Abstract
Context
Generalized anxiety disorder (GAD) is one of the most common psychiatric disorders in older adults; however, few data exist to guide clinicians in efficacious and safe treatment. Selective serotonin reuptake inhibitors (SSRIs) are efficacious for younger adults with GAD, but benefits and risks may be different in older adults.
Objective
To examine the efficacy, safety, and tolerability of the SSRI escitalopram in older adults with GAD.
Design, Setting, and Participants
A randomized controlled trial in primary care practices and related specialty clinics in Pittsburgh, Pennsylvania, of 177 participants aged 60 years or older with a principal diagnosis of GAD randomized to receive either escitalopram or placebo and conducted between January 2005 and January 2008.
Interventions
Twelve weeks of 10 to 20 mg/d of escitalopram (n=85) or matching placebo (n=92).
Main Outcome Measures
Cumulative response defined by Clinical Global Impressions-Improvement score of much or very much improved; time to response; and anxiety and role functioning changes measured by the Clinical Global Impressions-Improvement scale, Hamilton Anxiety Rating Scale, Penn State Worry Questionnaire, Late-Life Function and Disability Instrument activity limitations subscale, and the role-emotional impairment and social function subscales of the Medical Outcome Survey 36-item Short Form.
Results
In the primary analytic strategy in which participants (n=33) were censored at the time of dropout, mean cumulative response rate for escitalopram was 69% (95% confidence interval [CI], 58%-80%) vs 51% (95% CI, 40%-62%) for placebo (P=.03). A conservative intention-to-treat analysis showed no difference in mean cumulative response rate between escitalopram and placebo (57%; 95% CI, 46%-67%; vs 45%; 95% CI, 35%-55%; P=.11). Participants treated with escitalopram showed greater improvement than with placebo in anxiety symptoms and role functioning (Clinical Global Impressions-Improvement scale: effect size, 0.93; 95% CI, 0.50-1.36; P<.001; Penn State Worry Questionnaire: 0.30; 95% CI, 0.23-0.48; P=.01; activity limitations: 0.32; 95% CI, 0.01-0.63; P=.04; and the role-emotional impairment and social function: 0.96; 95% CI, 0.03-1.90; P=.04). Adverse effects of escitalopram (P<.05 vs placebo) were fatigue or somnolence (35 patients [41.1%]), sleep disturbance (12 [14.1%]), and urinary symptoms (8 [9.4%]).
Conclusions
Older adults with GAD randomized to escitalopram had a higher cumulative response rate for improvement vs placebo over 12 weeks; however, response rates were not significantly different using an intention-to-treat analysis. Further study is required to assess efficacy and safety over longer treatment durations.
Trial Registration
clinicaltrials.gov Identifier: NCT00105586
Generalized anxiety disorder (GAD) is the most common anxiety disorder in primary care1,2 and is defined by chronic, difficult-to-control worry and anxiety. Related somatic and psychiatric symptoms include muscle tension, sleep disturbance, and fatigue. Individuals with GAD have poorer quality of life,3 with impairments in role functioning (encompassing social, occupational, and family functioning) on par with those observed in major depressive disorder and other common medical problems such as arthritis and diabetes.4,5
The prevalence of GAD is as high as 7.3% in community-dwelling older adults6 and higher in primary care7 where they are most likely to present for treatment.8 In older adults, GAD is associated with poorer quality of life,9-11 increased health care utilization,11 and cognitive impairment.12,13 Additionally, chronic anxiety disorders are associated with increased risk and severity of medical illnesses such as cardiovascular disease, asthma, and cancer.14,15 Thus, by virtue of demographic shift and absent effective management, GAD in older adults will become an increasing human and economic burden.
Effective treatment options for GAD include selective serotonin reuptake inhibitors (SSRIs), benzodiazepines, buspirone, venlafaxine, duloxetine, and psychotherapy.16 Older adults have typically been excluded from GAD treatment research; however, only benzodiazepines have received study in a large-scale, prospective controlled trial in this age group.17 Although benzodiazepines are widely used for anxiety in older adults,18 the adverse effects of these medications in this age group include falls,19 cognitive impairment,20 and motor vehicle crashes,21 and their long-term use for GAD is discouraged.22
SSRIs are an alternative for treating GAD, but few data exist regarding their efficacy, tolerability, and safety in anxious older adults. Our group previously conducted a small, 8-week, placebo-controlled trial of citalopram.23 This study and another study24 preliminarily demonstrated the efficacy of SSRIs for older adults with anxiety disorders. We examined the efficacy, safety, and tolerability of the SSRI escitalopram for the acute treatment of GAD in older adults.
METHODS
Patient Population
Participants were aged 60 years or older with a principal diagnosis of GAD according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria,25 with clinically significant anxiety symptoms defined as a score of 17 or more in the Hamilton Anxiety Rating Scale.26 Participants were mainly recruited from primary care and related specialty medical care (eg, arthritis, geriatric medicine) clinics and practices in Pittsburgh, Pennsylvania (50% of participants) using screening and referrals generated by automated prompts.27 Other recruitment sources were specialty mental health practices (10% of participants) and self-referral from advertisements (40% of participants). Patients with comorbid unipolar depression or anxiety disorders were included if GAD was the principal diagnosis (based on severity and duration), as were patients with a past history of alcohol or substance abuse that was in full remission for at least 3 months.
Exclusion criteria were lifetime psychosis or bipolar disorder, dementia, increased suicide risk (eg, current ideation), medical instability according to review of medical chart data; ongoing psychotherapy; and current antidepressant or anxiolytic use, with the exception of benzodiazepines up to 2 mg/d equivalent of lorazepam. This was allowed because many anxious older adults take benzodiazepines18 and it would not be common practice to taper them before starting an SSRI.
Randomization and Study Drug Administration
The University of Pittsburgh institutional review board approved the study. Potential participants were assessed initially by telephone with a screening measure for GAD in older adults. If the participants screened positive and were receptive to study participation, they were assessed in person, after providing written informed consent, with a diagnostic interview (structured clinical interview for DSM-IV Axis I disorders),28 interview ratings of anxiety and depressive symptom severity (Hamilton Anxiety and Depression29 Rating Scales), and a screening test of cognition (Mini-Mental State Examination [MMSE]).30 Medical evaluation included interview for medical conditions and medications, review of medical records, discussion with participants' primary care physicians when indicated, and vital signs, with laboratory tests if needed. Using these data, the Cumulative Illness Rating Scale for Geriatrics31 quantified medical burden. Race and ethnicity were classified by participants using the National Institutes of Health ethnic origin and race form, for the purpose of characterizing the sample and examining ethnicity as a possible moderator of treatment efficacy.
Participants who met eligibility criteria were randomized to escitalopram or placebo using a permuted-block, 1:1 randomized list generated by a study statistician. The research pharmacy performed the randomization and assigned individuals in the order of their enrollment; otherwise, all study personnel, investigators, and participants were blinded to treatment assignment until completion of the entire study. Study medication consisted of identically appearing pills containing either 10 mg of escitalopram or placebo. Patients took 1 pill daily. For patients who did not achieve response after 4 weeks, the dosage was increased to 2 pills daily, as tolerated. Participants took study medication for 12 weeks or until they dropped out; 1 participant was removed from the study due to medical concerns (bacterial endocarditis).
Data Collection and Outcome Measures
Participants were recruited between January 2005 and October 2007, with the last participants completing the study in January 2008 (after 12 weeks). Participants were assessed weekly for the first 4 weeks and then every other week by trained, bachelor's degree–educated raters.
The main outcome assessment was the Clinical Global Impressions-Improvement scale32 for which raters synthesized anxiety rating scale scores, participant self-reports, and the rater's determination of degree of improvement. Other measures at each assessment point were the Hamilton Anxiety Rating Scale, adverse effects assessed by patient responses to an open-ended, nonspecific question, seated pulse and blood pressure measurements using a digital blood pressure monitor, and weight. At baseline and weeks 4, 8, and 12, participants were assessed using the self-report Penn State Worry Questionnaire.33 At baseline and week 12, participants were assessed using the Late-Life Function and Disability Instrument and Medical Outcome Survey 36-item Short Form to measure self-reported function and quality of life.34,35
Interrater reliability was established with training at the study onset, maintained with retraining throughout the study, and tested yearly (intraclass correlation coefficient for Clinical Global Impressions-Improvement scale, 0.89; and for Hamilton Anxiety Rating Scale, 0.93).
Statistical Analysis
Analyses were conducted by using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina) and Stata version 9 (StataCorp LP, College Station, Texas). We hypothesized that escitalopram would be better than placebo in achieving response, improving anxiety symptoms, and reducing anxiety-related impairments in function and quality of life.
The primary outcome was response, defined as Clinical Global Impressions-Improvement scale of 1 (very much improved) or 2 (much improved).32 The primary analytic strategy was cumulative incidence of response and the secondary strategy was time to response, using Kaplan-Meier survival analysis and the Greenwood formula for estimates of standard error. This strategy censored participants at the time of dropout. We also calculated intention-to-treat (ITT) rates of cumulative incidence of response in which participants who dropped out were considered to be nonresponders. We performed a standard ITT analysis including all randomized participants (except for 2 participants who did not receive any study medication) and a modified ITT analysis including only participants who provided at least 1 follow-up data point.36
The sample size was calculated to have 80% power to detect a difference of 25% in incident response, based on recruitment feasibility. Additionally, potential covariates were examined in a Cox proportional hazards regression model of time to response. We included referral source and clinical and demographic covariates that had the potential to affect medication efficacy, based on studies in the late-life mood disorder literature.37 After checking the proportional hazards assumption, Cox proportional hazards regression model was also fitted in the data set to study the potential covariates. The analysis was adjusted for age, sex, referral source, age of GAD onset, MMSE score, comorbid major depressive disorder, comorbid benzodiazepine use, and Cumulative Illness Rating Scale-Geriatrics score. We used sex and referral source as categorical data and age, age of GAD onset, and MMSE as continuous data.
The secondary analytical strategy was an evaluation of clinical changes using a mixed-effect repeated measures approach and examining effect sizes (Cohen d where 0.2=small, 0.5=medium, and 0.8=large).38 Outcomes were changes in symptoms of anxiety (Clinical Global Impressions-Improvement scale, Hamilton Anxiety Rating Scale, and Penn State Worry Questionnaire) and role functioning. Role functioning outcomes were the activity limitations subscale of the Late-Life Function and Disability Instrument and the role-emotional impairment and social function subscales of the 36-item Short Form. We chose these specific domains because they are most negatively impacted by GAD.3,9
For safety and tolerability evaluation, we compared the escitalopram and placebo groups in terms of (1) proportions with self-reported adverse effects, (2) dropouts due to adverse effects, (3) serious adverse events, and (4) rates of bradycardia and hypotension, using Fisher exact tests. All reported P values are 2-tailed, with significance level for all tests at P≤.05.
RESULTS
Of 179 participants randomized to treatment (Figure 1), 2 withdrew consent before receiving study medication and were excluded from all analyses. Additionally, 7 withdrew consent after receiving study medication but without providing any follow-up data and were included in the ITT analysis but excluded from the modified ITT analysis. Of the 177 randomized patients who received study medication, 85 were randomized to escitalopram and 92 to placebo.
Figure 1. Flow of Patients Through the Trial.
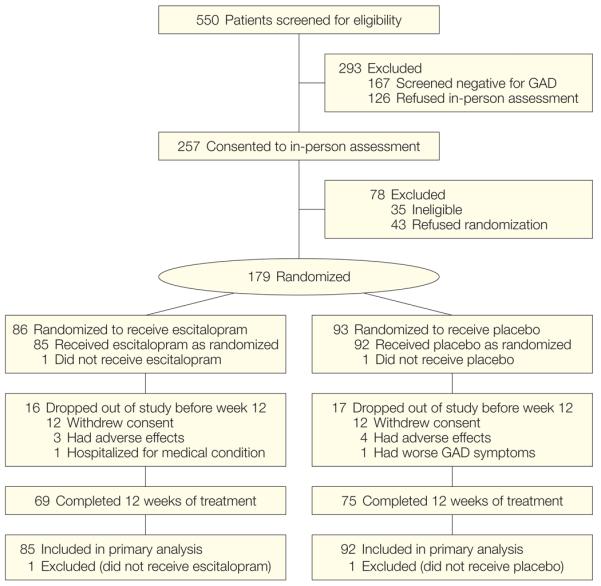
GAD indicates generalized anxiety disorder.
Randomized patients did not significantly vary from the entire eligible sample in any demographic characteristic (Table 1). Proportions of participants recruited from primary/specialty medical (n=88), mental health (n=17), or self-referral (n=72) did not differ between the escitalopram and placebo arms. Of the participants who received escitalopram, 16 (18.5%) dropped out of the study before week 12 (3 dropouts [3%] were due to adverse effects and 1 [1%] was due to hospitalization for a medical condition). Of the participants who received placebo, 17 (18.4%) dropped out before week 12 (4 dropouts [4%] were due to adverse effects and 1 [1%] was due to worsening GAD). All other dropouts were due to refusing to continue the study (ie, did not want to continue to participate in the trial).
Table 1.
Baseline Characteristics by Treatment Group
| Characteristic | Escitalopram (n = 85) |
Placebo (n = 92) |
χ2 or Kruskal-Wallis Testsa |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), y | 71.1 (7.4) | 72.2 (8.2) | 0.35 | .56 |
|
| ||||
| White, No. (%) | 68 (80.0) | 78 (84.8) | 0.41 | .49 |
|
| ||||
| Men, No. (%) | 24 (28.3) | 34 (37.0) | 1.53 | .22 |
|
| ||||
| Education, mean (SD), y | 14.0 (2.5) | 13.8 (3.3) | 0.51 | .47 |
|
| ||||
| Recruitment site, No. (%) | ||||
| Self-referral via word-of-mouth and advertisements | 36 (41.3) | 38 (43.4) | 0.03 | .92 |
|
| ||||
| Referral from mental health setting | 8 (8.7) | 6 (7.2) | ||
|
| ||||
| Screening/referral from primary care setting | 46 (50.0) | 41 (49.4) | ||
|
| ||||
| Clinical scales, mean (SD)b | ||||
| Hamilton Anxiety Rating Scale | 22.9 (4.3) | 23.1 (4.9) | 0.004 | .92 |
|
| ||||
| Hamilton Depression Rating Scale | 11.8 (3.4) | 12.3 (4.3) | 0.004 | .98 |
|
| ||||
| Cumulative Illness Rating Scale for Geriatrics (medical comorbidity score) | 9.3 (4.3) | 8.7 (3.9) | 0.74 | .39 |
|
| ||||
| Mini-Mental State Examination | 28.4 (1.6) | 27.8 (2.2) | 3.85 | .05 |
|
| ||||
| Penn State Worry Questionnaire | 55.5 (11.8) | 57.8 (13.1) | 1.49 | .22 |
|
| ||||
| Current comorbidity, No. (%) | ||||
| MDD | 10 (12.1) | 14 (15.2) | 0.97 | .62 |
|
| ||||
| Any depressive diagnosis (including MDD) | 22 (26.5) | 22 (23.9) | 0.16 | .69 |
|
| ||||
| Any anxiety disorder (other than GAD) | 19 (22.9) | 18 (19.6) | 0.29 | .59 |
|
| ||||
| Clinical history | ||||
| Age of onset of GAD, mean (SD), y | 43.3 (25.9) | 37.8 (27.8) | 1.78 | .18 |
|
| ||||
| Duration of current GAD, mean (SD), mo | 327.5 (314.6) | 404.0 (334.4) | 1.80 | .13 |
|
| ||||
| Concomitant benzodiazepine use at randomization, No. (%) | 14 (17.1) | 12 (13.2) | 1.19 | .48 |
Abbreviations: GAD, generalized anxiety disorder; MDD, major depressive disorder.
χ2 Test (for categorical variables) and Kruskal-Wallis test (for continuous variables) were used to compare all baseline variables between treatment groups.
Higher scores on the Hamilton Anxiety Rating Scale, Hamilton Depression Rating Scale, and Penn State Worry Questionnaire indicate greater symptoms.39 Published means on each scale for older adults with GAD vs anxiety-free comparisons are 19.9 vs 3.2 (range, 0-56) for Hamilton Anxiety Rating Scale; 16.6 vs 1.6 (range, 0-51) for Hamilton Depression Rating Scale; and 59.7 vs 28.2 (range, 16-80) for Penn State Worry Questionnaire.
There were no statistically significant differences between the escitalopram and placebo groups in overall dropouts, timing of dropouts, or dropouts due to adverse effects. Participants who dropped out before week 12 were significantly different from completers in terms of baseline severity on Hamilton Anxiety Rating Scale (25.1; 95% confidence interval [CI], 22.9-27.2; vs 22.6; 95% CI, 21.9-23.2; P=.003) and Hamilton Depression Rating Scale (14.0; 95% CI, 12.3-15.7; vs 11.6; 95% CI, 11.0-12.2; P=.02), co-prescription of benzodiazepine (dropout rate for benzodiazepine users, 33.3% [n=9/27]; and for nonusers, 16.0% [n=24/150]; P=.04), and race (dropout rate for white patients, 15.9% [n=23/145]; and for black patients, 31.2% [n=10/32]; P=.04); however, none of these variables differed in terms of proportions of dropouts in the escitalopram vs placebo groups.
For study completers, a patient-reported treatment guess was obtained to determine the success of blinding. Treatment guesses were obtained in 140 patients; of those randomized to escitalopram, 36 of 66 (55%) guessed correctly; of those randomized to placebo, 43 of 74 (58%) guessed correctly (Fisher exact, P=.18). These results suggest adequate success of blinding. Of patients randomized to escitalopram, 34 of 36 (94%) correct guessers were responders, while in the placebo group, 14 of 43 (33%) were responders (Fisher exact, P<.001), suggesting that correct guesses were associated with response in the active treatment group only.
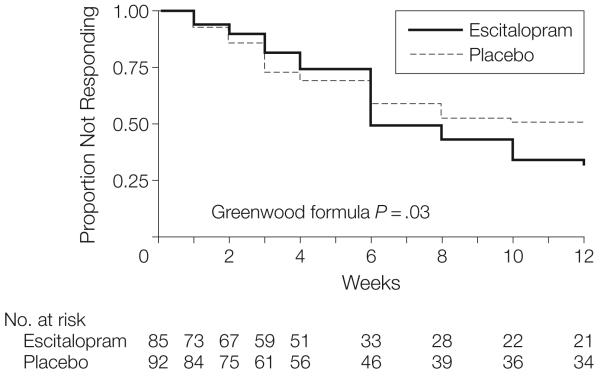
Clinical Response
Estimates of the cumulative incidence of response and time to response are shown in Figure 2. The cumulative incidence of response was higher in the escitalopram group than in the placebo group (mean response rate, 69%; 95% CI, 58%-80%; vs 51%; 95% CI, 40%-62%; P=.03). In the ITT analysis, the response was not different between groups (57%; 95% CI, 46%-67% for escitalopram; vs 45%; 95% CI, 35%-55% for placebo; P=.11); however, in a modified ITT analysis, response was higher in the escitalopram group than in the placebo group (60%; 95% CI, 50%-71%; vs 45%; 95% CI, 36%-56%; P=.048). In an exploratory analysis, 37 of 49 escitalopram responders (76%) and 30 of 42 placebo responders (71%) had at least 1 subsequent Clinical Global Impressions-Improvement scale score in the response range, suggesting that response typically persisted.
Figure 2. Kaplan-Meier Survival Curve With Escitalopram or Placebo.
Overall time to response is not different between groups. Subgroup analysis of 2 intervals (before week 4 and week 4 to 12) shows no difference between treatments initially (P=.43), a crossing over of the curves at week 6, and then subsequent superiority of escitalopram (P=.01).
In contrast with the cumulative incident response results, time to response (6 weeks median in the escitalopram group vs 10 weeks in placebo) did not significantly differ (P=.19). Examination of time-to-response showed a crossover in hazard functions (crossover of escitalopram and placebo curves between weeks 4 and 6). Therefore, as an exploratory analysis, we separately examined time-to-response analyses for the 2 intervals (baseline to week 4 and week 4 to week 12) using the Gail and Simon method.40 The initial 4-week interval showed no difference between escitalopram and placebo (P=.43), while the final 8-week interval showed a significant difference favoring escitalopram (P=.01) (Figure 2). Week 4 was the point of the titration of study medication to 2 pills, which was done in 55 of 71 patients (77%) in the escitalopram group and 66 of 83 patients (80%) in the placebo group (Fisher exact, P=.84).
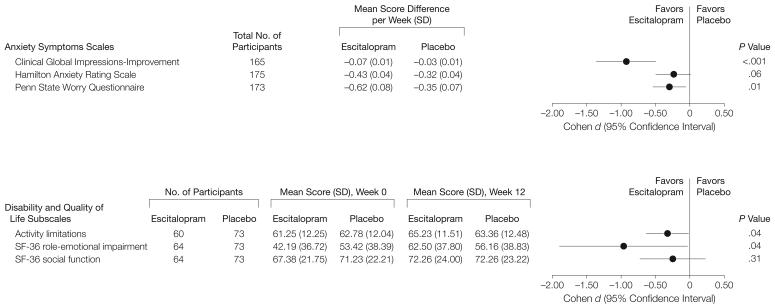
Figure 3 shows results from mixed-effects models of change in anxiety symptoms and role functioning. Escitalopram was significantly better than placebo for Clinical Global Impressions-Improvement scale, Penn State Worry Questionnaire, activity limitations subscale, and role-emotional impairment and social function subscales. Figure 3 also displays effect size (Cohen d) with 95% CIs, showing that the largest effect size was observed with Clinical Global Impressions-Improvement scale (0.93; 95% CI, 0.50-1.36; P<.001) and role-emotional impairment subscale (0.96; 95% CI, 0.03-1.90; P=.04); the rest of the scales showed effect size in the small-to-moderate range (Penn State Worry Questionnaire: 0.30; 95% CI, 0.23-0.48; P=.01; activity limitations: 0.32; 95% CI, 0.01-0.63; P=.04).
Figure 3. Improvements in Anxiety Symptoms and Disability and Quality of Life Measures.
Effect sizes are expressed as Cohen d where 0.2=small, 0.5=medium, and 0.8=large. Activity limitations is a subscale of the Late-Life Function and Disability Instrument. SF-36 role-emotional impairment and SF-36 social function are the role-emotional impairment and social function subscales of the Medical Outcome Survey 36-item Short Form.
Covariate Analyses
Baseline variables significantly associated with poorer overall time to response were presence of comorbid major depressive disorder (χ2=3.97; hazard ratio [HR], 0.58; 95% CI, 0.34-0.99; P=.046) and higher medical comorbidity scores in the Cumulative Illness Rating Scale for Geriatrics (χ2=4.16; HR, 0.94; 95% CI, 0.89-1.00; P=.04). However, a multivariate model including these variables as well as age, sex, referral source, comorbid benzodiazepine use, age of GAD onset, and MMSE score did not affect the significant association of treatment group with response (ie, no significant covariate×treatment group interactions, P values ranged from .22-.99, and no change in the significance of the treatment group main effect). Therefore, no baseline variable appeared to moderate medication efficacy.
Tolerability and Safety
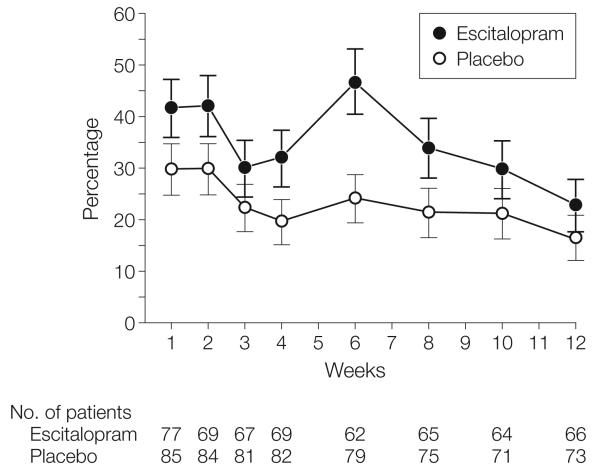
Adverse effects data are shown in Table 2. Most participants reported at least 1 adverse effect during the study. Of individual adverse effects, the most common in individuals taking escitalopram was fatigue or somnolence. Figure 4 shows the course of adverse effect endorsement over time. The only single time in which a significantly higher proportion of participants receiving escitalopram had adverse effects (P=.007) was at week 6, which was the appointment after most participants had a dosage increase from 10 to 20 mg/d.
Table 2.
Proportion of Participants Who Experienced at Least 1 Adverse Effect by Treatment Group
| No. (%) of Participants |
|||
|---|---|---|---|
| Escitalopram (n = 85) |
Placebo (n = 92) |
P Valuea |
|
| ≥1 Adverse effect | 65 (76.5) | 59 (64) | .10 |
|
| |||
| Withdrew due to adverse effects | 3 (3.5) | 4 (4.3) | .99 |
|
| |||
| Most common adverse effects | |||
| Fatigue or somnolence | 35 (41.1) | 10 (10.9) | <.001 |
|
| |||
| Gastrointestinal upset | 22 (25.9) | 26 (28.3) | .73 |
|
| |||
| Headache | 13 (15.3) | 7 (7.6) | .15 |
|
| |||
| Sleep disturbance | 12 (14.1) | 2 (2.2) | .004 |
|
| |||
| Sweating | 11 (12.9) | 5 (5.4) | .11 |
|
| |||
| Sexual | 9 (10.6) | 3 (3.3) | .07 |
|
| |||
| Urinary symptomsb | 8 (9.4) | 0 | .002 |
|
| |||
| Increased anxiety or depression | 7 (8.2) | 9 (9.8) | .80 |
|
| |||
| Light-headedness | 7 (8.2) | 7 (7.6) | .99 |
|
| |||
| Tremor | 7 (8.2) | 2 (2.2) | .09 |
|
| |||
| Aches | 5 (5.9) | 14 (15.2) | .05 |
|
| |||
| Rash or pruritus | 5 (5.9) | 6 (6.5) | .99 |
Two-tailed Fisher exact tests.
Increased or decreased frequency and urgency.
Figure 4. Rates of Overall Self-reported Adverse Effects Over Time in the Escitalopram and Placebo Groups.
Error bars indicate SE. Proportions did not significantly differ at any point except at week 6 (escitalopram vs placebo, P=.007), which was the visit after the titration to 20 mg/d.
No participants in either group had a serious or unexpected adverse event. One patient (randomized to receive escitalopram) was hospitalized for bacterial endocarditis and was removed from the study after week 3. With respect to vital sign changes, we found a significant effect by treatment group on pulse, systolic blood pressure, and diastolic blood pressure, with a greater decrease in participants receiving escitalopram than those receiving placebo.
In an exploratory, post hoc evaluation of changes in pulse and blood pressure as a function of baseline values, the greater decrease in the escitalopram group in blood pressure was isolated to those participants with high baseline values (Table 3). Although the escitalopram group had a greater decrease in pulse, prevalence of bradycardia (pulse < 60/min) did not change over time in either the escitalopram or placebo groups (19/69 [27%] at baseline vs 22/62 [35%]; t test, P=.33; vs 14/82 [17%] at baseline vs 16/69 [23%]; t test, P=.35). Weight changes over time were not significant in the overall sample or between groups by mixed-effect model (for time: standard estimate = 0.07, F1632=0.36, P=.55; and for group × time interaction: standard estimate=−0.12, F1632=0.41, P=.52).
Table 3.
Changes in Blood Pressure and Pulse by Treatment Group and Baseline Valuesa
| Mean (95% Confidence Interval) |
||||||
|---|---|---|---|---|---|---|
| Escitalopram |
Placebo |
Analysis of Changes Between Groupsb |
||||
| Baseline | Changec | Baseline | Changec | F(Time × Group) |
P Value |
|
| Systolic blood pressure, mm Hg | ||||||
| Entire group (n = 158) | 140.4 (134.4 to 146.4) | −3.1 (−9.3 to 3.1) | 136.2 (132.0 to 140.3) | −0.7 (−4.6 to 3.3) | 4.7 (1669) | .03 |
|
| ||||||
| Top quartile (n = 41) | 173.7 (163.5 to 184.0) | −23.3 (−39.4 to −7.1) | 159.9 (155.4 to 164.4) | −12.4 (−19.4 to −5.4) | 10.2 (1152) | .002 |
|
| ||||||
| Bottom 3 quartiles (n = 117) | 128.9 (124.8 to 133.0) | 3.4 (−2.4 to 9.2) | 127.7 (124.3 to 131.2) | 3.3 (−0.6 to 7.3) | 0.07 (1516) | .79 |
|
| ||||||
| Diastolic blood pressure, mm Hg | ||||||
| Entire group (n = 158) | 78.2 (75.0 to 81.5) | −3.9 (−7.6 to −0.2) | 74.6 (72.2 to 77.0) | −1.0 (−3.4 to 1.3) | 8.9 (1559) | .003 |
|
| ||||||
| Top quartile (n = 42) | 94.9 (89.5 to 100.2) | −12.7 (−22.6 to −2.8) | 88.5 (85.9 to 91.0) | −7.5 (−12.9 to −2.2) | 6.9 (1156) | .01 |
|
| ||||||
| Bottom 3 quartiles (n = 116) | 71.7 (69.5 to 73.8) | −1.0 (−4.1 to 2.1) | 69.9 (67.9 to 72.0) | 1.2 (−0.9 to 3.2) | 2.7 (1512) | .10 |
|
| ||||||
| Pulse, beats/min | ||||||
| Entire group (n = 151) | 70.7 (66.7 to 74.1) | −5.9 (−9.0 to −2.6) | 68.3 (66.2 to 70.4) | 0.51 (−1.8 to 2.8) | 10.4 (1653) | .001 |
|
| ||||||
| Top quartile (n = 40) | 88.0 (80.7 to 96.0) | −10.0 (−18.3 to −1.7) | 82.0 (79.2 to 84.8) | −6.4 (−11.7 to −1.1) | 5.5 (1153) | .02 |
|
| ||||||
| Bottom 3 quartiles (n = 111) | 62.6 (60.5 to 64.8) | −3.9 (−6.4 to −1.4) | 64.5 (62.8 to 66.1) | 2.5 (0.3 to 4.8) | 5.6 (1499) | .02 |
For systolic blood pressure, a range of 151 to 220 mm Hg indicated top quartile group and 94 to 150 mm Hg indicated the bottom 3 quartiles group; for diastolic blood pressure, 82 to 130 mm Hg and 47 to 81 mm Hg, respectively; and for pulse, 75 to 156 beats/min and 48 to 74 beats/min, respectively.
Calculated from group × time interaction from mixed-effects models.
Modeled data from the mixed-effects models.
COMMENT
In this clinical trial of SSRI treatment for GAD in older adults, we found that escitalopram was better than placebo in terms of cumulative response and improvements in anxiety symptoms and self-reported role functioning. Treatment with escitalopram was associated with a higher rate of several adverse effects, particularly fatigue or somnolence, vs placebo. In spite of this, adverse effects tended to be benign, not leading to serious events and rarely leading to dropout. Moreover, safety appeared favorable with respect to vital signs data.
The efficacy of escitalopram in this study is consistent with smaller prospective studies of SSRIs in late-life anxiety disorders,23,24 as well as retrospective pooled analyses of adults aged 60 years or older participating in phase 3 industry studies of venlafaxine extended-release or duloxetine for GAD.41,42 The lack of efficacy of escitalopram in the ITT analysis is consistent with its overall modest efficacy, diminished further by nonadherence. Given that patients with anxiety disorders are often poorly adherent to pharmacotherapy, these negative results may more accurately portray the results of treatment in clinical settings.
In a subsequent post hoc analysis, escitalopram separated from placebo only after week 4 of treatment. This finding is consistent with research showing that comorbid anxiety predicts slower antidepressant response in late-life depression43,44; another potential explanation is that escitalopram required a dose titration to 20 mg/d to be efficacious. With respect to subgroup comparisons, we found no baseline characteristic that altered the efficacy finding, including medical or psychiatric comorbidity or source of recruitment (ie, primary care setting vs other source). This suggests that the efficacy findings in this study are generalizable to late-life GAD cases typically observed in primary care and other medical settings.
Analyses of symptomatic change using mixed-effects models found escitalopram to be better than placebo. Additionally, because GAD is associated with impairments in role function similar to major depressive disorder or other common chronic medical conditions such as diabetes and arthritis,3 we examined treatment-attributable changes in role functioning. The activity limitations and role-emotional impairment subscales, which measure impairments in role function, indicated that escitalopram was better than placebo. These results substantiate the benefits of treating GAD for improving role impairments in this age group, as has been shown in young adults.45 However, the effect size for most outcomes was in the low to moderate range and is consistent with SSRI effect size for GAD in young adults.46 Because of this modest effect size, many older adults will fail to achieve adequate resolution of GAD-related symptoms and role impairments with SSRI monotherapy, so there is great need for augmentation or switch strategies in this condition and also for efficacious nonpharmacologic treatments.
Escitalopram was well-tolerated with a rate of dropouts from adverse effects (3%) comparable with that of younger adults.47 Fatigue or somnolence was the most common adverse effect, which is notable because this adverse effect is not mentioned in published placebo-controlled evaluations of escitalopram in young adults with GAD46 or older adults with major depressive disorder.48 Thus, health care professionals need to inform their older patients with GAD of this and other potential adverse effects during acute treatment. Another clinically salient point is that overall adverse effects increased at week 6, coinciding with the increase in escitalopram to 20 mg/d. Thus, health care professionals need to make their patients aware of the increased likelihood of adverse effects with dose increases.
With respect to safety, no patient had a medication-related serious adverse event. Although some studies have raised concerns about hypotension and bradycardia with antidepressants, particularly in older adults and cardiac high-risk groups,49,50 we did not find evidence of either problem. Instead, individuals with hypertension at baseline had a decrease in blood pressure, while those with low to normal blood pressure at baseline had no overall change. This finding might represent a benefit of treating anxiety in older persons, but this examination was not the original intent of our study and these findings should be considered exploratory and hypothesis-generating only. Given the strong association between anxiety disorders and cardiovascular disease,15 further studies should examine whether improved blood pressure control is a benefit of treatment for anxiety disorders in older adults and by what mechanism. Possibilities may include a direct effect of reduced anxiety vs an indirect effect via patients becoming more adherent at managing their blood pressure.
The primary limitation of our study was its relatively brief duration. Generalized anxiety disorder is a chronic disorder and longer-term evaluations are needed to determine the benefits and risks of treatment, including whether response once obtained acutely is sustained over the long term. With respect to treatment-attributable improvements in anxiety symptoms and role functioning, we examined several outcome variables. Although this clarifies some of the domains in which treatment effects can be expected, it also increases the risk of type I error. Additionally, we cannot make an unqualified claim of escitalopram's safety. We examined safety over 12 weeks, a relatively brief duration, using self-reports and vital signs but not laboratory test results. SSRIs may cause acute hyponatremia51 and pharmacoepidemiological studies have associated their use in older patients with an increased risk of fractures, upper gastrointestinal tract bleeding, and completed suicide in the first month of treatment.52-54 Further research is needed to clarify the risks of these medications in this age group.
Our study is one of the few placebo-controlled acute treatment studies of an SSRI in this age group. Because pharmacokinetic and pharmacodynamic changes with aging may alter the risks and benefits of psychotropic medications,55 research performed in healthy young adults does not necessarily apply to older adults. Our data are further strengthened by a sample that is representative of typical older adults with GAD, in terms of comorbidities and sources of recruitment.
In conclusion, older adults with GAD randomized to escitalopram had a higher cumulative response rate for improvement compared with placebo over 12 weeks. Response rate was not higher with escitalopram in an ITT analysis, suggesting that the medication's modest efficacy is further diminished by nonadherence. It is important for clinicians to emphasize to their anxious older patients the need for an adequate trial in which to observe any benefits, as well as the expectation and nature of adverse effects. Given the high human and economic burden of GAD, these data should provide impetus to detect and treat this common disorder. Further study is required to assess efficacy and safety over longer treatment durations.
Acknowledgments
Funding/Support: This work was supported by grants R01 MH070547 from the National Institutes of Health, P30 MH068579 from the Center for Mental Health Services Research (Enola Proctor, PhD, principal investigator), and P30 MH71944 from the Advanced Center for Interventions and Services Research in Late-Life Mood Disorders (Dr Reynolds, principal investigator); the John A. Hartford Center of Excellence in Geriatric Psychiatry (Dr Reynolds, principal investigator); and the University of Pittsburgh Medical Center endowment in geriatric psychiatry (Dr Reynolds). Forest Laboratories Inc, which holds the US patent for escitalopram, provided escitalopram and matching placebo for the study.
Role of the Sponsors: Neither the National Institutes of Health nor Forest Laboratories Inc had a role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Additional Contributions: We thank the patients for their participation. Kris Koenig, MBA (employee of University of Pittsburgh), provided management of the study and Linda Ball, PhD (employee of Department of Psychiatry, Washington University), provided critical review of the manuscript. Neither received any additional compensation outside of their paid position.
Footnotes
Financial Disclosure: Dr Lenze reported being a consultant for Fox Learning Systems; being a consultant for the Veteran's Medical Research Foundation from 2007-2008; receiving research support from Forest Laboratories (current), Pfizer, Novartis, and OrthoMcNeill Neurologics (until 2007); and receiving an honorarium from Eli Lilly for a talk in 2005. Dr Rollman reported serving on a primary care consultant panel for Wyeth in 2004. Dr Shear reported receiving investigator-initiated research support from Forest (until 2005), being a consultant for Forest (until 2007), and being on an advisory board for Pfizer (until 2006). Dr Pollock reported serving on the advisory board of Forest Laboratories and being a faculty member of the Lundbeck International Institute; currently being a consultant for Lundbeck and Wyeth; serving as a consultant for Takeda in 2007; and before 2004, participating in speakers bureaus for Forest and Sepracor pharmaceuticals. Dr Reynolds reported receiving research support (drug only) from Forest, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, Wyeth, and Eli Lilly. No other authors reported any financial disclosures.
REFERENCES
- 1.Ormel J, VonKorff M, Ustun TB, Pini S, Korten A, Oldehinkel T. Common mental disorders and disability across cultures: results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272(22):1741–1748. doi: 10.1001/jama.272.22.1741. [DOI] [PubMed] [Google Scholar]
- 2.Wittchen HU, Kessler RC, Beesdo K, Krause P, Höfler M, Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(suppl 8):24–34. [PubMed] [Google Scholar]
- 3.Hoffman DL, Dukes EM, Wittchen H-U. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25(1):72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):19–39. doi: 10.1016/s0193-953x(05)70204-5. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund PA, Dewit DJ, Ustün TB, Wang PS, Wïttchen HU. Distinguishing generalized anxiety disorder from major depression: prevalence and impairment from current pure and comorbid disorders in the US and Ontario. Int J Methods Psychiatr Res. 2002;11(3):99–111. doi: 10.1002/mpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beekman AT, Bremmer MA, van Balkom AJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study, Amsterdam. Int J Geriatr Psychiatry. 1998;13(10):717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Tolin DF, Robison JT, Gaztambide S, Blank K. Anxiety disorders in older Puerto Rican primary care patients. Am J Geriatr Psychiatry. 2005;13(2):150–156. doi: 10.1176/appi.ajgp.13.2.150. [DOI] [PubMed] [Google Scholar]
- 8.Ettner SL, Hermann RC. Provider specialty choice among Medicare beneficiaries treated for psychiatric disorders. Health Care Financ Rev. 1997;18(3): 43–59. [PMC free article] [PubMed] [Google Scholar]
- 9.Wetherell JL, Thorp SR, Patterson TL, Golshan S, Jeste DV, Gatz M. Quality of life in geriatric generalized anxiety disorder: a preliminary investigation. J Psychiatr Res. 2004;38(3):305–312. doi: 10.1016/j.jpsychires.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71(2):309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach GJ, Hopko DR, Feigon S, et al. “Minor GAD”: characteristics of subsyndromal GAD in older adults. Behav Res Ther. 2003;41(4):481–487. doi: 10.1016/s0005-7967(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 12.Mantella RC, Butters MA, Dew MA, et al. Cognitive impairment in late-life generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15(8): 673–679. doi: 10.1097/JGP.0b013e31803111f2. [DOI] [PubMed] [Google Scholar]
- 13.Caudle DD, Senior AC, Wetherell JL, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Ormel J, Demler O, Stang PE. Co-morbid mental disorders account for the role impairment of commonly occurring chronic physical disorders: results from the National Comorbidity Survey. J Occup Environ Med. 2003;45(12):1257–1266. doi: 10.1097/01.jom.0000100000.70011.bb. [DOI] [PubMed] [Google Scholar]
- 15.Byrne PP, Davidson KW, Kessler RC, et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Rynn MA, Brawman-Mintzer O. Generalized anxiety disorder: acute and chronic treatment. CNS Spectr. 2004;9(10):716–723. doi: 10.1017/s1092852900022367. [DOI] [PubMed] [Google Scholar]
- 17.Koepke HH, Gold RL, Linden ME, Lion JR, Rickels K. Multicenter controlled study of oxazepam in anxious elderly outpatients. Psychosomatics. 1982;23(6):641–645. doi: 10.1016/S0033-3182(82)73363-8. [DOI] [PubMed] [Google Scholar]
- 18.tez CI, Smith K, Vasile RG, Rende R, Edelen MO, Keller MB. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5–13. doi: 10.1097/JGP.0b013e31815aff5c. [DOI] [PubMed] [Google Scholar]
- 19.Herings RMC, Stricker BHC, deBoer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med. 1995;155(16):1801–1807. [PubMed] [Google Scholar]
- 20.Hanlon JT, Horner RD, Schmader KE, et al. Benzodiazepine use and cognitive function among community-dwelling elderly. Clin Pharmacol Ther. 1998;64(6):684–692. doi: 10.1016/S0009-9236(98)90059-5. [DOI] [PubMed] [Google Scholar]
- 21.Hemmelgarn B, Suissa S, Huang A, Boivin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278(1):27–31. [PubMed] [Google Scholar]
- 22.Allgulander C, Bandelow B, Hollander E, et al. World Council of Anxiety. WCA recommendations for the long-term treatment of generalized anxiety disorder. CNS Spectr. 2003;8((8)(suppl 1)):53–61. doi: 10.1017/s1092852900006945. [DOI] [PubMed] [Google Scholar]
- 23.Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162(1):146–150. doi: 10.1176/appi.ajp.162.1.146. [DOI] [PubMed] [Google Scholar]
- 24.Schuurmans J, Comijs H, Emmelkamp PM, et al. A randomized, controlled trial of the effectiveness of cognitive-behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry. 2006;14(3):255–263. doi: 10.1097/01.JGP.0000196629.19634.00. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 26.The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Rollman BL, Fischer GS, Zhu F, Belnap BH. Comparison of electronic physician prompts versus wait-room case-finding on clinical trial enrollment. J Gen Intern Med. 2008;23(4):447–450. doi: 10.1007/s11606-007-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version: Administration Booklet. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health, Education, and Welfare publication (ADM) 76-338 . Guy W: ECDEU Assessment Manual for Psychopharmacology. National Institute of Mental Health; Rockville, MD: 1976. Rev ed. [Google Scholar]
- 33.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 34.Jette AM, Haley SM, Coster WJ, et al. Late life function and disability instrument, I: development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57(4):M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. The Medical Outcomes Trust; Boston, MA: 1993. [Google Scholar]
- 36.Gillings D, Koch G. The application of the principle of intention-to-treat to the analysis of clinical trials. Drug Inf J. 1991;25:411–424. [Google Scholar]
- 37.Whyte EM, Dew MA, Gildengers A, et al. Time course of response to antidepressants in late-life major depression: therapeutic implications. Drugs Aging. 2004;21(8):531–554. doi: 10.2165/00002512-200421080-00004. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates Inc; Hillsdale, NJ: 1988. [Google Scholar]
- 39.Beck JG, Stanley MA, Zebb BJ. Characteristics of generalized anxiety disorder in older adults: a descriptive study. Behav Res Ther. 1996;34(3):225–234. doi: 10.1016/0005-7967(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 40.Chen YH, Liu GH. Testing for crossover of two hazard functions using Gail and Simon's method. J Biopharm Stat. 2006;16(3):313–326. doi: 10.1080/10543400600614791. [DOI] [PubMed] [Google Scholar]
- 41.Katz IR, Reynolds CF, III, Alexopoulos GS, Hackett D. Venlafaxine ER as a treatment for generalized anxiety disorder in older adults: pooled analysis of five randomized placebo-controlled clinical trials. J Am Geriatr Soc. 2002;50(1):18–25. doi: 10.1046/j.1532-5415.2002.50003.x. [DOI] [PubMed] [Google Scholar]
- 42.Davidson J, Allgulander C, Pollack MH, et al. Efficacy and tolerability of duloxetine in elderly patients with generalized anxiety disorder: a pooled analysis of four randomized, double-blind, placebo-controlled studies. Hum Psychopharmacol. 2008;23(6):519–526. doi: 10.1002/hup.949. [DOI] [PubMed] [Google Scholar]
- 43.Steffens DC, McQuoid DR. Impact of symptoms of generalized anxiety disorder on the course of late-life depression. Am J Geriatr Psychiatry. 2005;13(1):40–47. doi: 10.1176/appi.ajgp.13.1.40. [DOI] [PubMed] [Google Scholar]
- 44.Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- 45.Endicott J, Russell JM, Raskin J, et al. Duloxetine treatment for role functioning improvement in generalized anxiety disorder: three independent studies. J Clin Psychiatry. 2007;68(4):518–524. doi: 10.4088/jcp.v68n0405. [DOI] [PubMed] [Google Scholar]
- 46.Hidalgo RB, Tupler LA, Davidson JR. An effectsize analysis of pharmacologic treatments for generalized anxiety disorder. J Psychopharmacol. 2007;21(8):864–872. doi: 10.1177/0269881107076996. [DOI] [PubMed] [Google Scholar]
- 47.Goodman WK, Bose A, Wang Q. Treatment of generalized anxiety disorder with escitalopram: pooled results from double-blind, placebo-controlled trials. J Affect Disord. 2005;87(2-3):161–167. doi: 10.1016/j.jad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Bose A, Li D, Gandhi C. Escitalopram in the acute treatment of depressed patients aged 60 years or older. Am J Geriatr Psychiatry. 2008;16(1):14–20. doi: 10.1097/JGP.0b013e3181591c09. [DOI] [PubMed] [Google Scholar]
- 49.Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20(3):277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- 50.Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry. 2006;51(14):923–929. doi: 10.1177/070674370605101408. [DOI] [PubMed] [Google Scholar]
- 51.Fabian TJ, Amico JA, Kroboth PD, et al. Paroxetine-induced hyponatremia in older adults: a 12-week prospective study. Arch Intern Med. 2004;164(3): 327–332. doi: 10.1001/archinte.164.3.327. [DOI] [PubMed] [Google Scholar]
- 52.Richards JB, Papaioannou A, Adachi JD, et al. Canadian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167(2):188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 53.Abajo FJ, Garcia-Rodriguez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry. 2008;65(7):795–803. doi: 10.1001/archpsyc.65.7.795. [DOI] [PubMed] [Google Scholar]
- 54.Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reup-take inhibitors in the elderly. Am J Psychiatry. 2006;163(5):813–821. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 55.Pollock BG. Geriatric psychiatry: psychopharmacology: general principles. In: Sadock BJ, Sadock VA, editors. Kaplan & Sadock's Comprehensive Textbook of Psychiatry/VIII. Williams & Wilkins; Baltimore, MD: 2005. pp. 3716–3720. [Google Scholar]