Abstract
Objective
Electroencephalographic seizures have been shown to occur in 5% to 20% of neonates and infants after biventricular repair of a variety of cardiac defects. Occurrence of a seizure is a predictor of adverse long-term neurodevelopmental sequelae. The contemporary incidence of postoperative seizures after repair of cardiac defects such as hypoplastic left heart syndrome and other forms of single ventricle is not known.
Methods
A prospective study of 178 patients less than 6 months of age undergoing cardiopulmonary bypass with or without deep hypothermic circulatory arrest (DHCA) was conducted at a single institution from September 2001 through March 2003 to identify postoperative seizures assessed by 48-hour continuous video electroencephalographic monitoring.
Results
Cardiac defects included transposition of the great arteries with or without a ventricular septal defect (n = 12), ventricular septal defect with or without coarctation (n = 28), tetralogy of Fallot (n = 24), hypoplastic left heart syndrome or variant (n = 60), other functional single ventricle (n = 14), and other defects suitable for biventricular repair (n = 40). Median age at the time of the operation was 7 days (range, 1–188 days) and was 30 days or less in 110 (62%) patients. DHCA was used in 117 (66%) patients, with multiple episodes in 9 patients. Median total duration of DHCA was 40 minutes (range, 1–90 minutes). Electroencephalographic seizures were identified in 20 (11.2%) patients. Seizures occurred in 15 (14%) of 110 neonates and 5 (7%) of 68 older infants. Seizures occurred in 1 (4%) of 24 patients with tetralogy of Fallot, 1 (8%) of 12 with transposition of the great arteries, and 11 (18%) of 60 with hypoplastic left heart syndrome or variant. By stepwise logistic regression analysis, once increasing duration of total DHCA (P = .001) was considered, no other variable improved prediction of occurrence of a seizure. Patients with DHCA duration of more than 40 minutes had an increased incidence of seizures (14/58 [24.1%]) compared with those with a DHCA duration of 40 minutes or less (4/59 [6.8%], P = .04). The incidence of seizures for patients with a DHCA duration of 40 minutes or less was not significantly different from those in whom DHCA was not used (2/61 [3.3%], P = .38).
Conclusions
In the current era, continuous electroencephalographic monitoring demonstrates early postoperative seizures in 11.2% of a heterogeneous cohort of neonates and infants with complex congenital heart defects. Increasing duration of DHCA was identified as a predictor of seizures. However, the incidence of seizures in children with limited duration of DHCA was similar to that in infants undergoing continuous cardiopulmonary bypass alone.
Occurrence of a seizure in the early postoperative period after repair or palliation of congenital heart defects (CHDs) is a marker for a central nervous system (CNS) injury and has been associated with adverse neurodevelopmental sequelae.1–3 However, use of neuromuscular blockade and sedation in the early postoperative period renders clinical diagnosis of seizures difficult. The standard for detection and quantification of seizures remains continuous electroencephalographic (EEG) monitoring. In the Boston Circulatory Arrest Study, conducted between 1988 and 1992, continuous EEG monitoring in the first 48 hours after the arterial switch operation demonstrated seizures in 27 (20%) of 136 neonates and infants.1 Assignment to a bypass strategy of predominantly deep hypothermic circulatory arrest (DCHA) was associated with an increased risk of postoperative seizures, although the difference was not statistically different. A more recent study (1992–1996) evaluating use of alpha-stat versus pH-stat blood gas management strategy during cardiopulmonary bypass (CPB) documented EEG seizures in 6 (5%) of 116 neonates and infants undergoing biventricular repair of a variety of cardiac defects.4 Assignment to pH-stat strategy was associated with a trend toward fewer EEG seizures; however, the difference did not reach statistical significance. Neither study included infants with hypoplastic left heart syndrome (HLHS) or other forms of functional single ventricle. Follow-up evaluation of the patients in the Circulatory Arrest Study demonstrated that occurrence of a postoperative EEG seizure was associated with a worse neurodevelopmental outcome at 1, 4, and 8 years of age (personal communication, J. Newburger, 2005).2,3 This study was undertaken to evaluate the current incidence of postoperative EEG seizures in a heterogeneous population of neonates and infants, including those with HLHS and other forms of functional single ventricle.
Methods
Sample
A subgroup of children enrolled in a prospective study evaluating polymorphisms of apolipoprotein E (APOE) as a risk factor for neurodevelopmental dysfunction also underwent continuous video EEG monitoring in the early postoperative period.5 A previous study demonstrated that the presence of the APOE ε2 allele is a risk factor for worse neurodevelopmental outcome at 1 year of age after infant cardiac surgery.5 Patients younger than 6 months undergoing CPB with or without DHCA for repair of CHD were eligible. Exclusion criteria included (1) multiple congenital anomalies, (2) recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletions, and (3) language other then English spoken in the home. The study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from the parent or guardian.
Operative Management
Surgical intervention was performed by 5 cardiac surgeons with a dedicated team of cardiac anesthesiologists. Alpha-stat blood gas management was used. Pump flow rates were not standardized for this study. DHCA was used at the surgeon’s discretion. Before DHCA, patients underwent core cooling with topical hypothermia of the head to a nasopharyngeal temperature of 18°C. Modified ultrafiltration was performed in all patients.
Video EEG Examination
Video EEGs were recorded on 1 of 3 identical, dedicated, portable Telefactor “Millenium Beehive” machines (Conshohocken, Pa) capturing time-synchronized video images and digital EEG data. Surface electrodes were applied with collodion glue before surgical intervention according to the international 10–20 system modified for neonates. Ten leads were used for neonates, and 19 leads were used for older infants. Tracings were recorded at 15 mm/s, with a recording sensitivity of 7 µV/mm. The records were frequently evaluated for technical adequacy. A brief 15-minute preoperative baseline study was recorded. Recording was reinitiated postoperatively immediately after admission to the cardiac intensive care unit. Video EEGs were recorded continuously for the first 48 hours after surgical intervention. Studies were terminated only for early death or at parental request.
Each record was visually reviewed in its entirety every 24 hours by the recording EEG technologist and independently by a pediatric neurologist (RRC). A seizure was recognized by the appearance of a sudden, repetitive, evolving, stereotyped, ictal pattern with a definite beginning, middle, and end; a minimum duration of 10 seconds; and a minimum amplitude of 2 µV. If a seizure appeared soon after a prior one, a minimum period of 10 seconds was required between the 2 events to consider them separate seizures. Rhythmic EEG artifact mimicking seizures caused by nursing care or other interventions was easily determined by review of the coincident video files. After confirmation of the presence of seizures, the attending cardiac intensive care unit physician was informed of the occurrence of a seizure. All treatment decisions were made at the discretion of the attending physician.
Data Collection
Preoperative factors, including gestational age, birth head circumference, birth weight, Apgar scores, and preoperative intubation, were obtained from birth and hospital records (Table 1). Weight, age at the time of surgical intervention, and type of surgical intervention were recorded along with perfusion data, including CPB time, aortic crossclamp time, and duration of DHCA (Table 1). Total support time was calculated as CPB time plus DCHA time. Total DHCA time was calculated as the sum of the duration of each episode of DHCA. Neuroimaging studies, including magnetic resonance imaging (MRI) of the brain, were performed for clinical indications in some patients with EEG seizures.
TABLE 1.
Logistic regression of each predictor of EEG seizure incidence
| Predictors | Significance |
|---|---|
| Preoperative factors | |
| Apgar score at 1 min | .339 |
| Apgar score at 5 min | .287 |
| Birth head circumference percentile | .292 |
| Birth weight percentile | .840 |
| APOE 22 or 23 | .896 |
| APOE 34 or 44 | .929 |
| Gestational age | .851 |
| Sex | .388 |
| Race | .706 |
| Type of delivery (vaginal or cesarean section) | .341 |
| Operative factors | |
| Age at the time of surgical intervention | .130 |
| Neonate vs infant | .204 |
| Weight at surgical intervention | .266 |
| Cardiac defect | .049* |
| Operative class | .112 |
| Surgeon | .293 |
| Preoperative intubation | .494 |
| Use of prostaglandin | .013* |
| Lowest nasopharyngeal temperature | .025* |
| Duration of cooling | .121 |
| Hematocrit on CPB | .574 |
| Total support time | .004* |
| Total CPB time | .343 |
| Total DHCA time | .001* |
| No. of episodes of DHCA | .006* |
| Maximum single period of DHCA | .002* |
| Total DHCA ≥40 min | .001* |
| No. of times ECMO support or VAD was required | .107 |
| Delayed sternal closure | .278 |
APOE, Apolipoprotein E; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device.
P < .10.
Data Analysis
Patients were grouped according to the specific cardiac defect as transposition of the great arteries (TGA; with or without ventricular septal defect [VSD]), tetralogy of Fallot (TOF), VSD (with or without coarctation), other defects suitable for biventricular repair, HLHS (and variants), and other forms of functional single ventricle. Codes were also assigned according to a previously described classification incorporating cardiac anatomy and perioperative physiology, which has been shown to be predictive of perioperative mortality.6 Class I is 2 ventricles with no aortic arch obstruction, class II is 2 ventricles with aortic arch obstruction, class III is a single ventricle with no arch obstruction, and class IV is a single ventricle with arch obstruction. Patients with TOF and TGA are defined as class I, whereas patients with HLHS and variants are defined as class IV. Data are presented as medians and ranges.
Statistical Methods
Potential predictors of the occurrence of a seizure were evaluated. Occurrence of a seizure was considered as a categorical (yes/no) variable, regardless of the number of seizures identified. The relationship of length of DCHA to seizure occurrence was also investigated. Preliminary analyses used logistic regression to investigate the marginal predictive power of potential covariates. The covariates considered are listed in Table 1. Two quantitative predictors were also considered categorically: age at surgical intervention was grouped as neonate (up to 30 days of age) or not (31 days of age or older), and use of DHCA was grouped as 0 to 40 minutes or longer than 40 minutes. All predictors listed in Table 1 were tested by means of logistic regression for univariate prediction of seizures. The presence of EEG seizures was coded as 1, and the absence was coded as 0. Categorical variables were treated as one or more 0–1 dummy variables. The 3 APOE alleles result in 6 genotypes. These were grouped into the ε2 group (ε2ε2 and ε3ε2), the ε3ε3 group, and the ε4 group (ε3ε4 and ε4ε4). The subjects in the ε2ε4 group were excluded from the APOE analyses because the effects of the ε2 and ε4 alleles are opposing in Alzheimer disease.
All covariates that predicted seizure outcome with a P value of .1 or less by logistic regression were retained for the stepwise regressions to identify a model for seizure prediction. All analyses used SPSS 10.0 for Windows (SPSS, Inc, Chicago, Ill).
Results
Study Population
Between September 16, 2001, and April 2, 2003, 238 eligible infants underwent cardiac surgery. Of these, 209 (88%) were enrolled in the study of APOE genotype. Continuous postoperative EEG monitoring was performed in 183 (88%) patients. All patients enrolled in the APOE study were approached for enrollment in the EEG study. Reasons for not undergoing EEG monitoring included parental refusal and unavailability of an EEG monitor.
There were 73 female and 105 male patients, with a median gestational age of 39 weeks (range, 28–41 weeks) and a median birth weight of 3090 g (range, 956–4460 g). Thirty-one (18%) patients were less than 37 weeks’ gestational age. Microcephaly (birth head circumference less than the fifth percentile for gestational age) was present in 27%. Eighty-three patients were in class I, 21 were in class II, 14 were in class III, and 60 were in class IV. TOF was present in 24 patients, VSD (with or without coarctation) was present in 28 patients, and TGA (with or without VSD) was present in 12 patients. Patients with a variety of other defects suitable for biventricular repair, such as atrioventricular septal defects, total anomalous pulmonary venous connection, and truncus arteriosus, were also enrolled (n = 40). HLHS or a variant was present in 60 patients, with other forms of functional single ventricle present in 14 patients.
The median age at surgical intervention was 7 days (range, 1–188 days). Surgical intervention was performed on 110 (62%) patients as neonates (age ≤30 days). The median weight at the time of the operation was 3.5 kg (range, 1.5–7.3 kg). The median hematocrit level after hemodilution on CPB was 31% (range, 20%–43%) and was less than 25% in 7 patients. When DHCA was used, the median nasopharyngeal temperature was 18°C (range, 15°C−21°C). The median duration of cooling for patients undergoing DCHA was 18 minutes (range, 15–21 minutes). When DHCA was not used, the median nasopharyngeal temperature was 28°C (range, 17°C−34°C). The median duration of total support was 80 minutes (range, 18–232 minutes). DHCA was used in 117 (66%) patients, with multiple episodes in 9 patients. The median duration of total DHCA was 40 minutes (range, 1–93 minutes).
Incidence and Predictors of Seizures
Complete 48-hour EEG studies were obtained in 178 (97%) patients. Reasons for noncompletion of the EEG study included death in 1 patient, parental request in 1 patient, technical issues resulting in loss of the EEG recording in 2 patients, and need for a second period of CPB in 1 patient. No seizures were identified on the preoperative EEG recordings. Postoperative EEG seizures were identified in 20 (11.2%) patients. The median time to the initial seizure was 22.5 hours postoperatively (range, 10–36 hours). The median number of seizures per patient in the 48-hour monitoring period was 36 (range, 1–217). No EEG seizure was associated with evidence of a clinical seizure on concurrent video monitoring. There were episodes of abnormal vital signs, including blood pressure or increased heart rate, which could be interpreted as possible seizures; however, none occurred in conjunction with an EEG seizure.
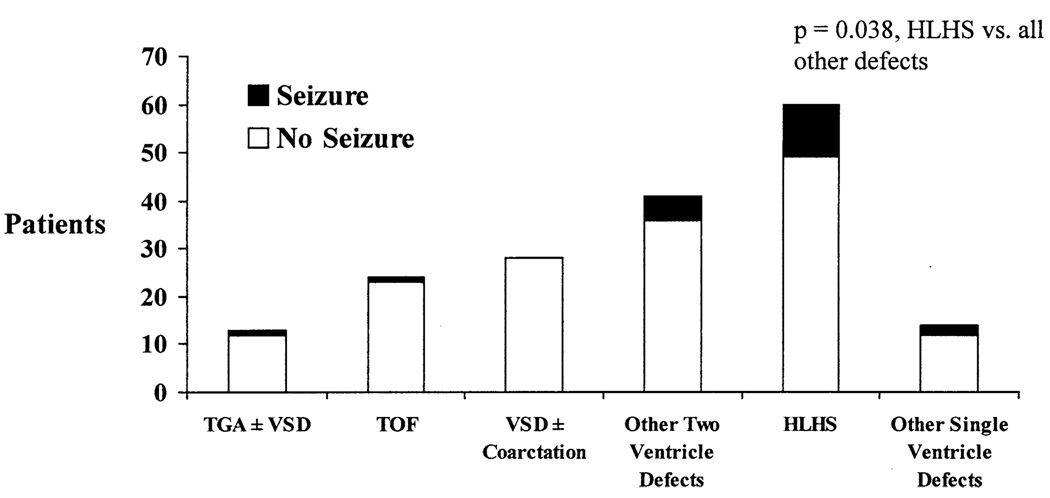
Marginal predictors of seizures included cardiac diagnosis (P = .049, df = 1), prostaglandin use (P = .013), lowest nasopharyngeal temperature (P = .025), and various measures of total support time and DHCA time (Table 1). No APOE genotype effect was identified. Overall, the incidence of seizures tended to be lower in patients undergoing biventricular repair compared with those with HLHS. Seizures were identified in 1 (4%) of 24 patients with TOF, 1 (8%) of 12 patients with TGA, 0 of the 28 patients with VSD, 5 (12%) of the 41 patients with other biventricular defects, 11 (18%) of the 60 patients with HLHS or variant, and 2 (14%) of the 14 patients with other forms of functional single ventricle (Figure 1). Seizures were most frequent in patients with HLHS or a variant compared with all other diagnoses (P = .038, df = 1). By analysis of variance, total DHCA time significantly varied among these diagnoses (P = .002, df = 5), with means of 34, 31, 32, 44, 46, and 33 minutes, respectively. Seizures occurred in 4 (4.8%) of 83 of the class I patients, 3 (14.3%) of 21 of the class II patients, 2 (14.3%) of 14 of the class III patients, and 11 (18.3%) of 60 of the class IV patients; however, grouping by class did not significantly predict seizures (P = .112). Seizures occurred in 15 (14%) of 110 neonates and 5 (7%) of 68 older infants, but this difference was not statistically significant (P = .15) at this sample size.
Figure 1.
Relationship of EEG seizures to cardiac defect. The number of patients in each group with and without seizures is shown on the y-axis.
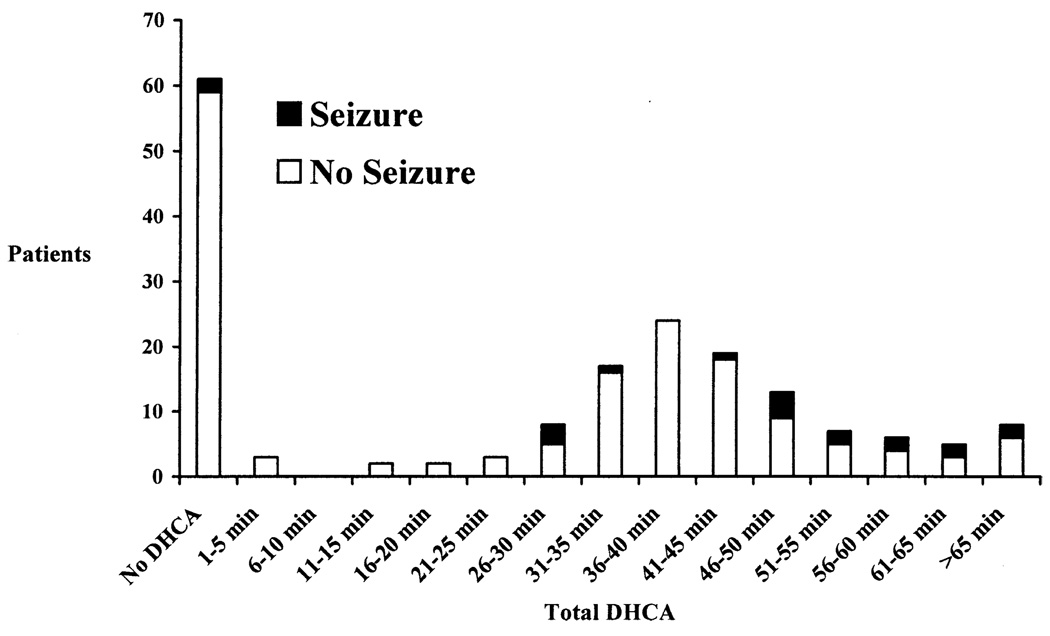
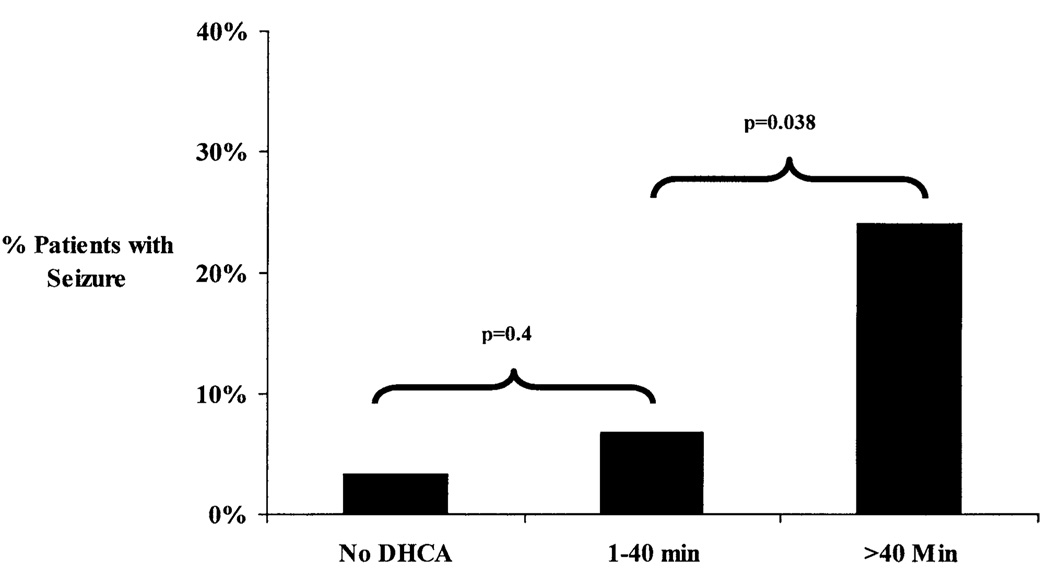
The incidence of seizures increased with increasing maximum single or cumulative total duration of DHCA (both P < .002, Figure 2). Seizures occurred more significantly when the duration of DHCA exceeded 40 minutes (Figure 2). However, use of continuous CPB did not eliminate the risk of seizures. To further characterize the relationship between DHCA duration and the incidence of seizures, patients were divided into 3 groups according to total DHCA time: continuous CPB alone, DHCA for 40 minutes or less, and DHCA for more than 40 minutes (Figure 3). Patients in whom the duration of DHCA was more than 40 minutes had a greater occurrence of seizures (14/58 [24.1%]) than those with a DHCA duration of 40 minutes or less (4/59 [6.8%], P = .038). The incidence of seizures for patients in whom the duration of DHCA was 40 minutes or less was not significantly different from those in whom DHCA was not used (2/61 [3.3%], P = .4).
Figure 2.
Relationship between duration of DHCA and occurrence of an EEG seizure. Patients are grouped according to total DHCA time in 5-minute intervals. The number of patients in each group with and without an EEG seizure is shown on the y-axis.
Figure 3.
Comparison of incidence of seizures among continuous CPB alone, DHCA for 40 minutes or less, and DHCA for greater than 40 minutes.
When stepwise logistic regression was used to jointly consider those factors found to marginally predict seizures at a P value of .1 (Table 1), only increasing duration of total DHCA could be identified as an independent predictor of occurrence of a seizure (P = .001). Inclusion of no other variable in the model improved prediction of the occurrence of a seizure. This was true whether the total DHCA time was considered as a continuous variable or dichotomized at 0 to 40 versus greater than 40 minutes.
Neuroimaging
MRI of the brain was performed for clinical indications in 16 (80%) of 20 of the patients in whom an EEG seizure was identified. Abnormalities were found in 14 (88%) of the 16 patients, including periventricular leukomalacia (PVL) in 7 (44%), localized infarction in 5 (31%), and watershed infarction in 3 (23%). Both PVL and a watershed infarction were present in 1 patient.
Discussion
The current study demonstrates that postoperative seizures documented by continuous EEG monitoring occur in 11% of neonates and infants undergoing repair of complex CHDs. The incidence of seizures varied according to cardiac defect and was lowest in children undergoing biventricular repair of lesions such as TOF, VSD, and TGA. The occurrence of seizures was highest in those undergoing reconstructive surgery for HLHS and variants. By multivariable analysis, the strongest predictor of the risk of a seizure was increasing total duration of DCHA. The incidence of seizures increased when the duration of DHCA exceeded 40 minutes (Figure 2 and Figure 3). However, use of continuous CPB did not eliminate the risk of seizures. In addition, many children underwent periods of DHCA for longer than 40 minutes and did not have seizures, whereas seizures did occur in some children after briefer periods of DHCA. These findings suggest that the use, duration, or both, of DHCA alone do not fully account for the risk of seizures in this patient population. Other patient- and procedure-specific variables might also be important risk factors.
Visual detection of clinical seizure activity clearly underestimates the incidence of seizures, as documented by continuous EEG monitoring.1 Clinical assessment of seizure activity in the early postoperative period is clouded by the use of sedation and neuromuscular blockade. Studies that evaluate only clinical seizure activity will not identify sub-clinical seizures, which are also markers of CNS injury.7,8 In the current study no postoperative EEG seizure was associated with clinical seizure activity. The Boston Circulatory Arrest Study evaluated early and late neurologic outcomes in neonates and infants undergoing the arterial switch operation for TGA with or without a VSD.1–3 EEG seizures were 3 times more frequent than clinical seizure activity. Seizures documented by EEG monitoring occurred in 20% of these infants and were more frequent among children assigned to DHCA, although the difference was not statistically significant. Longer duration of DHCA was associated with a higher risk of EEG seizures. In addition, an anatomic factor (the presence of a VSD) increased the risk of EEG seizures. Follow-up evaluation of this cohort demonstrated that occurrence of an early postoperative seizure was a significant risk factor for adverse neurodevelopmental outcomes at 1, 4, and 8 years of age (personal communication, J. Newburger, 2005).2,3
The current study, as well as published data from studies using EEG monitoring, suggests that the incidence of post-operative seizures is decreasing, especially among children undergoing biventricular repair. The incidence of EEG seizures in the circulatory arrest study was 20%.1 In a later study evaluating blood gas management strategies, investigators at Boston Children’s Hospital identified EEG seizures in 6 (5%) of 116 patients undergoing biventricular repair of a variety of cardiac defects, including TOF, TGA, VSD, total anomalous pulmonary venous connection, and truncus arteriosus.4 Fewer seizures occurred in infants assigned to the pH-stat group; however, the difference was not significant. In the current study the incidence of EEG seizures in comparable patients was 3% (2/63). Neither the Boston Circulatory Arrest Study nor the blood gas management study included patients with more complex forms of heart disease, such as functional single ventricle and HLHS.
In the current study, seizures were more common in patients with HLHS and variants than in those with other heart defects. However, type of cardiac defect was not a predictor of seizures in the multivariable analysis. At our institution, DHCA is used uniformly during stage 1 reconstruction. DHCA is less commonly used for repair of biventricular defects without arch obstruction. Thus, the use and duration of DHCA is linked to a diagnosis of HLHS or variant (class IV).
Many factors likely contribute to the risk of postoperative seizure. Previous studies and the current study have focused on intraoperative management. However, children with CHD are an at-risk population for neurodevelopmental problems. Development of the brain in these children is often abnormal, with a high incidence of microcephaly and congenital structural abnormalities, as well as acquired lesions. 9,10 Cerebral hypoperfusion and low cerebral oxygen saturation are present in some children before surgical intervention. 11 Postoperative events, such as low cardiac out-put, hypoxia, and hypotension, can cause CNS injury or exacerbate existing injury.12 The findings of the current study suggest that the use, duration, or both, of DHCA alone does not explain the incidence of postoperative seizures. Avoidance of DHCA did not completely eliminate the risk of seizure. Seizures occurred in infants even after brief periods of DHCA and did not occur in many infants with longer periods of DHCA. The heterogeneity of findings on postoperative neuroimaging studies suggests that multiple factors, including localized infarctions, PVL, and watershed infarctions, might be implicated as causes of seizures.13
Identification of an EEG seizure after surgical intervention can serve as a marker for CNS injury and perhaps as a surrogate for later neurodevelopmental difficulties. However, occurrence of a seizure likely identifies only the most severely injured children. Developmental abnormalities can be identified in approximately 50% of school-aged survivors after the arterial switch operation.14 The 8-year follow-up evaluation of patients in the Boston Circulatory Arrest Study demonstrated that academic achievement, overall neurologic performance, and academic performance were lower than population norms regardless of treatment assignment.15 Other studies have shown that brain MRI in the early postoperative period demonstrates that acquired structural CNS abnormalities are more common than the incidence of EEG seizures in the current study.7,12 Occurrence of an EEG seizure is a relatively specific marker for perioperative CNS injury; however, sensitivity for CNS injury is low, and only the most severely injured children are identified. Thus, occurrence of an EEG seizure will not identify all children with perioperative CNS injury who might be at risk for long-term neurodevelopmental difficulties.
The long-term neurodevelopmental sequelae of a postoperative seizure are likely secondary, in large part, to the underlying CNS injury provoking the seizures, such as hypoxic-ischemic injury or stroke. However, recent research demonstrates that recurrent seizures induced by proconvulsant drugs might have long-term undesirable effects on brain structure, learning, and susceptibility to spontaneous seizures.16–20 Similarly, neonatal seizures might create long-term changes in the profile of GABAA subunit composition, the brain’s principal inhibitory neurotransmitter receptor site.21,22 Although the major force behind the ultimate neurodevelopmental abnormalities after postoperative seizures might largely be their underlying cause, the magnitude of the contribution of the seizures themselves to the final extent of disability is not known in human newborns.
The findings of the current study suggest that there might be a threshold for the duration of DHCA below which the incidence of seizure activity is relatively low and not significantly different from that of patients in whom DHCA is not used. Previous studies have attempted to define a safe threshold for use of DHCA to prevent CNS injury and subsequent neurodevelopmental problems. However, there is increasing evidence that even use of continuous CPB is not entirely safe and is associated with CNS injury and adverse developmental sequelae. Studies in adults document significant neurocognitive decline after CPB.23 Follow-up evaluation of the patients in The Boston Circulatory Arrest Study demonstrated that both treatment groups were significantly impaired compared with population norms.15 Interestingly, the authors were able to demonstrate that the neurodevelopmental outcome at 8 years in multiple domains was not adversely affected unless the duration of DHCA exceeded 35 to 40 minutes.24 This apparent threshold is similar to that seen in the current study. However, there is considerable interindividual variability in the response to CPB. Factors such as polymorphisms in genes regulating the inflammatory response or neuronal repair are likely important determinants of this variability.5 It is probably not possible to define a safe threshold for exposure to DHCA, CPB, or both for all children below which no injury will occur. Assessment of neurologic function and identification of neurodevelopmental disabilities can be difficult in children. Inability to identify or quantify an injury and its sequelae does not mean an injury has not occurred. As children mature, subtle deficits affecting learning, attention, behavior, and other higher functions become apparent.
There are several limitations to the study. Management of CPB is highly surgeon and institution specific. Many factors that might increase the risk of CNS injury and thus seizures were not standardized for this study. These include pump flow rates, cannulation technique, and use of vents. Alpha-stat blood gas management was used. Some studies have suggested a benefit to use of pH-stat blood gas management. However, the largest clinical trial of blood gas management in patients undergoing biventricular repair does not demonstrate an advantage of either technique in terms of neurodevelopmental outcomes.25 In that trial EEG seizures occurred in 5.1% of patients (9% of those randomized to alpha-stat and 2% of those randomized to pH-stat).4 In the current study the incidence of seizures in similar patients was 3%, suggesting that blood gas management does not alter the risk. Finally, this study does not establish a safe upper limit for the duration of DHCA. A duration of 40 minutes was selected on the basis of review of the data, as well as the recent reports for the Boston Circulatory Arrest Study suggesting that long-term neurodevelopmental outcomes are not adversely affected by DHCA duration of less than 40 minutes.24 The incidence of seizures does increase with increasing duration of DHCA. Many additional factors also alter the risk of CNS injury for a specific patient. It is important to note that avoidance of DHCA does not eliminate the risk of seizures. There is evidence that prolonged exposure to CPB enhances the systemic inflammatory response and might be associated with worse organ injury.20 Use of DHCA might decrease the inflammatory response and lessen organ injury in some cases.26 The optimal technique for use of either CPB or continuous DHCA has not been identified, and neither strategy should be completely avoided.26
In conclusion, the current study demonstrates that EEG seizures occur in 11% of neonates and infants undergoing repair of complex CHD. Increasing duration of DHCA is a risk factor for seizures. However, use of continuous CPB did not eliminate the risk of seizures. Use of limited periods of DHCA did not significantly increase the risk of a seizure. Because of the possibility that seizures are not merely a marker for CNS injury but might exacerbate injury, identification of patients at increased risk is important. Future studies are necessary to determine whether aggressive EEG monitoring and therapy, including prophylactic treatment, can reduce the incidence of seizures and diminish the adverse long-term sequelae.
Acknowledgments
Supported by a grant from the Fannie E. Rippel Foundation, an American Heart Association National Grant-in-Aid (9950480N), and a grant HL071834 from the National Institutes of Health.
Discussion
Dr Ross M. Ungerleider (Portland, Ore). You and your colleagues should be congratulated on contributing a very thought-fully designed and labor-intensive study that provides several insights. Most prominently, you emphasize once again a disturbing finding that was demonstrated, as you referenced, by the Boston Circulatory Arrest Trial, and that is that the appearance of clinical seizures is an insensitive barometer by which to determine whether infants have had neurologic injury.
You discovered subclinical EEG seizures in 11% of your patients and in 18% of your neonatal patients undergoing the Norwood procedure, which might be important in light of the neurobehavioral outcome issues that have been identified for that subgroup of patients. Perhaps we are approaching an irreducible minimum of neurologic injury because you are no longer seeing the same incidence of clinical seizures, but I believe, and I am sure you do too, that there is still room for much understanding and potential for improvement.
I would like you to please address several issues for us. First of all, should we all routinely look for subclinical seizures and, if we find them, should they be treated? Would that provide us with a way to further improve neurologic outcome for patients we might not otherwise recognize as being at risk?
Dr Gaynor. The first thing we need to understand is the effect of a seizure in this population. These children just recently completed 1-year evaluations about a month ago, and we are analyzing those data, and the whole cohort is coming back for a detailed evaluation at 4 years. Therefore, I have a feeling that a seizure as a marker of injury will be a predictor of a worse outcome. What we do not know is this: Can we prevent seizures if we identify children at risk and prophylactically treat them, or if we aggressively treat them once they are identified, does that change the outcome? These are questions for research studies, and I think we need carefully designed protocols to address these questions before we recommend routine EEG monitoring.
But a seizure is a marker of CNS injury. Therefore, there is an injury that causes a seizure; however, there is a lot of evidence from animal models and other forms of seizures that a seizure itself can extend or exacerbate the injury. Therefore, it is likely that treatment and prevention of repetitive seizures will improve the outcome, but I do not think there are data to recommend routine monitoring. However, I think it is clearly a topic for further research studies.
Dr Ungerleider. Second, the incidence of subclinical seizures in the hypoplastic patients is disturbing. As the title of your article indicates, perhaps it has to do with the fact that those patients probably had exposure to the longest periods of circulatory arrest. Could you comment on other reasons that you think the hypoplastic patients had a high incidence of seizures? Did they have anything else in the preoperative findings? Did the APOE findings show anything? As our group has emphasized, postoperative patients with cyanosis, with hypoxemia, are particularly at risk if they have inadequate cerebral oxygen delivery. Were there any differences in the postoperative hemodynamics in the hypoplastic patients who had seizures?
Dr Gaynor. We did not evaluate the postoperative period as a risk factor, but the HLHS patients do tend to have lower blood pressure and are hypoxic. The 2-ventricle patients usually have good cardiac outputs and are not hypoxic. I think it is important also to look at preexisting factors.
We have conducted a study looking at cerebral blood flow before surgical intervention in children, and what is frightening is that many children have very, very low levels of cerebral blood flow, even before surgical intervention. Furthermore, there is a significant incidence of structural abnormalities, both congenital and acquired, such as periventricular leukomalacia, even before surgical intervention. These might be more common in, for example, children with HLHS; microencephaly appears to be more common in children with HLHS, possibly because of the arch anomalies affecting cerebral blood flow in utero. Therefore, they might be a particular at-risk population both on preoperative factors and on postoperative factors, as you suggested.
Dr Ungerleider. Do you think the cause of the seizures that occur after continuous bypass is different from that after circulatory arrest?
Dr Gaynor. I think it might be. I think it is multifactorial. I did not present the data, but we looked at neuro-imaging studies, and 16 of the 20 children had MRIs obtained for clinical reasons. There were a variety of lesions seen, both consistent with hypoperfusion, such as watershed infarcts, hypoxic ischemia, PVL, and localized embolic strokes. Therefore, I think all of those could be the cause of the seizure, and the cause might be different in the children who had continuous bypass.
Again, a weakness of this study is that we do not know the status before surgical intervention. We did perform baseline EEGs, and no child had a seizure identified on a preoperative EEG, but we did not do neuro-imaging studies in every child preoperatively to know whether there was underlying structural CNS disease.
Dr Ungerleider. Finally, your application of circulatory arrest leaves many questions. What were your cooling times, and did you alter them for patients who you expected might have longer exposures to arrest? Despite the significance in clinical trials, there is ample laboratory support for the use of pH-stat cooling, and this is especially true for some high-risk patient groups like those with cyanosis, such as hypoplastic patients. I am curious why you chose to use alpha-stat.
Finally, although you chose 40 minutes as your cutoff point for safe circulatory arrest—and I recognize that is a time frame that was corroborated by the recently published Boston data—there are numerous laboratory studies that show significant histologic and metabolic derangements after 15 to 20 minutes of circulatory arrest and certainly as long as 30 minutes. Interestingly, the data that you presented showed no seizures for circulatory arrest between 1 and 26 minutes. Could you comment on that?
Dr Gaynor. Back to the pH-stat, the data from Boston show that there is no difference in long-term neurodevelopment outcome on the basis of blood gas management. There was a trend toward fewer seizures when pH-stat was used. I evaluated the patients from our study who met the criteria for entry into the Boston pH-stat versus alpha-stat study, and there were 63 patients. In the pH-stat subgroup in the Boston study, 1 of 59 patients had seizures; in our group 2 of 63 had seizures. Therefore, I do not think that incidence is different.
For cooling for DHCA, we generally cool for 20 minutes, and we use topical hypothermia. Therefore, we try to have a prolonged cooling period, and it is a very uniform period for all the children in the study.
Dr Ungerleider. Maybe you did not hear the final question, but in the patients between 1 and 26 minutes of circulatory arrest, there were no seizures. I realize that was a small number of patients, but I wonder whether you could speculate whether reperfusion every 15 minutes might have helped avoid some seizures for the patients who had longer total durations of exposure to circulatory arrest.
Dr Gaynor. I think there are newer techniques—intermittent cerebral perfusion, regional cerebral perfusion—and these techniques have met with a lot of acceptance and widespread use in some centers, but there are no data yet, and as we introduce new techniques, we need to carefully evaluate them as we go forward. Therefore, I think it is speculative, and it might decrease the incidence, but we need data.
Dr Ungerleider. These questions and your answers are simply meant to help formalize our questions that will lead to future investigation in this field.
Dr Richard A. Jonas (Boston, Mass). I have a comment and a question. I also am very surprised that you used the alpha-stat strategy for this study because laboratory work at your own hospital has demonstrated advantages for the pH-stat approach, and in our prospective randomized trial, death approached significance; in fact, the P value was .058 for a higher mortality with the alpha-stat strategy. The pH-stat strategy suppresses metabolic rate, so that it makes sense that it would be a preferable approach for circulatory arrest. My question is this: How has this study influenced your clinical approach to handling children with these types of problems?
Dr Gaynor. We still use DHCA. We attempt to limit the periods of DHCA, if possible. Ten years ago, DHCA was frequently used for switches and biventricular repairs, and now those are almost always done during continuous bypass. We attempt to limit the duration for patients having the Norwood procedure, but we have not gone to intermittent cerebral perfusion or regional cerebral perfusion for those patients.
Dr Bradley Allen (Houston, Tex). Just to follow up on something that Dr Ungerleider asked: Was one of your variables cyanosis, or did you only look at specific lesions, such as HLHS? As we know, cyanotic or hypoxic infants are less tolerant to myocardial ischemia during cardioplegic arrest. Moreover, hypoxia might predispose the infant to a reoxygenation injury, which along with ischemia might lead to tissue injury, both in the brain and else-where. Therefore, I was wondering whether you used hypoxia as one of your variables in the multivariate analysis.
Dr Gaynor. We did not look at that specifically. it is hard to define consistently. What level of cyanosis do you use? Some children have intermittent cyanosis. How long? Some of these children had TOF and had had 4 months of cyanosis versus a newborn who had been cyanotic for just a few days. We did not stratify according to cyanosis alone. But the incidence of cyanosis among those with TOF before surgical intervention was low.
Footnotes
Read at the Eighty-fourth Annual Meeting of The American Association for Thoracic Surgery, Toronto, Ontario, Canada, April 25-28, 2004.
References
- 1.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Karl CK, et al. Comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KCK, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 4.du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, et al. Perioperative effects of alpha-stat versus ph-stat stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1001. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor JW, Gerdes M, Zachai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 6.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–357. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 7.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73:1752–1758. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 8.Clancy RR, McGaurn SA, Wernovsky G, Gaynor JW, Spray TL, Norwood WI, et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111:592–601. doi: 10.1542/peds.111.3.592. [DOI] [PubMed] [Google Scholar]
- 9.Mahle WT, Zimmerman RA, Nicolson SC, Galli K, Gaynor JW, Wernovsky G, et al. Serial magnetic resonance imaging of the brain in newborns undergoing congenital heart surgery. Circulation. 2000;102(19 Suppl 3):III136–III141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 10.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. 1999;103:402–408. doi: 10.1542/peds.103.2.402. [DOI] [PubMed] [Google Scholar]
- 11.Kurth CD, Steven JM, Montenegro LM, Watzman HM, Gaynor JW, Spray TL, et al. Cerebral oxygen saturation before congenital heart surgery. Ann Thorac Surg. 2001;72:187–192. doi: 10.1016/s0003-4975(01)02632-7. [DOI] [PubMed] [Google Scholar]
- 12.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 13.Ichord R, Smith S, Licht D, Sharif U, Raffini L, Gay nor JW, et al. Stroke and acute cerebral injuries presenting as subclinical seizures after newborn heart surgery. Ann Neurol. 2003;54:110–111. [Google Scholar]
- 14.Hovels-Gurich HH, Seghaye C, Schnitker R, Wiesner M, Huber W, Minkenberg R, et al. Long-term neurodevelopmental outcomes in school-ages children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2002;124:448–458. doi: 10.1067/mtc.2002.122307. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 16.Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- 17.McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 19.Wasterlain CG. Neonatal seizures and brain growth [abstract] Neuropaediatrics. 1978;9:213–228. doi: 10.1055/s-0028-1091482. [DOI] [PubMed] [Google Scholar]
- 20.Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Dev Neurosci. 2002;24:367–381. doi: 10.1159/000069049. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Hsu FC, Raol YH, Coulter DA, Brooks-Kayal AR. Selective alterations of GABA A receptor subunit expression and function in hippocampal dentate granule cells after seizures in the developing brain [abstract] Epilepsia. 2001;42 suppl 7:224. [Google Scholar]
- 22.Zhang G, Raol YH, Brooks-Kayal AR. Selective alteration of excitatory and inhibitory receptors and transporters in hippocampal dentate granule cells after seizures in the developing brain. Epilepsia. 2002;43 suppl 7:27. [Google Scholar]
- 23.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 24.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1340. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 25.Bellinger DC, Wypij D, du Plessis AJ, Rappaport LA, Riviello J, Jonas RA, et al. Developmental and neurologic effects of alpha-stat versus ph-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 2001;121:374–383. doi: 10.1067/mtc.2001.111206. [DOI] [PubMed] [Google Scholar]
- 26.Ungerleider RM, Gaynor JW. The Boston Circulatory Arrest Study. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/j.jtcvs.2003.12.037. [DOI] [PubMed] [Google Scholar]