Abstract
OBJECTIVE
The goal was to evaluate polymorphisms of the APOE gene as modifiers of neurobehavioral outcomes for preschool-aged children with congenital heart defects, after cardiac surgery.
METHODS
A prospective observational study with neurodevelopmental evaluation between the fourth and fifth birthdays was performed. Attention and behavioral skills were assessed through parental report.
RESULTS
Parents of 380 children completed the neurobehavioral measures. Child Behavior Checklist scores for the pervasive developmental problem scale were in the at-risk or clinically significant range for 15% of the cohort, compared with 9% for the normative data (P < .00001). Attention problem scores were in the at-risk or clinically significant range for 12% of the cohort, compared with 7% for the normative data (P = .0002). The Attention-Deficit/Hyperactivity Disorder Rating Scale-IV, Preschool Version, was completed for 378 children; 30% scored in the clinically significant range for inattention and 22% for impulsivity. After adjustment for covariates, the APOE ε2 allele was significantly associated with higher scores (worse problems) for multiple Child Behavior Checklist indices, including somatic complaints (P = .009), pervasive developmental problems (P = .032), and internalizing problems (P=.009). In each case, the ε4 allele was associated with a better outcome. APOE ε2 carriers had impaired social skills, compared with ε4 carriers (P = .009).
CONCLUSIONS
For preschool-aged children with congenital heart defects requiring surgery, parental rating scales showed an increased prevalence of restricted behavior patterns, inattention, and impaired social interactions. The APOE ε2 allele was associated with increased behavior problems, impaired social interactions, and restricted behavior patterns.
Keywords: congenital heart defects, genetic predisposition to disease, apolipoprotein E, behavioral symptoms, attention-deficit/hyperactivity disorder, impulsive behavior, autistic disorder
Congenital heart defects (CHDs) are the most common birth defects in humans, affecting 8 per 1000 live births (~30 000–40 000 children each year in the United States), with one third of affected children requiring intervention in early infancy. Neurodevelopmental dysfunction is the most common and potentially most disabling outcome of CHDs and their treatment. Improved survival rates, combined with expectations for independence and behavioral self-regulation as the children mature, have led to increasing recognition of neurobehavioral symptoms and impaired functional outcomes for some survivors. Recent reports identified a high prevalence of inattention and hyperactivity/impulsivity behaviors, consistent with the behavioral phenotype of attention-deficit/hyperactivity disorder (ADHD).1 Apolipoprotein E is an important regulator of cholesterol metabolism, and APOE has an important role as a susceptibility gene that modifies outcomes after central nervous system (CNS) injury. In 1998, we initiated a prospective study evaluating the association between neurodevelopmental dysfunction and APOE genotype in 550 neonates and infants who were undergoing surgery for treatment of CHDs. The APOE ε2 allele was associated with significantly worse neurodevelopmental outcomes at 1 year of age.2
METHODS
Study Design
This was a prospective trial assessing the effects of APOE polymorphisms on neurobehavioral outcomes for preschool-aged patients (4 –5 years of age) after cardiac surgery in infancy.2 Patients ≤6 months of age who were undergoing surgical treatment of CHDs with cardiopulmonary bypass, with or without deep hypothermic circulatory arrest (DHCA), were eligible. Exclusion criteria included (1) multiple congenital anomalies, (2) recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletion syndrome, and (3) language other than English spoken in the home. The study was approved by the institutional review board at the Children’s Hospital of Philadelphia. Informed consent was obtained from parents or guardians.
Operative Management
Alpha-stat blood gas management was used. Pump flow rates were not standardized. DHCA was used at the surgeon’s discretion. Before DHCA, patients underwent core cooling, with topical hypothermia of the head, to a nasopharyngeal temperature of 18°C. Modified ultrafiltration was performed for all patients.
Data Collection
Data on preoperative factors that might affect neurobehavioral outcomes independently, including gestational age, birth head circumference, and birth weight, were obtained from hospital records. Weight and age at surgery were recorded for the initial operation and for subsequent procedures with cardiopulmonary bypass. Operative variables were recorded, including the durations of cardiopulmonary bypass and DHCA, lowest nasopharyngeal temperature, and hematocrit level after hemodilution.
APOE Genotype Determinations
Whole blood or buccal swab samples were obtained before the operation and were stored at 4°C. Genomic DNA was prepared and was used for determination of APOE genotypes by using a previously published method.3
Four-Year Neurodevelopmental Examinations
Neurodevelopmental evaluations were performed between the fourth and fifth birthdays. Growth measurements (weight, length, and head circumference) were recorded. Maternal education, Hollingshead socioeconomic status, and ethnicity were determined through parental report.4 A health history was obtained, focusing on the incidence of interim illnesses, rehospitalizations, neurologic events or interim evaluations, current medication use, and parental concerns about health. Parents completed behavior questionnaires and rating scales. Parents were asked specifically whether they had ever been told that their child had autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, or ADHD.
Patients were evaluated by a genetic dysmorphologist at the 1-year and/or 4-year evaluations. Additional genetic analyses were performed as indicated. Neonatal recognition of dysmorphic features may be difficult; therefore, some patients for whom the diagnosis of a genetic syndrome was made at a later evaluation were enrolled. Patients were classified as having no definite genetic syndrome or chromosomal abnormality (normal), a suspected genetic syndrome (suspect), or a definite genetic syndrome or chromosomal abnormality (abnormal).
Cognitive skills were assessed as the full-scale IQ from the Wechsler Preschool and Primary Scale of Intelligence, Third Edition.5 Core language skills were assessed by using the Preschool Language Scale-4 (PLS-4) total language score (TLS). Executive function was assessed with the Neuro-PSYchology (NEPSY) statue test, which targets inhibition and motor persistence (component processes of attention and executive function).6 Details are provided in the Appendix.
Attention and other behavioral skills were assessed through parental report by using the Child Behavior Checklist for ages 1.5 to 5 years (CBCL/1.5–5), the ADHD Rating Scale-IV, Preschool Version, and the Preschool and Kindergarten Behavior Scales (PKBS).7–9 The CBCL/1.5–5 is a questionnaire used to obtain parental reports of behavior problems and prosocial adaptive skills demonstrated within the previous 6 months.7 Responses are grouped to produce 7 narrow-band problem scores and 5 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)– oriented scores. Results include a total problem score and 2 broad-band indices (internalizing and externalizing problems). Children were classified as having normal, at-risk, or clinically significant scores. The ADHD Rating Scale-IV, Preschool Version, is an 18-item questionnaire that requires parents to rate the frequency of occurrence of ADHD symptoms, as defined in the DSM-IV.9 Respondents rate each item on a Likert scale of 0 (not at all) to 3 (very often). The PKBS provide a social skills score (assessing social cooperation, social interaction, and social independence), externalizing problems and internalizing problems scores (assessing problem behaviors, ie, self-centered/explosive, attention problems/overactive, antisocial/aggressive and social withdrawal, and anxiety/somatic), and a total problem behavior score.9 Details are provided in the Appendix.
A potential problem with behavior questionnaires and rating scales involves the accuracy of parental responses and potential bias. Parents may consciously or subconsciously portray their child in a positive manner, perhaps to improve perceptions of their parenting skills or because their child is a “survivor” overcoming a chronic disease. The defensive responding score from the Parenting Stress Index-Short Form was used to evaluate the candor of parental responses across parental report measures. Low scores (≤10) are associated with a parent who may be giving socially desirable responses.10
Data Analyses
Comparisons of outcomes and variables between groups of subjects were performed by using Fisher’s exact test or Student’s t test. Linear regression analysis was used to characterize the adjusted associations between APOE genotype and 4-year outcomes. APOE genotype was coded for the presence of the ε2 or ε4 allele. Subjects with the ε2ε4 genotype were excluded from the analysis of APOE genotype effects. Subjects were categorized in 3 groups, that is, ε2 (ε2ε2 or ε2ε3), ε3 (ε3ε3), and ε4 (ε3ε4 or ε4ε4). APOE group was coded as a dummy variable, with the ε3 group as the reference. The final model considered demographic covariates with a set of operative covariates. The APOE genotype group effect is reported as the difference between the ε2 or ε4 group and the reference ε3 group. In addition, P values generated through analysis of variance comparing all 3 APOE genotype groups (df = 2) are reported.
RESULTS
Study Group
Between September 1998 and April 2003, 675 eligible infants underwent cardiac surgery. Twenty-three infants died before consent was obtained, parents of 102 infants declined participation, and 550 infants (81%) were enrolled. There were 21 deaths during the initial hospitalization and an additional 43 deaths before 5 years of age. The study population was 65% white, 23% black, and 12% of other ethnic origins; 58% of subjects were male. The largest diagnostic groups included hypoplastic left heart syndrome (n = 121), tetralogy of Fallot (n = 83), ventricular septal defects with or without coarctation (n = 77), and transposition of the great arteries (n = 45). APOE genotyping was completed in 540 (98%) of 550 cases. The APOE genotype distribution was as follows: ε2ε2, n = 3; ε2ε3, n = 64; ε2ε4, n = 14; ε3ε3, n = 323; ε3ε4, n = 124; ε4ε4, n = 12 (not different from Hardy-Weinberg proportions).
Four hundred eighty-six patients were alive and eligible for the 4-year evaluation, which was completed by 381 patients (78% of eligible patients). Baseline characteristics were compared for patients who returned for the 4-year evaluation (n = 381), those who did not return (n = 105), and those who died before 4 years of age (n = 64) (Table 1). The only significant difference in baseline characteristics between returning and nonreturning patients was underrepresentation of black patients among returning patients (21% vs 29%).
TABLE 1.
Comparison of Baseline and Operative Characteristics of Patients According to Return Status
| Baseline Characteristics | Returned (N = 381) |
Alive, Did Not Return (N = 105) |
Died (N = 64) |
P | |
|---|---|---|---|---|---|
| Returned vs Did Not Return |
Returned vs Died |
||||
| Gender, n (%) | .44 | .28 | |||
| Female | 166 (44) | 41 (39) | 26 (41) | ||
| Male | 215 (56) | 64 (61) | 41 (64) | ||
| Ethnicity, n (%) | .0002 | <.0001 | |||
| Asian/Pacific Islander, Hispanic, Native American, or other |
40 (10) | 15 (14) | 11 (17) | ||
| Black, not Hispanic | 80 (21) | 30 (29) | 16 (25) | ||
| White, not Hispanic | 261 (69) | 60 (57) | 67 (58) | ||
| Gestational age, mean ± SD, wk (N) | 38.5 ± 2.1 (378) | 38.5 ± 2.4 (102) | 37.6 ± 2.9 (62) | .97 | .03 |
| Birth weight, mean ± SD, g (N) | 3119 ± 636 (379) | 3136 ± 664 (101) | 2799 ± 721 (62) | .82 | .002 |
| Birth head circumference, mean ± SD, cm (N) |
33.6 ± 2.1 (372) | 33.5 ± 2.3 (96) | 32.6 ± 2.8 (60) | .81 | .01 |
| APOE genotype, n | .13 | .342 | |||
| ε2 | 44 | 13 | 10 | ||
| ε3 | 225 | 68 | 30 | ||
| ε4 | 101 | 17 | 18 | ||
| ε24 | 9 | 3 | 2 | ||
| Unknown | 2 | 4 | 4 | ||
| Diagnostic class, n (%) | .13 | .04 | |||
| I: 2 ventricles, no arch obstruction | 202 (53) | 59 (56) | 14 (22) | ||
| II: 2 ventricles, arch obstruction | 47 (13) | 9 (8) | 3 (5) | ||
| III: 1 ventricle, no arch obstruction | 36 (9) | 10 (10) | 8 (12) | ||
| IV: 1 ventricle, arch obstruction | 96 (25) | 27 (26) | 39 (61) | ||
| Preoperative intubation, n (%) | 117 (31) | 29 (28) | 25 (39) | .63 | .19 |
| Preoperative length of stay, mean ± SD, d | 2.2 ± 3.0 | 2.7 ± 4.9 | 5.1 ± 14.4 | .18 | .40 |
| Age at first operation, mean ± SD, d | 42.4 ± 53.9 | 45.1 ± 52.6 | 26.5 ± 47.6 | .64 | .017 |
| Weight at first operation, mean ± SD, kg | 3.88 ± 1.26 | 3.84 ± 1.25 | 3.11 ± 0.93 | .80 | <.0001 |
| Total cardiopulmonary bypass time in first operation, mean ± SD, min |
65.7 ± 39.3 | 67.0 ± 37.9 | 71.7 ± 46.8 | .76 | .34 |
| Use of DHCA, n (%) | 217 (57) | 65 (62) | 58 (91) | ||
| Total DHCA time in first operation, mean ± SD, min |
39.1 ± 16.5 | 41.3 ± 21.2 | 50.3 ± 18.5 | .24 | <.0001 |
| Hematocrit level after hemodilution in first operation, mean ± SD, % |
27.9 ± 4.0 | 27.3 ± 4.2 | 28.3 ± 4.0 | .21 | .45 |
| Postoperative length of stay after first operation, mean ± SD, d |
12.2 ± 13.0 | 12.3 ± 14.2 | 33.3 ± 38.9 | .28 | .12 |
Neurodevelopmental Testing
Mean scores for many domains, including cognitive and core language skills, were in the normal or low normal range. However, the distributions were shifted downward, so that a significant number of children had scores >1 SD below the mean (moderate impairment) or >2 SD below the mean (significant impairment). Table 2 shows the prevalence of impairments in the study cohort for selected domains. Almost 30% of children had at least moderate cognitive impairment (full-scale IQ >1 SD below the mean). At least moderate core language impairment (PLS-4 TLS >1 SD below the mean) was present for 25% of children. Executive function was moderately impaired (NEPSY statue scoreϡ SD below the mean) for 33% of children and severely impaired (NEPSY statue score #x0003E2; SD below the mean) for 11%.
TABLE 2.
Four-Year Outcomes for Selected Domains
| Domain | Test | Score, Mean ± SD |
Proportion >1 SD Below Mean, % |
Proportion >2 SD Below Mean, % |
Proportion at Risk or Clinically Significant, % |
|---|---|---|---|---|---|
| Cognition | Full-scale IQ | 95.02 ± 19.14 | 29 | 11 | |
| Core language | PLS-4 total language | 97.05 ± 18.67 | 24 | 10 | |
| Attention | ADHD Rating Scale-IV inattention | 6.34 ± 5.40 | 30 | ||
| ADHD Rating Scale-IV impulsivity | 7.19 ± 5.55 | 22 | |||
| CBCL/1.5–5 attention problems | 54.64 ± 6.9 | 12 | |||
| Executive function | NEPSY statue | 9.35 ± 3.93 | 33 | 11 | |
| Behavior | CBCL/1.5–5 internalizing problems | 49.57 ± 11.56 | 22 | ||
| CBCL/1.5–5 total problems | 48.74 ± 11.58 | 18 | |||
| Social skills | CBCL/1.5–5 withdrawn | 55.32 ± 7.32 | 11 | ||
| CBCL/1.5–5 PDPs | 55.36 ± 7.73 | 15 | |||
| PKBS social skills | 106.07 ± 12.98 | 10 |
Parental Reports of Behavior
The prevalence of at-risk or clinically significant scores for some CBCL/1.5–5 scales was greater in the whole APOE cohort than the general population (Table 3). Attention problem scores were in the at-risk or clinically significant range for 12% of the cohort, compared with 7% for the normative data (P = .0002). Inattention and impulsivity were also assessed by using the ADHD Rating Scale-IV, Preschool Version, which was completed by parents of 378 children. Of the entire cohort, 30% scored in the clinically significant range for inattention and 22% for impulsivity on the ADHD Rating Scale-IV, Preschool Version. When only nondefensive parental reports (n = 244) were considered, 35% scored in the clinically significant range for inattention and 25% for impulsivity. These findings, in conjunction with the CBCL/1.5–5 findings, suggest that the prevalence of ADHD in this cohort likely is significantly greater than the 4% to 7% reported in the DSM-IV.11
TABLE 3.
CBCL/1.5–5 Scores in Comparison With Normative Data
| Proportion With at-Risk or Clinically Significant Scores, % | P | ||||
|---|---|---|---|---|---|
| Normative Data |
APOE Cohort (N = 380) |
APOE Cohort, Defensive Reporters (N = 246) |
APOE Cohort vs Normative Data |
APOE Cohort, Defensive Reporters, vs Normative Data |
|
| Emotionally reactive | 10.0 | 10.5 | 14.2 | .73236 | .02709 |
| Anxious/depressed | 8.0 | 7.4 | 10.6 | .64996 | .13747 |
| Somatic complaints | 9.0 | 15.4 | 18.3 | .00001 | <.00001 |
| Withdrawn | 7.0 | 10.8 | 15.0 | .00379 | <.00001 |
| Sleep problems | 5.0 | 9.0 | 9.8 | .00041 | .00062 |
| Attention problems | 7.0 | 11.8 | 15.5 | .00022 | <.00001 |
| Aggressive behavior | 7.0 | 6.3 | 6.9 | .60115 | .95616 |
| Affective problems | 7.0 | 13.2 | 17.9 | <.00001 | <.00001 |
| Anxiety problems | 8.0 | 9.5 | 13.0 | .28964 | .00379 |
| PDPs | 7.0 | 15.0 | 21.1 | <.00001 | <.00001 |
| ADHD problems | 9.0 | 5.8 | 6.9 | .02875 | .25216 |
| Oppositional-defiant problems | 7.1 | 6.6 | 8.5 | .74769 | .34488 |
| Internalizing problems | 21.0 | 22.4 | 29.7 | .51252 | .00084 |
| Externalizing problems | 17.0 | 16.3 | 21.5 | .72253 | .05775 |
| Total problems | 18.0 | 18.2 | 24.8 | .93615 | .00552 |
The large proportion of children scoring in the at-risk or clinically significant range on the pervasive developmental problem (PDP) scale (15% in the APOE cohort, compared with 9% for the normative data; P<.00001) is particularly concerning. The PDP scale was developed to incorporate some of the behavioral symptoms the DSM-IV lists as criteria for the diagnosis of an autism spectrum disorder (autism, Asperger syndrome, or pervasive developmental disorder not otherwise specified).12 Scores in the at-risk or clinically significant range on the PDP scale are indicative of problems in the area of reciprocal social interactions and restricted behaviors (eg, repetitive behavior or disturbed by change). High scores on the PDP scale do not confirm the diagnosis of an autism spectrum disorder but suggest that further evaluation is warranted. The prevalence of concerning scores was also increased for the somatic complaints, withdrawn, sleep problems, and affective problems subscales.
Of note, 35% of parents demonstrated a pattern of defensive responding on the Parenting Stress Index-Short Form. When only nondefensive parent reporters were considered, the prevalence of at-risk or clinically significant scores increased (Table 3). In the defensive reporter subgroup, the prevalence of at-risk or clinically significant scores was 21% for PDPs, 30% for internalizing problems, and 25% for total problems (all significantly increased in comparison with the normative data; all P<.006). This suggests that parental report instruments may underrepresent behavioral symptoms for some children. Parents were asked whether they had ever been told that their child had an autism spectrum disorder. Affirmative answers were reported by 9 of 381 parents. These findings suggest that the prevalence of an autism spectrum disorder in the cohort is possibly >1 in 50, a threefold increase in comparison with the national average of 1 in 150 reported by the Autism and Developmental Disabilities Monitoring Network.13
Social skills and problem behaviors were assessed with the PKBSs, which were completed by 377 parents. At least moderate impairment of social skills (including social cooperation, interaction, and independence) was reported by 10% of parents (Table 4). At least moderate incidence of problem behaviors (including attention/overactive problems, social withdrawal, self-centered, and social withdrawal) was reported by 15% to 18% of parents. The prevalence of social impairment and problem behaviors increased when only nondefensive parent reporters were considered. In this subgroup (n = 244), almost 1 of 4 parents reported at least moderate incidence of problem behaviors.
TABLE 4.
PKBS Impairment
| n (%) | ||||
|---|---|---|---|---|
| Entire Cohort (N = 377) |
Defensive Reporters (N = 244) |
|||
| Moderate Impairment |
Severe Impairment |
Moderate Impairment |
Severe Impairment |
|
| Social skills | 36 (9.5) | 10 (2.7) | 29 (11.9) | 8 (3.3) |
| Externalizing problems | 58 (15.4) | 18 (4.8) | 48 (19.7) | 14 (5.7) |
| Internalizing problems | 64 (17.0) | 21 (5.6) | 58 (23.8) | 20 (8.2) |
| Total problem behavior |
66 (17.5) | 16 (4.2) | 54 (22.1) | 15 (6.1) |
Coexisting cognitive and language impairments were associated with behavior problems. In this cohort, cognitive function (full-scale IQ) and language skills (PLS-4 TLS) correlated with the incidence and severity of problem behaviors and social skills. The correlations were weak to moderate (r = 0.2–0.5) but significant. Higher full-scale IQ and PLS-4 TLS values correlated with lower scores for behavior and attention problems, as well as higher scores for social skills.
APOE Genotype and Behavior Problems
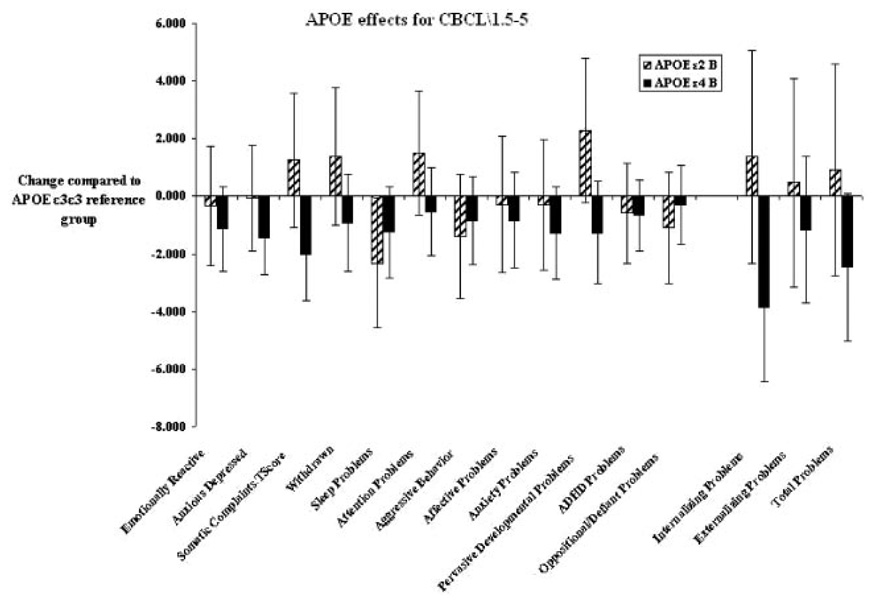
There were significant APOE effects for multiple behavior domains (Table 5). The effects of the APOE ε2 and ε4 alleles, compared with the reference ε3 group, on CBCL/1.5–5 scores are shown in Fig 1. Higher scores on the CBCL/1.5–5 scales indicate worse outcomes. In Fig 1, a positive value indicates a worse outcome in comparison with the reference group. After adjustment for demographic and operative covariates, the ε2 allele was significantly associated with worse problems (higher scores) in multiple indices, including somatic complaints (P = .009), PDPs (P = .032), and internalizing problems (P = .009). In each of these cases, the ε4 allele was associated with a better outcome in comparison with the ε3 reference group (Table 5). APOE ε2 carriers had impaired social skills, with lower PKBS social skill scores, compared with ε4 carriers (P = .0009). A significant improvement in executive function (NEPSY statue scores) was observed for ε4 carriers, compared with the ε3 reference group (P = .002); however, ε2 allele carriers did not show a decrease in performance, compared with the reference group, in this test. In addition, head circumference was smaller (P = .036) in the APOE ε2 group, after adjustment for covariates (including gender, ethnicity, birth weight, and birth head circumference), which suggests impaired postnatal brain growth.
TABLE 5.
APOE Genotype Effects
| Outcome | P | ε2 | ε4 | ||
|---|---|---|---|---|---|
| B | SE | B | SE | ||
| Head circumference at 4 y | .0361 | −0.7129 | 0.2815 | −0.0092 | 0.1989 |
| Full-scale IQ | .4686 | 0.3198 | 2.7477 | 2.3476 | 1.9213 |
| PLS-4 TLS | .2201 | −2.3985 | 2.6563 | 2.3367 | 1.8785 |
| Attention/impulsivity (ADHD Rating Scale-IV) | |||||
| Inattentive | .2998 | 0.9084 | 0.8851 | −0.5746 | 0.6269 |
| Hyperactivity/impulsivity | .1835 | −1.0109 | 0.9095 | −1.0852 | 0.6452 |
| Behavior (CBCL/1.5–5) | |||||
| Emotionally reactive | .3778 | −0.2497 | 1.0552 | −1.0872 | 0.7481 |
| Anxious/depressed | .0703 | 0.0891 | 0.9281 | −1.4666 | 0.6573 |
| Somatic complaints | .0091 | 1.5501 | 1.1750 | −2.0052 | 0.8293 |
| Withdrawn | .1813 | 1.6568 | 1.2038 | −0.7818 | 0.8518 |
| Sleep problems | .0698 | −2.1188 | 1.1512 | −1.2346 | 0.8144 |
| Attention problems | .1980 | 1.5528 | 1.0980 | −0.6092 | 0.7773 |
| Aggressive behavior | .2354 | −1.3357 | 1.1006 | −0.9227 | 0.7771 |
| Affective problems | .4650 | 0.0265 | 1.1821 | −1.0081 | 0.8387 |
| Anxiety problems | .2014 | −0.1839 | 1.1435 | −1.5321 | 0.8098 |
| PDPs | .0324 | 2.4156 | 1.2733 | −1.2316 | 0.8981 |
| ADHD problems | .4736 | −0.5374 | 0.8807 | −0.7287 | 0.6235 |
| Oppositional-defiant problems | .3664 | −1.1151 | 0.9909 | −0.3706 | 0.6974 |
| Internalizing problems | .0093 | 1.4324 | 1.8801 | −3.9978 | 1.3234 |
| Externalizing problems | .5494 | 0.5396 | 1.8454 | −1.2605 | 1.3051 |
| Total problems | .0807 | 1.0139 | 1.8624 | −2.6556 | 1.3149 |
| Social skills (PKBSs) | |||||
| Social skills total score | .0087 | −4.3912 | 2.1242 | 2.7860 | 1.5174 |
| Externalizing problems proportion | .2238 | 2.0399 | 4.2638 | −4.5846 | 3.0235 |
| Internalizing problems proportion | .1621 | 1.9621 | 4.7187 | −4.7305 | 3.3973 |
| Problem behavior total score | .2392 | 0.8414 | 2.2370 | −2.4271 | 1.5829 |
| Attention/executive function (NEPSY) | |||||
| Visual attention | .9603 | −0.0783 | 0.4501 | 0.0568 | 0.3169 |
| Statue | .0016 | 0.6175 | 0.6254 | 1.5700 | 0.4345 |
FIGURE 1.
APOE genotype effects, after correction for covariates, on the CBCL/1.5–5 narrow-band problem scores, DSM-IV–oriented scores, broad-band indices (internalizing and externalizing problems), and total problem score. Higher scores indicate worse performance. The APOE ε2 and ε4 groups were compared with the reference ε3ε3 group.
DISCUSSION
In this prospective observational study, we demonstrate that parent-reported behavioral symptoms, including inattention, impaired social interactions, and restricted behavior patterns, are increased in preschool-aged children after cardiac surgery in infancy. Deficits in language development and executive function are common. In general, the frequency of abnormal findings is two- to fourfold higher than that seen in the general population. Our findings also suggest that a pattern of defensive reporting is common for the parents of some children, leading to an incomplete picture of behavioral symptoms.
The risk of behavior problems is modified by APOE genotype. After correction for covariates, the APOE ε2 allele is associated with impaired executive function, impaired social interactions, and restricted behavior patterns. The APOE ε2 allele also is associated with a smaller head circumference, which suggests that APOE genotype may modify brain growth and development. Conversely, the APOE ε4 allele is associated with more-favorable outcomes.
Multiple studies in the past 10 to 15 years have drawn attention to behavioral symptoms in children after cardiac surgery.14–25 The most commonly used instrument has been the CBCL.7,13 In most studies, parents of preadolescent children with CHDs reported worse scores for many domains (withdrawn, social problems, attention problems, internalizing problems, externalizing problems, and total problems), compared with control subjects. 14–25 In a recent study, >30% of children with CHDs scored within the clinically significant range for attention problems.18 Children with CHDs scored lower for social involvement, school performance, and total competence than did control subjects.21 The Boston Circulatory Arrest Study examined cognitive and behavioral outcomes after repair of transposition of the great arteries.22,23,26–28 At the 8-year follow-up evaluation, parents and teachers identified many areas of psychosocial vulnerability. According to parental reports, 1 of 4 children was functioning in the borderline/clinical range for an index integrating activities, social relationships, and school performance. Both parents and teachers identified attention and executive function as challenges for the children. The children tended to become “lost in details,” unable to complete a task and fit pieces together into a cohesive whole, such as assembling story elements into a narrative. Lower-level skills were largely intact, but children had difficulty integrating or coordinating the skills to accomplish higher-order goals, such as producing connected discourse or applying math concepts to solve problems.
Our findings suggest that individuals’ responses to surgery for treatment of CHDs are related to their APOE genotype. Apolipoprotein E-containing lipoproteins are the primary lipid-transport vehicles in the CNS, with an important role in mobilization and redistribution of cholesterol and phospholipids during remodeling of neuronal membranes.29,30 The most common allele in humans is ε3, with 2 other common alleles (ε2 and ε4) whose protein products differ from that of ε3 by single amino acid substitutions. APOE genotype is an important modulator of the response to traumatic brain injury and neurodevelopment. 29–31 The APOE ε4 allele is associated with increased risks of Alzheimer disease and worse prognosis after traumatic brain injury in adults. However, the effects of APOE genotype on recovery are not the same for the immature developing brain and the aging brain. Better functional recovery after traumatic brain injury was reported for children with the ε4 allele, compared with children without that allele. 32 In a study of lead exposure, the ε4 allele was associated with a 4.4-point higher score on the Mental Developmental Index of the Bayley Scales of Infant Development-II. APOE genotype modified the adverse effects of lead exposure. 33 Negative effects of lead exposure on the Mental Developmental Index were fourfold greater for ε2 and ε3 carriers, compared with ε4 carriers. Chronic diarrhea with malnutrition is associated with deficits in cognition and executive function. In children who suffered multiple diarrheal episodes early in life, the ε4 allele was associated with better visual working memory and semantic fluency.34 APOE ε2 carriers had a 12-fold increased risk of cerebral palsy and ε4 carriers a five-fold increase.35
This study confirms the findings of previous studies and demonstrates that infant cardiac surgery is associated with distinctive neurodevelopmental sequelae characterized by mild cognitive impairment, impaired executive function, inattention, impulsive behavior, and impaired core language skills, as well as decreased social skills. These neurobehavioral deficits may be associated with significant functional morbidity as children grow older, including poor school performance, increased strain in interpersonal relationships, and inappropriate behavior. The study demonstrates that the APOE ε2 allele is associated with increased behavior problems at 4 years of age, which confirms our previous report that the ε2 allele is associated with worse neurodevelopmental outcomes at 1 year of age after repair of CHDs in infancy. This APOE genotype-environment interaction demonstrates that genetic polymorphisms that impair neuroresiliency and CNS recovery may explain some of the interindividual variation in developmental outcomes after surgery for treatment of CHDs.
WHAT’S KNOWN ON THIS SUBJECT
Behavior problems are frequent after cardiac surgery in infancy. No data concerning the role of APOE have been published.
WHAT THIS STUDY ADDS
This study provides evidence of a role for APOE genotype in determining the risk of behavior problems after cardiac surgery in infancy.
ACKNOWLEDGMENTS
This work was supported by a grant from the Fannie E. Rippel Foundation, an American Heart Association National Grant-in-Aid (grant 9950480N), and grant HL071834 from the National Institutes of Health.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- CBCL/1.5–5
Child Behavior Checklist for ages 1.5 to 5 years
- CHD
congenital heart defect
- DHCA
deep hypothermic circulatory arrest
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders; Fourth Edition
- NEPSY
Neuro-PSYchology
- PKBS
Preschool and Kindergarten Behavior Scale
- PLS-4
Preschool Language Scale-4
- TLS
total language score
- PDP
pervasive developmental problem
- CNS
central nervous system
APPENDIX
The CBCL/1.5–5 is a questionnaire used to obtain parental reports of behavior problems and prosocial adaptive skills demonstrated within the previous 6 months. Responses are grouped to produce 7 narrow-band problem scores and 5 DSM-IV–oriented scores. Results include a total problem score and 2 broad-band indices (internalizing and externalizing problems). Children are classified as having normal, at-risk, or clinically significant scores. The internalizing problems score is determined from the scores of the following subtests: emotionally reactive, anxious/depressed, somatic complaints, and withdrawn. The externalizing problems score is determined from the attention problems and aggressive behavior scores. The total problems score consists of the sum of the scores for the 99 specific problem items on the form plus the highest scores for any written-in responses to item 100.
The ADHD Rating Scale-IV, Preschool Version, is an 18-item questionnaire that requires parents to rate the frequency of occurrence of ADHD symptoms, as defined in the DSM-IV.9 The respondent rates each item on a Likert scale of 0 (not at all) to 3 (very often). This scale was developed specifically for children 3 to 6 years of age. Normative data were collected from a stratified sample of 907 children. Mean scores are provided for inattention and hyperactivity/impulsivity. Percentile rankings were used to identify children rated by their parents as scoring significantly high in inattention or hyperactivity.
The PKBS provide 3 scores for social skills, including social cooperation, social interaction, and social independence, and 5 scores for problem behaviors, including self-centered/explosive, attention problems/overactive, antisocial/aggressive and social withdrawal, anxiety/somatic, and total.10 Normative data were obtained from 2855 preschool-aged children (3–6 years of age). Reliability was reported to range from 0.81 to 0.97 in internal consistency and in the moderate to high range (0.58–0.87) in test-retest reliability. Predictive validity studies suggested that PKBS scores were able to predict need for special education services. Validity studies comparing the PKBS with other tests of social skills indicated strong correlations.
The Wechsler Preschool and Primary Scale of Intelligence, Third Edition, is a standardized test of intelligence for children 3.5 to 7 years of age.5 It is commonly used in both clinical settings and research settings; it takes ~45 minutes to administer and yields 3 summary scores and 12 subtest scores. Scores include full-scale IQ, verbal IQ, and performance IQ, with means of 100 and SDs of 15. The test covers a wide range of cognitive tasks. There is a large body of data explaining the meaning of test findings. The Wechsler Preschool and Primary Scale of Intelligence Revised proved to have moderate to strong reliability (coefficients for verbal IQ, performance IQ, processing speed, full-scale IQ, and general language were 0.92, 0.87, 0.93, 0.92, and 0.90, respectively) and validity (correlation with other cognitive tests in the positive and significant range of 0.74–0.90) in a variety of studies.
The PLS-4 is a general test of early language skills.36 It provides a measure of language comprehension and expressive communication. Standard scores are derived on the basis of age and performance. The TLS (mean: 100; SD: 15) is derived on the basis of performance on the receptive and expressive sections.
The NEPSY is a developmental neuropsychological assessment tool that was published in 1998.6 The NEPSY statue subtest assesses inhibition and motor persistence. The NEPSY subtests yield scale scores with means of 10 and SDs of 3. Reliability ranges from 0.50 to 0.81. Validity studies indicate that there is weak correlation between the attention/executive function subtests and tests of general intelligence. The use of the NEPSY with clinical populations of children diagnosed as having ADHD indicates that identified children score significantly more poorly on the tests of attention.
The theoretical model of the Parenting Stress Index-Short Form reflects the concept that parental distress, a difficult child, and a dysfunctional parent-child interaction combined have an impact on parenting behaviors, which then affect the child’s outcomes. Through 36 questions, which parents answer by using 5-point scales, the test yields 4 scores, that is, defensive responding, parental distress, parent-child dysfunctional interaction, and difficult child. This measure has been used frequently in research on medically ill infants and children. The defensive responding scale assesses the extent to which the respondent approaches the questionnaire with a strong bias to present the most favorable impression and to minimize indications of problems or stress in the parent-child relationship. The score is determined on the basis of a pattern of possible false-negative responses to 7 questions (for example, questions about doing new things, giving up parts of a lifestyle, and handling new things). A pattern of disagreement indicates a potential pattern of false reporting, because of a defensive personality style, dishonesty, or disengaged parents.
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Reprints Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4) doi: 10.1542/peds.2007-1066. Available at: www.pediatrics.org/cgi/content/full/121/4/e759. [DOI] [PubMed]
- 2.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126(6):1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 3.Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. Ann Thorac Surg. 1997;64(3):715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 4.Hollingshead A. Four-Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 5.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. San Antonio, TX: Psychological Corp; 2002. [Google Scholar]
- 6.Korkman M, Kirk U, Kemp S. NEPSY. 2nd ed. San Antonio, TX: Psychological Corp/Harcourt Assessment; 2007. [Google Scholar]
- 7.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 8.McGoey KE, DuPaul GJ, Haley E, Shelton TL. Parent and teacher ratings of attention-deficit/hyperactivity disorder in preschool: the ADHD Rating Scale-IV, Preschool Version. J Psychopathol Behav Assess. 2007;29(4):269–276. [Google Scholar]
- 9.Merrell K. Preschool and Kindergarten Behavior Scales. Austin, TX: Psychological Corp; 1994. [Google Scholar]
- 10.Uzark K, Jones K. Parenting stress and children with heart disease. J Pediatr Health Care. 2003;17(4):163–168. doi: 10.1067/mph.2003.22. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 12.Achenbach T, Rescorla LA. Child Behavior Checklist for Ages 1½–5. Burlington, VT: University of Vermont; 2000. [Google Scholar]
- 13.Center for Disease Control and Prevention. Autism and developmental disabilities monitoring network. [Accessed May 18, 2009]; Available at: www.cdc.gov/ncbddd/autism/
- 14.Oates RK, Turnbull JA, Simpson JM, Cartmill TB. Parent and teacher perceptions of child behaviour following cardiac surgery. Acta Paediatr. 1994;83(12):1303–1307. doi: 10.1111/j.1651-2227.1994.tb13021.x. [DOI] [PubMed] [Google Scholar]
- 15.Casey FA, Sykes DH, Craig BG, Power R, Mulholland HC. Behavioral adjustment of children with surgically palliated complex congenital heart disease. J Pediatr Psychol. 1996;21(3):335–352. doi: 10.1093/jpepsy/21.3.335. [DOI] [PubMed] [Google Scholar]
- 16.McCusker CG, Doherty NN, Molloy B, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child. 2007;92(2):137–141. doi: 10.1136/adc.2005.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Behavior and self-perception in children with a surgically corrected congenital heart disease. J Dev Behav Pediatr. 2007;28(4):294–301. doi: 10.1097/DBP.0b013e3180cabc3c. [DOI] [PubMed] [Google Scholar]
- 18.Miatton M, De Wolf D, Francois K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. 2007;151(1):73–78. doi: 10.1016/j.jpeds.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Hövels-Gürich HH, Konrad K, Skorzenski D, Herpertz-Dahlmann B, Messmer BJ, Seghaye M-C. Attentional dysfunction in children after corrective cardiac surgery in infancy. Ann Thorac Surg. 2007;83(4):1425–1430. doi: 10.1016/j.athoracsur.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 20.Hövels-Gürich HH, Konrad K, Skorzenski D, et al. Long-term behavior and quality of life after corrective cardiac surgery in infancy for tetralogy of Fallot or ventricular septal defect. Pediatr Cardiol. 2007;28(5):346–354. doi: 10.1007/s00246-006-0123-z. [DOI] [PubMed] [Google Scholar]
- 21.Hövels-Gürich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87(6):506–510. doi: 10.1136/adc.87.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332(9):549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 23.Bellinger DC, Wypij D, Kuban KC, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 24.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114(5) doi: 10.1542/peds.2003-0983-L. Available at: www.pediatrics.org/cgi/content/full/114/5/e572. [DOI] [PubMed]
- 25.Visconti KJ, Saudino KJ, Rappaport LA, Newburger JW, Bellinger DC. Influence of parental stress and social support on the behavioral adjustment of children with transposition of the great arteries. J Dev Behav Pediatr. 2002;23(5):314–321. doi: 10.1097/00004703-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Visconti KJ, Bichell DP, Jonas RA, Newburger JW, Bellinger DC. Developmental outcome after surgical versus interventional closure of secundum atrial septal defect in children. Circulation. 1999;100(19 suppl):II145–II150. doi: 10.1161/01.cir.100.suppl_2.ii-145. [DOI] [PubMed] [Google Scholar]
- 27.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329(15):1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 28.Bellinger DC, Wypij D, duPlesis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 29.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18(5):465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Roses AD. Apolipoprotein E, a gene with complex biological interactions in the aging brain. Neurobiol Dis. 1997;4(3–4):170–185. doi: 10.1006/nbdi.1997.0161. [DOI] [PubMed] [Google Scholar]
- 31.Ariza M, Pueyo R, Matarin MdM, et al. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77(10):1191–1193. doi: 10.1136/jnnp.2005.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackman JA, Worley G, Strittmatter WJ. Apolipoprotein E and brain injury: implications for children. Dev Med Child Neurol. 2005;47(1):64–70. doi: 10.1017/s0012162205000113. [DOI] [PubMed] [Google Scholar]
- 33.Wright RO, Hu H, Silverman EK, et al. Apolipoprotein E genotype predicts 24-month Bayley Scales of Infant Development score. Pediatr Res. 2003;54(6):819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 34.Oriá RB, Patrick PD, Blackman JA, Lima AAM, Guerrant RL. Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses. 2007;68(5):1099–1107. doi: 10.1016/j.mehy.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda MM, Weck ME, Sarwark JF, Hamidullah A, Wainwright MS. Association of apolipoprotein E genotype and cerebral palsy in children. Pediatrics. 2007;119(2):306–313. doi: 10.1542/peds.2006-1083. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale-4. San Antonio, TX: Harcourt Assessment; 2002. [Google Scholar]