renal denervation on mean arterial pressure (MAP) in a rat model of intrauterine growth restriction (IUGR) induced by reduced uterine perfusion. MAP was measured at 6 weeks of age in conscious chronically instrumented animals that underwent either sham (Sham) or bilateral renal denervation (RDNX) at 4 weeks of age. * P<0.05 vs. control sham, † P<0.05 vs. IUGR sham., All data are expressed as mean ± SEM.
Verification of Renal Denervation
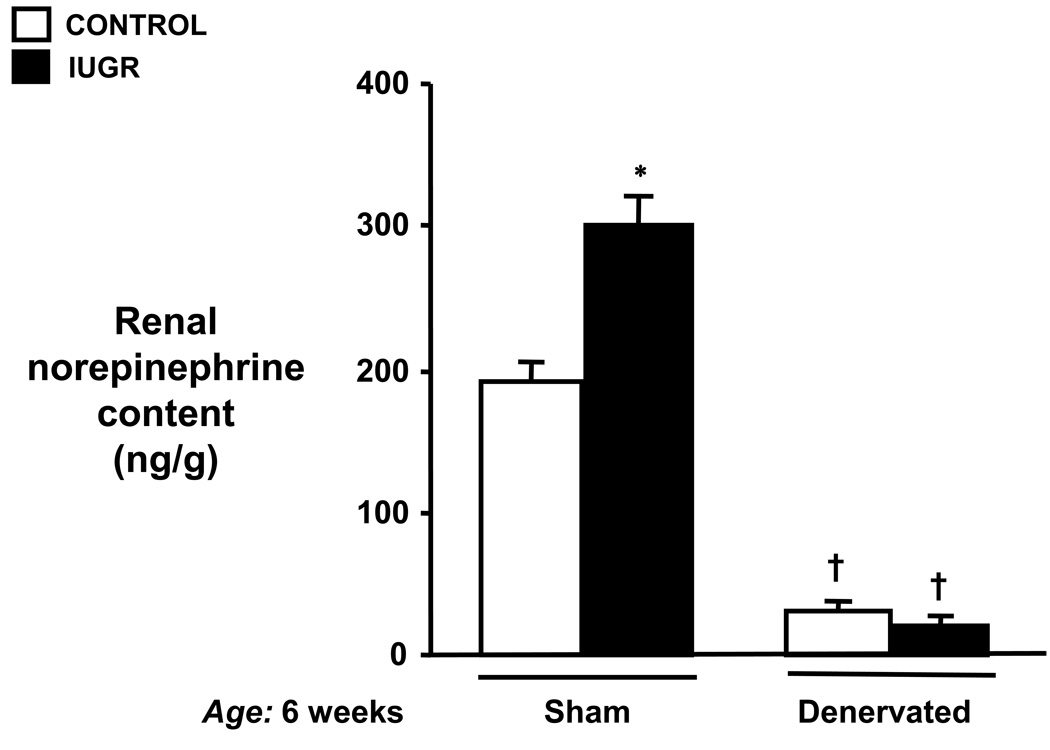
Renal norepinephrine content as shown in Figure 2 was determined to verify completeness of renal denervation. Renal norepinephrine content was markedly reduced following bilateral renal denervation in both control and IUGR offspring as compared to their intact counterparts at 6 weeks (control: 193±12 vs. 31±6 ng/g and IUGR: 301±22 vs. 22±4 ng/g, sham vs. RDNX, respectively) Additionally, the hypertension in pre-pubertal IUGR offspring was associated with a significant increase in renal norepinephrine content at 6 weeks of age. (Figure 2).
Figure 2.
Renal norepinephrine content in control or intrauterine growth restricted (IUGR) offspring that underwent either sham (Sham) or bilateral renal denervation (RDNX) at 4 weeks of age followed by measure of mean arterial pressure and collection of kidneys for determination of renal norepinephrine content at 6 weeks of age. * P<0.05 vs. control sham, † P<0.05 vs. sham counterpart. All data are expressed as mean ± SEM.
Plasma Renin Activity
There were no differences in plasma renin activity (PRA) between IUGR offspring and intact control offspring (6.2±1.0 vs. 4.7±0.3 AI/L/Hr, IUGR vs. control, respectively). Bilateral renal denervation did not affects PRA (4.6±0.2 and 3.5±1.9 AI/L/Hr; IUGR vs. control respectively).
DISCUSSION
One regulatory system that may be altered during fetal programming is the SNS. Activation of the SNS is observed in LBW humans and in animal models of IUGR (22–25, 31–35). Since the renal nerves influence fetal renal development (36), exposure to an elevated level of SNS activity during the critical period of renal development may impair kidney development and affect the kidney’s normal ability to regulate blood pressure. Our laboratory has previously reported that an adverse fetal environment due to placental insufficiency results in IUGR offspring predisposed to development of hypertension (12). Although hypertension develops in both pre-pubertal male and female IUGR offspring, only male IUGR offspring remain hypertensive after puberty (12). A role for SNS activation is suggested as bilateral renal denervation abolishes hypertension in post-pubertal male IUGR offspring (21). Thus, the renal nerves contribute to the maintenance of established hypertension in adulthood in this model of IUGR (21). However, the temporal role of the renal nerves in the development of hypertension in IUGR offspring remains unknown. Therefore, the purpose of this study was to determine whether the renal nerves play a role in the early onset of hypertension, as well as the maintenance of hypertension in adulthood in this model of IUGR.
The present study demonstrates that the renal nerves play an important causative role in the initiation of hypertension observed in this model of IUGR induced hypertension. Early renal denervation had no effect on arterial pressure in control offspring, yet reduced or normalized arterial pressure in IUGR offspring relative to control. Renal norepinephrine content was increased in intact IUGR offspring as compared to intact control offspring at 6 weeks of age. Thus, increased renal sympathetic innervation may be present in pre-pubertal IUGR offspring. Animal studies suggest that chronic exposure to hypoxia during development can serve as a potent stimulator of hyperinnervation (37, 38) and long-lasting alterations in renal nerve activity (35). Thus, reduced uterine perfusion and subsequent fetal hypoxia may induce altered renal nerve development in IUGR offspring resulting in hypertension.
Sympathetic activation, as suggested by increased plasma levels of catecholamines, has been reported in animal models of IUGR induced by placental insufficiency (24, 25). Determination of sympathetic activity has not been made in the present model of IUGR. However, based on the MAP responses to renal denervation, we hypothesize that sympathetic activation also occurs in this model of IUGR and includes increased sympathetic outflow to the kidney. Thus, an important question in future studies will be to determine the stimulus for sympathoexcitation. As indicated above, hypoxia is a potent stimulator of hyperinnervation (34, 37, 38). Hypoxia during fetal development may also serve as a stimulus for increased renal sympathetic outflow. Hypoxia can increase renal sympathetic activation by stimulating tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of catecholamines (39, 40). The gene for tyrosine hydroxylase is regulated by hypoxia-inducible factor-1 (HIF-1), a transcription factor involved in oxygen and energy homeostasis (41). Thus, activation of tyrosine hydroxylase in response to hypoxia (42, 43) may lead to alterations in renal norepinephrine synthesis resulting in increased norepinephrine release from nerve terminals.
Increased sympathetic outflow including sustained increases in renal sympathetic nerve activity can also occur as a result of the actions of angiotensin II on the central nervous system (44). In the low protein model of fetal programming, marked increases in MAP are associated with increased expression of angiotensin II receptors in areas of the brain involved in cardiovascular regulation (45). In addition, intracerebroventricular administration of an angiotensin-converting enzyme inhibitor or an AT1 specific antagonist significantly reduces MAP in low protein offspring (45). Therefore, it is possible that the central actions of angiotensin are involved in the fetal programming of hypertension and may serve as a stimulus for SNS activation.
In the present study, PRA did not differ between pre-pubertal control and IUGR offspring. Bilateral renal denervation had no effect on PRA in either control or IUGR offspring. Intra-renal levels of the RAS also do not differ at this age in this model of IUGR (29). However, in models of programming induced by protein restriction during gestation, activation of the intra-renal RAS is observed as early as 4 weeks of age (46, 47). Furthermore, RAS blockade abolishes hypertension in protein restriction models of programming in both pre-pubertal (48, 49) and in adult low protein offspring (50) suggesting the RAS plays a critical role in both the development and maintenance of established hypertension in this model of hypertension programmed by prenatal insult. Although we do not observe activation of the RAS in pre-pubertal IUGR offspring, intra-renal levels of the RAS are activated in post-pubertal adult male IUGR offspring from reduced uterine perfusion dams (29). Furthermore, ACE inhibition abolishes hypertension in post-pubertal adult male IUGR offspring (51). Established hypertension in adult male IUGR offspring is also associated with a two-fold higher level of plasma testosterone and castration abolishes hypertension in adult male IUGR offspring (51). Thus, other mechanisms, such as the RAS and testosterone, in addition to the renal nerves, contribute to the established phase of hypertension in this model of fetal programming induced by placental insufficiency.
Several animal models of programmed hypertension demonstrate that a prenatal insult can impair the renal sympathetic nerves, thus resulting in permanent effects on renal development and renal function leading to hypertension. (33–35). Whether alterations in renal morphology and histology are present in this model of IUGR is unknown. Renal weight when normalized to body weight did not differ in this study. However, we previously reported a reduction in kidney weight was present in IUGR offspring at 4 weeks of age (12). A reduction in nephron number is observed in models of IUGR induced by placental insufficiency in the rat (15, 18) and is associated with increased proteinuria (18). Maternal protein restriction in the rat is also associated with a reduction in nephron number (14, 16, 17) and proteinuria (50). Thus, a renal mechanism may contribute to increased blood pressure in this model of IUGR-induced hypertension.
The fetal environment is an important factor in fetal development and contributes to postnatal consequences. The present study demonstrates that the renal nerves play a causative role in the early onset of hypertension observed in a model of IUGR-induced hypertension induced by placental insufficiency. Thus, activation of the SNS may be a mechanism by which placental insufficiency programs IUGR-induced hypertension. Although established hypertension involves interaction of other regulatory systems in addition to the renal nerves, the renal nerves play a critical role in the early onset of hypertension in pre-pubertal IUGR offspring.
ACKNOWLEDGEMENTS
The authors would like to Dr. Thomas E. Lohmeier for his critical evaluation of this manuscript. This work was supported by grant HL074927 of the Heart, Lung, and Blood Institute of the National Institutes of Health.
Abbreviations
- LBW
low birth weight
- IUGR
intrauterine growth restriction
- RAS
renin angiotensin system
- RDNX
bilateral renal denervation
- SNS
sympathetic nervous system
- MAP
mean arterial pressure
- RAS
renin angiotensin system
REFERENCES
- 1.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 2.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 3.Primatesta P, Falaschetti E, Poulter NR. Birth weight and blood pressure in childhood: results from the Health Survey for England. Hypertension. 2005;45:75–79. doi: 10.1161/01.HYP.0000150037.98835.10. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The fetal origins of hypertension. J Hypertens Suppl. 1996;14:S117–S120. [PubMed] [Google Scholar]
- 5.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 6.Morley R, Owens J, Blair E, Dwyer T. Is birthweight a good marker for gestational exposures that increase the risk of adult disease? Paediatr Perinat Epidemiol. 2002;16:194–199. doi: 10.1046/j.1365-3016.2002.00428.x. [DOI] [PubMed] [Google Scholar]
- 7.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 8.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein PS, Divon MY. Etiologies of fetal growth restriction. Clin Obstet Gynecol. 1997;40:723–729. doi: 10.1097/00003081-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJP. Mothers, babies, and disease in later life. London: BMJ Publishing Group; 1994. [Google Scholar]
- 12.Alexander BT. Placental insufficiency leads to development of hypertension in growth restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 13.Holemans K, Aerts L, Van Assche FA. Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig. 2003;10:392–399. doi: 10.1016/s1071-5576(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 14.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 15.Merlet-Benichou C, Gilbert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pedaitr Nephrol. 1994;8:175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 16.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 18.Schreuder MF, Nyengaard JR, Fodor M, van Wijk JAE, Delemarre-van de Waal HA. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J Am Soc Nephrol. 2005;16:2913–2919. doi: 10.1681/ASN.2004100875. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 20.Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–114. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander BT, Hendon AE, Ferrell G, Dwyer TM. Renal denervation abolishes hypertension in low birth weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 22.IJzerman RG, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation. 2003;108:566–571. doi: 10.1161/01.CIR.0000081778.35370.1B. [DOI] [PubMed] [Google Scholar]
- 23.Phillips DI, Barker DJ. Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med. 1997;14:673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka T, Kudo T, Kishimoto Y. Catcholamines in experimentally growth-retarded rat fetus. Asia Oceania. J Obstet Gynaecol. 1991;17:341–348. doi: 10.1111/j.1447-0756.1991.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones CT, Robinson JS. Studies on experimental growth retardation in sheep. Plasma catecholamines in fetuses with small placenta. J Dev Physiol. 1983;5:77–87. [PubMed] [Google Scholar]
- 26.Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 28.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R828–R836. doi: 10.1152/ajpregu.00591.2004. [DOI] [PubMed] [Google Scholar]
- 29.Grigore D, Ojeda N, Robertson E, Alexander BT. Hypertension in adult growth restricted offspring is associated with activation of the renal renin angiotensin system. The FASEB J. 2006;20:A757. [Google Scholar]
- 30.Haber E, Koerner T, Page LB, Kliman B, Purnode A. Application of a radioimmunoassay for Angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocr Metab. 1969;29:1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- 31.Kuzawa CW. Fetal origins of developmental plasticity: are fetal clues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 32.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sanders MW, Fazzi GE, Jansen GMJ, de Leeuw PW, Blanco CE, De Mey JGR. Reduced uteroplacental blood flow alters renal arterial reactivity and glomerular properties in the rat offspring. Hypertension. 2004;43:1–7. doi: 10.1161/01.HYP.0000127787.85259.1f. [DOI] [PubMed] [Google Scholar]
- 34.Soukhova-O’Hare GK, Roberts AM, Gozal D. Impaired control of renal sympathetic nerve activity following neonatal intermittent hypoxia in rats. Neuroscience Letteres. 2006;399:181–185. doi: 10.1016/j.neulet.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 35.Maduwegedera D, Kett MM, Flower RA, Lanbert GW, Bertram JF, Wintour EM, Denton KM. Sex differences in postnatal growth and renal development in offspring of rabbit mothers with chronic secondary hypertension. Am J Physiol Regulatory Intergrative Com Physiol. 2006;292:706–714. doi: 10.1152/ajpregu.00458.2006. [DOI] [PubMed] [Google Scholar]
- 36.Robillard JE, Guillery EN, Segar JL, Merrill DC, Jose PA. Influence of renal nerves on renal function during development. Pediatr Nephrol. 1993;7:667–671. doi: 10.1007/BF00852576. [DOI] [PubMed] [Google Scholar]
- 37.Rouwet EV, Tintu AN, Schellings MW, et al. Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation. 2002;105:2791–2796. doi: 10.1161/01.cir.0000017497.47084.06. [DOI] [PubMed] [Google Scholar]
- 38.Ruijtenbeek K, Le Noble FA, Janssen GM, et al. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation. 2000;102:2892–2897. doi: 10.1161/01.cir.102.23.2892. [DOI] [PubMed] [Google Scholar]
- 39.Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 40.Teitelman G, Baker H, Joh TH, Reis DJ. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: Possible role of tissue environment. Proc Natl Acad Sci USA. 1979;76:509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: Hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 42.Leclere N, Andreeva N, Fuchs J, Kietzmann T, Gross J. Hypoxia-induced long-term increase of dopamine and tyrosine hydroxylase mRNA levels. Prague Med Rep. 2004. 2004;105:291–300. [PubMed] [Google Scholar]
- 43.Norris ML, Millhorn DE. Hypoxia-induced protein binding to O2-responseive sequences on the tyrosine hydroxylase gene. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 44.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 45.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 46.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and antiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287:F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 47.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 2003;104:607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- 48.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 49.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- 50.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 51.Ojeda NB, Grigore D, Yanes LL, et al. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00311.2006. in press, epub: Articles in Press. [DOI] [PubMed] [Google Scholar]