Abstract
Background
The prevalence of perioperative stroke in infants undergoing operations for congenital heart disease has not been well described. The objectives of this study were to determine the prevalence of stroke as assessed by postoperative brain magnetic resonance imaging (MRI), characterize the neuroanatomic features of focal ischemic injury, and identify risk factors for its development.
Methods
Brain MRI was performed in 122 infants 3 to 14 days after cardiac operation with cardiopulmonary bypass, with or without deep hypothermic circulatory arrest. Preoperative, intraoperative, and postoperative data were collected. Risk factors were tested by logistic regression for univariate and multivariate associations with stroke.
Results
Stroke was identified in 12 of 122 patients (10%). Strokes were preoperative in 6 patients and possibly intraoperative or postoperative in the other 6 patients, and were clinically silent except in 1 patient who had clinical seizures. Arterial-occlusive and watershed infarcts were identified with equal distribution in both hemispheres. Multivariate analysis identified lower birth weight, preoperative intubation, lower intraoperative hematocrit, and higher blood pressure at admission to the cardiac intensive care unit postoperatively as significant factors associated with stroke. Prematurity, younger age at operation, duration of cardiopulmonary bypass, and use of deep hypothermic circulatory arrest were not significantly associated with stroke.
Conclusions
The prevalence of stroke in infants undergoing operations for congenital heart disease was 10%, half of which occurred preoperatively. Most were clinically silent and undetected without neuroimaging. Mechanisms included thromboembolism and hypoperfusion, with patient-specific, procedure-specific, and postoperative contributions to increased risk.
Advances in perioperative and operative management have markedly decreased the mortality of congenital heart disease (CHD) and facilitated anatomic repair at earlier ages. Neurodevelopmental disability, however, affects as many as 50% of infants undergoing interventions for congenital heart lesions. Early postoperative seizures, cognitive impairment, delays in speech, language, visual-motor, and visual-spatial skills, attention deficit/hyperactivity disorders, and learning disabilities have all been described [1, 2]. The mechanisms leading to these sequelae have not been fully elucidated.
The immature brain in infants with CHD is vulnerable to diffuse ischemic injury. The heart disease itself, as well as the open heart procedure used for repair or palliation, may expose these infants to global ischemia-reperfusion injury and the risk for localized injury from air embolism or thromboembolism [3, 4]. Neuropathologic studies have shown ischemic lesions and infarcts along arterial border zones, encephalomalacia, hemorrhage, and microscopic changes in gray and white matter [5]. The incidence of preoperative cranial ultrasound abnormalities in infants with CHD is as high as 59% and consists of cerebral atrophy and linear echo densities in the basal ganglia and thalamus [6]. Prospective MRI studies have identified periventricular leukomalacia (PVL) in up to 20% of neonates preoperatively, with additional injury occurring in the perioperative period [7, 8]. Postoperative hypoxemia and hypotension have been identified as risk factors for PVL [8].
The mechanisms of focal ischemic brain injury after infant cardiac procedures have not been well described. The objective of this study was to determine the prevalence and to characterize the neuroanatomic features of perioperative focal arterial ischemic injury as measured by postoperative brain magnetic resonance imaging (MRI) and to identify risk factors for its development.
Patients and Methods
Inclusion Criteria
Patients from two ongoing studies were prospectively enrolled to undergo brain MRI in the early postoperative period. Both studies were approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. The first study occurred between June 21, 2000, and November 5, 2001, and evaluated apolipoprotein E (APOE) genotype as a risk factor for neurodevelopmental dysfunction [8, 9]. The APOE study enrolled 169 of 206 eligible patients. The second study occurred between April 30 and August 4, 2004, and assessed the role of cytokine gene polymorphisms in perioperative brain injury. The cytokine study enrolled 20 of 42 eligible patients.
Patients 6 months or younger undergoing cardiopulmonary bypass (CPB), with or without deep hypothermic circulatory arrest (DHCA), for repair of CHD were eligible. Exclusion criteria were multiple congenital anomalies, recognizable genetic or phenotypic syndromes other than 22q11 deletion, and non-English-speaking caregivers, as previously reported [9]. All patients enrolled in the APOE and cytokine studies were eligible for enrollment in the supplementary MRI study. Early postoperative MRIs were performed in 105 of the 169 patients enrolled in the APOE genotype study. For the cytokine study, 17 of 20 patients underwent a postoperative MRI. Reasons for not undergoing MRI included death or discharge before the MRI, instability precluding MRI in the 14 days after the operation, and caregiver nonconsent. Thus, 122 patients comprised the study cohort. Informed consent was obtained from the parent or the guardian.
Operative Management
Before CPB, patients underwent surface cooling with topical hypothermia to the head. Alpha-stat blood gas management was used, and DHCA was used at the surgeon’s discretion. Before DHCA, patients underwent core cooling on CPB to a nasopharyngeal temperature of 18°C. After rewarming, modified ultrafiltration was performed in all patients. Operative and anesthetic techniques did not differ throughout the study period, other than a trend to maintaining a higher hematocrit during the latter part of the APOE and cytokine study. Postoperatively, all patients returned to the cardiac intensive care unit (CICU) with transthoracic right atrial catheters.
Data Collection
Gestational age, head circumference, birth weight, and Apgar scores were obtained from birth records. Weight, age at operation, and type of procedure were recorded along with the duration of CPB, aortic cross-clamping, and DHCA, if used. Total support time was calculated as CPB duration plus DHCA. Patients were grouped according to a previously described classification model incorporating cardiac anatomy and perioperative physiology [10]. Hemodynamic data including heart rate, right atrial pressure, and systolic (SBP) and diastolic blood pressure (DBP) were retrospectively collected from the CICU data records at 4-hour intervals for the first 48 hours postoperatively. Arterial blood gas data including pH, po2, and pco2 were recorded at similar intervals. The data from intracardiac catheters separate from the right atrial catheters were not analyzed because additional monitoring catheters were rarely used.
Postoperative MRI
MRI of the brain was performed between 3 and 14 days postoperatively with a 1.5 Tesla Magnetom magnet (Siemens, Erlangen, Germany). T1- and T2-weighted images were performed in the axial plane, with T1 imaging also acquired in the sagittal and coronal planes. Axial gradient-echos were done for the susceptibility effects of blood products, and axial diffusion was used to detect early pathophysiologic processes in cerebral infarction.
Two types of stroke were identified: focal arterial ischemic and vascular watershed. Focal arterial ischemic stroke was defined as a discrete demarcated lesion, with or without hemorrhagic transformation, conforming to the distribution of a focal arterial occlusion. Vascular watershed zones arise between end-arterial territories of two major branches of the circle of Willis. Arterial watershed infarcts were defined as discrete demarcated lesions within vascular watershed zones (Fig 1). Vascular territories were determined according to a standard published atlas of cerebral arterial territories [11].
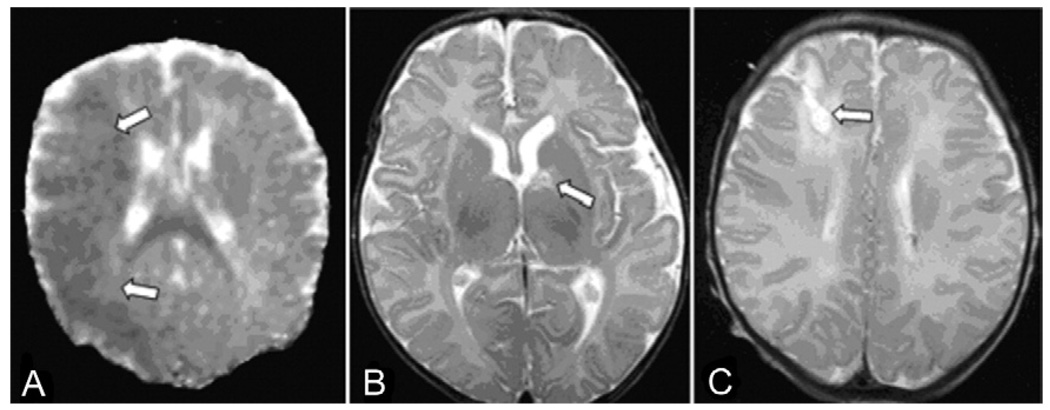
Fig 1.
Illustrative magnetic resonance imaging findings of infarcts (arrows). (A) Apparent diffusion coefficient map from axial diffusion-weighted image shows large acute middle cerebral artery territory infarct. (B) Left caudate lacunar infarct on axial T2 image. (C) Remote right middle cerebral-anterior cerebral artery watershed zone infarct on axial T2 image.
Independent analysis by a neuroradiologist blinded to the patient’s clinical course and genetic data confirmed the occurrence, type, and vascular distribution of focal ischemic lesions. Infarcts were classified as acute if associated with restricted diffusion (≤ 7 to 10 days after onset), subacute if not associated with restricted diffusion (> 7 to 10 days after onset), and chronic if associated with cystic encephalomalacia (> 2 weeks after onset).
In each case, the timing of the infarct in relationship to the operation was determined from the date of operation relative to the date of the MRI and estimated radiologic maturity of the infarct as acute, subacute, or chronic. An infarct was judged to be preoperative if the radiologic appearance was subacute or chronic and the MRI was obtained within 7 days after the operation. An infarct was judged to be possibly intraoperative or postoperative if the radiologic appearance was acute and the MRI was obtained within 7 days after the operation.
APOE Genotype Determination
Genomic DNA was prepared and APOE genotype determined by using a previously published method [12].
Statistical Methods
Data are presented as medians and ranges, where appropriate. Examined risk factors are listed in Table 1. For each of the postoperative hemodynamic variables (po2, pco2, pH, SBP, DBP, radial artery pressure, and heart rate), only admission (time zero), minimum, and maximum values were considered. APOE genotypes were sorted into three groups: the ε2 group (ε2ε2 and ε2ε3), the ε3ε3 group, and the ε4 group (ε3ε4 and ε4ε4). Patients with the ε2ε4 genotype were excluded because of opposing effects of ε2 and ε4 in Alzheimer disease [13]. In addition to analysis as continuous variables, three quantitative risk factors were also included as dichotomous variables: gestational age was considered as either premature (< 37 weeks) or term; age at operation was classified as neonatal (≤ 30 days old) or infant; and the use of DHCA was grouped as “yes” or “no.”
Table 1.
Univariate Logistic Regression of Separate Predictors of Stroke
| Risk Factors | β | SE | p Value |
|---|---|---|---|
| Preoperative factors | |||
| Race | |||
| Whitea | … | … | 0.007b |
| Blackc | −0.111 | 0.072 | 0.123 |
| Asianc | 0.556 | 0.168 | 0.001b |
| Hispanicc | −0.111 | 0.168 | 0.511 |
| Gestational age | −0.091 | 0.116 | 0.433 |
| Gestational age, < 37, ≥ 37 w | −0.491 | 0.833 | 0.556 |
| Delivery | 0.984 | 0.800 | 0.219 |
| Apgar 1 | −0.117 | 0.165 | 0.479 |
| Apgar 5 | −0.326 | 0.429 | 0.447 |
| Birth head circumference | −0.182 | 0.122 | 0.136 |
| Birth weight | −0.001 | 0.000 | 0.070b |
| APOE 22 or 23c | 0.140 | 0.110 | 0.206 |
| APOE 33a | 0.159 | ||
| APOE 34 or 44c | −0.077 | 0.064 | 0.227 |
| Intubation preoperatively | 1.253 | 0.644 | 0.052b |
| Prostaglandin E1 | 0.539 | 0.696 | 0.438 |
| Operative factors | |||
| Age at operation | 0.004 | 0.007 | 0.588 |
| Age at operation, <30, ≥30 d | 0.346 | 0.709 | 0.626 |
| Weight | −0.254 | 0.327 | 0.436 |
| Operative class | −0.031 | 0.225 | 0.892 |
| Cooling duration, min | 0.014 | 0.038 | 0.708 |
| DHCA, yes/no | 0.655 | 0.695 | 0.346 |
| DHCA duration, min | 0.021 | 0.012 | 0.098b |
| CPB duration, min | −0.010 | 0.009 | 0.282 |
| Total support, min | −0.001 | 0.008 | 0.891 |
| Hematocrit | −0.180 | 0.091 | 0.047b |
| Surgeona | … | … | 0.765 |
| Postoperative factors | |||
| Heart rate | |||
| At CICU admission | −0.024 | 0.014 | 0.080b |
| Maximum ≤ 48 h post-op | −0.025 | 0.020 | 0.218 |
| Minimum ≤ 48 h post-op | −0.022 | 0.017 | 0.189 |
| Systolic blood pressure | |||
| At CICU admission | 0.048 | 0.017 | 0.005b |
| Maximum ≤ 48 h post-op | 0.030 | 0.016 | 0.050b |
| Minimum ≤ 48 h post-op | 0.017 | 0.028 | 0.545 |
| Diastolic blood pressure | |||
| At CICU admission | 0.030 | 0.023 | 0.192 |
| Maximum ≤ 48 h post-op | 0.012 | 0.023 | 0.603 |
| Minimum ≤ 48 h post-op | −0.033 | 0.050 | 0.519 |
Reference group (p value represents analysis of variance results for comparison of all categories).
p < 0.10.
Comparison with reference group.
APOE = apolipoprotein E; β = regression coefficient; CICU = cardiac intensive care unit; CPB = cardiopulmonary bypass; DHCA = deep hypothermic circulatory arrest; SE = standard error.
All variables were tested by univariate logistic regression for association with stroke. Values of p for categoric variables were derived by analysis of variance methods, in which the p value indicates a measurement of overall difference between groups. Values of p reported for subcategories within a categoric variable represent the results of regression analysis testing for difference in the outcome variable between the subcategory and the reference subcategory. Any risk factor with a value of p ≤ 0.10 was considered in a multivariate stepwise logistic regression to determine association with stroke. All analyses used SPSS 10.0 software (SPSS Inc, Chicago, IL) and the R statistical environment.
Results
Patient demographics and intraoperative variables are summarized in Table 2. Perioperative stroke was identified in 12 of 122 patients (10%; Fig 2, Table 3). No identifiable postoperative clinical deficits or neurologic signs referable to the infarction were noted in 11 of 12 patients. Continuous electroencephalographic (EEG) monitoring confirmed seizures in 1 patient who demonstrated clinical events suspicious for seizures beginning 1 day after circulatory arrest and urgent initiation of venoarterial extracorporeal membrane oxygenation. EEG monitoring disclosed multifocal seizure discharges and EEG seizures persisting for several days despite escalating doses of anticonvulsants, maximally involving the region of the infarction in the right hemisphere (Fig 1A). Timing, severity, and localization of EEG seizure activity were consistent with the large size, localization, and age of the infarct demonstrated on MRI.
Table 2.
Patient Demographics and Intraoperative Variables
| Patient Data | Stroke (n = 12) Median (Range) |
No Stroke (n = 110) Median (Range) |
|---|---|---|
| Gestational age w | 39 (28–40) | 39 (29–42) |
| Birth weight, g | 2755 (739–3818) | 3123 (1230–4528) |
| Head circumference, cm (n = 120) |
33 (24–36) | 34 (27–38) |
| Age at operation, d | 6 (3–162) | 6 (1–177) |
| Weight at operation, g | 2900 (1900–5300) | 3300 (1520–6900) |
| Cooling duration, min | 15 (3–30) | 15 (0–57) |
| DHCA duration, min (n = 76) |
47 (2–86) | 38 (1–78) |
| CPB duration, min | 52 (40–116) | 61 (13–242) |
| Total support time, min | 87 (51–156) | 61 (13–242) |
| Hematocrit,a % | 26 (21–31) | 28 (20–38) |
After hemodilution on CPB.
CPB = cardiopulmonary bypass; DHCA = deep hypothermic circulatory arrest.
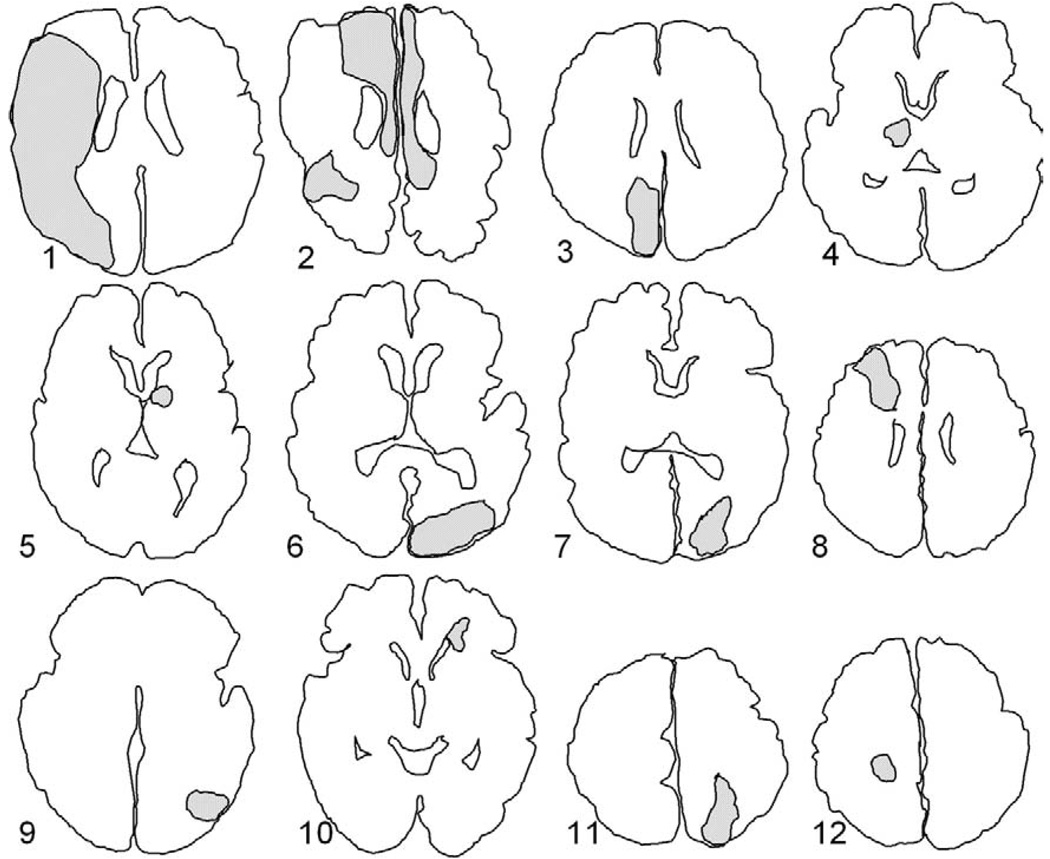
Fig 2.
Location of infarct (regions of shaded area) obtained by manual outline of lesion on representative axial T2 or diffusion-weighted image. See Table 3 for details.
Table 3.
Type, Location, and Vascular Territory of Ischemic Infarcts
| Patienta | Type and Location of Infarct | Infarct Ageb | Vascular Territory |
|---|---|---|---|
| Arterial occlusive (n = 5) | |||
| 1 | MCA, right | Acute | Proximal MCA |
| 2 | Frontal, bilateral & parietal, right | Remote | Bilateral ACA + MCA branch |
| 3 | Occipital, hemorrhagic, right | Acute | PCA |
| 4 | Thalamic, right | Acute | PCA, thalamic branch |
| 5 | Caudate, left | Acute | MCA lenticulostriate branch |
| Watershed (n = 7) | |||
| 6 | Parietal-occipital, hemorrhagic, left | Acute | MCA-PCA |
| 7 | Parietal-occipital, left | Subacute | MCA-PCA |
| 8 | Anterior frontal, right | Remote | ACA-MCA |
| 9 | Parietal-occipital, left | Subacute | MCA-PCA |
| 10 | Deep frontal white matter, left | Subacute | ACA-MCA |
| 11 | Parietal-occipital, left | Subacute | MCA-PCA |
| 12 | Parietal white matter, left | Subacute | MCA-PCA |
As depicted in Figure 2.
Acute indicates restricted diffusion; subacute, no restricted diffusion; remote, cystic encephalomalacia.
ACA = anterior cerebral artery; MCA = middle cerebral artery; PCA = posterior cerebral artery.
The type and location of infarctions are summarized in Table 3. The infarcts were all supratentorial, involving the carotid circulation in 10 patients and the vertebrobasilar circulation in 2 patients. Stroke conformed to large or medium vessel arterial occlusion in 5 patients and to arterial watershed zones in the remaining 7 patients, most commonly the middle cerebral artery (MCA)-posterior cerebral artery (PCA) watershed zone. Only one large complete MCA territory infarct was documented. Significant hemorrhagic transformation was seen in two infarcts. Infarcts were acute in 5 patients, subacute in 5, and remote in 2. PVL and extraaxial hemorrhage were also detected but are not included in the present analysis.
Univariate analysis of risk factors for infarcts is summarized in Table 1. There were no significant differences in cardiac anatomy (Table 4), type of operation, intracardiac shunting, or prostaglandin E1 (PGE1) use between patients with and without stroke. Multivariate analysis (Table 5) revealed lower birth weight, preoperative intubation, lower intraoperative hematocrit, and higher systolic blood pressure upon CICU admission as variables significantly associated with stroke. Aside from the initial systolic blood pressure upon admission to the CICU, there was no difference in blood pressure for the next 48 hours between patients with and without infarcts.
Table 4.
Cardiac Anatomy and Association With Stroke
| Anatomy | No. | Stroke | No Stroke | p Value |
|---|---|---|---|---|
| Class I | 50 | 5 | 45 | 0.751 |
| Class II | 15 | 2 | 13 | … |
| Class III | 9 | 0 | 9 | … |
| Class IV | 48 | 5 | 43 | … |
| 2 ventricles | 65 | 7 | 58 | 0.712 |
| 1 ventricle | 57 | 5 | 52 |
Class I = 2 ventricles with no arch obstruction; Class II = 2 ventricles with arch obstruction; Class III = single ventricle with no arch obstruction; Class IV = single ventricle with arch obstruction.
Table 5.
Statistically Significant Risk Factors for Perioperative Stroke
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | β | SE | p Value | β | SE | p Value |
| Asian race | 0.556 | 0.168 | 0.001 | 2.233 | 2.044 | 0.274 |
| Birth weight | −0.001 | 0.000 | 0.070 | −0.002 | 0.001 | 0.016 |
| Intubationb | 1.253 | 0.644 | 0.052 | 2.079 | 0.982 | 0.034 |
| Hematocritb | −0.180 | 0.091 | 0.047 | −0.247 | 0.126 | 0.051 |
| DHCA duration | 0.021 | 0.012 | 0.098 | 0.032 | 0.019 | 0.086 |
| At CICU admission | ||||||
| Heart rate | −0.024 | 0.014 | 0.080 | −0.049 | 0.028 | 0.074 |
| SBP | 0.030 | 0.016 | 0.050 | 0.089 | 0.036 | 0.012 |
Before the operation.
After hemodilution on cardiopulmonary bypass.
β = regression coefficient; CICU = cardiac intensive care unit; DHCA = deep hypothermic circulatory arrest; SBP = systolic blood pressure; SE = standard error.
Comment
We demonstrated a 10% prevalence of stroke in neonates and infants with CHD undergoing cardiac operations using CPB, half of which occurred preoperatively. This large, prospective cohort study evaluating stroke in infants with CHD, in which brain MRI was used for case ascertainment, shows that infarcts in these infants are almost always clinically covert, with a wide spectrum of size, chronicity, and anatomic distributions. In keeping with the clinically silent nature of these lesions acutely, we found that the most reliable assessment of stroke in this population is by prospective systematic imaging with MRI examination. Both arterial-occlusive and watershed infarcts were found, suggesting a multifactorial mechanism of stroke in patients with CHD that may include thromboembolism and hypoperfusion. The varying ages of the infarcts indicate multiple time points from birth, and possibly prenatally, when these children are vulnerable to brain injury.
Variables associated with stroke in our cohort included lower birth weight, preoperative intubation, lower hematocrit level after hemodilution on bypass, and elevated SBP upon CICU admission. Gestational age, PGE1 use, cardiac anatomy, and DHCA duration were not significantly different between patients with and without stroke. The number of patients with stroke was too small to differentiate between risk factors for focal arterial occlusive and watershed lesions. The association of low birth weight would suggest that fetal blood flow patterns and placental sufficiency might be involved in the pathogenesis of stroke in infants with CHD [14]. Preoperative mechanical ventilation might be a marker of increased cardiopulmonary instability in the preoperative period and has been identified as a risk factor for both death and CICU morbidity [15]. Hypotension and hypoxemia have previously been shown to be associated with PVL [8]; however, these risk factors were not significantly associated with stroke in our population. A possible explanation may be that cerebral blood flow patterns are different in children with PVL compared with those who with perioperative stroke. Variations in other factors that affect cerebral oxygen delivery, such as pH and Paco2, were not identified as risk factors for stroke.
In the Boston Circulatory Arrest Study (BCAS), limited to patients with transposition of the great arteries, brain MRI imaging at age 1 year revealed definite abnormalities in 15% and possible abnormalities in an additional 8% [16]. Stroke was not specifically reported. The only significant risk factor for MRI abnormalities was preoperative acidosis. Similarly, our data show no significant association of DHCA use or duration or total CPB time with stroke. The sole intraoperative variable associated with stroke was a lower hematocrit after hemodilution on CPB. Hemodilutional anemia during CPB has been shown to be associated with histologic evidence of cerebral ischemic damage in animal models and lower Psychomotor Developmental Index scores in children at 1 year [17, 18].
Other studies evaluating brain MRI abnormalities have also demonstrated varying ages of lesions relative to birth and procedures, with preoperative and postoperative periods presenting a continuum of risk in infants with CHD. Dent and colleagues [19] reported focal or diffuse ischemic lesions in 23% and 53% of patients in the preoperative and postoperative periods, respectively, after the Norwood procedure. In their study, a greater base deficit was associated with preoperative ischemia, whereas prolonged low regional cerebral oxygen saturation (< 45% for > 90 minutes) was associated with postoperative changes. In another study limited to patients with transposition, McQuillen and colleagues [20] found low Apgar scores at 5 minutes and need for balloon atrial septostomy to be associated with preoperative stroke. Risk factors for postoperative injury included lowest flow and largest base deficit during CPB and decreased mean blood pressure on postoperative day 1.
The current study has several limitations. The absence of preoperative MRIs precludes definitive identification of the timing of occurrence of stroke and limits the interpretation of cause and effect among potential risk factors. In addition, only 1 patient underwent a detailed neurologic examination in the immediate postoperative period, potentially limiting the clinical identification of strokes identified on the MRI studies.
This study was also not designed to systematically evaluate all patients for other risk factors for stroke such as thrombophilia or anatomic anomalies in the cervical or cranial circulation.
Finally, our results may have been affected by selection bias, because not all patients from the ongoing studies had postoperative MRIs. Patients who were discharged before undergoing a MRI would overestimate the prevalence of stroke, whereas patients with instability precluding a MRI might underestimate the prevalence.
In conclusion, we found a 10% prevalence of stroke on postoperative MRI imaging in neonates and infants undergoing operations for CHD that used CBP with or without DHCA. Almost all strokes were clinically silent. Focal arterial occlusive and watershed infarcts were present, suggesting thromboembolism and hypoperfusion were both mechanisms of injury. Risk factors for stroke included lower birth weight, preoperative mechanical ventilation, and lower hematocrit during CPB. Importantly, other operative variables, such as duration of CPB and the use of DHCA, were not significantly associated with an increased risk of stroke. In contrast to our study evaluating risk factors for PVL in the same cohort, postoperative hypoxemia and hypotension were not associated with an increased risk of stroke, suggesting that the mechanisms underlying the two forms of cerebral injury are different.
Ongoing neurodevelopmental studies will investigate the effect of stroke on short-term and long-term functional outcomes. More complete evaluation of stroke risk factors, including thrombophilia and vascular anomalies as well as serial neuroimaging, may provide additional insight concerning mechanisms and potential treatment strategies.
Acknowledgments
This study was supported by grants from the Fourjay and Ethel B. Foerderer Foundations, and the American Heart Association.
Footnotes
INVITED COMMENTARY
I read with interest the article by Chen and colleagues [1].
- Can fetal diagnosis and maternal care for all having CHD impact birth weight and thereby mitigate this as a risk?
- Can fetal triage to a congenital cardiac liaised high-risk obstetrics and neonatal team preclude cardiorespiratory instability in more babies and thus lessen preoperative stroke?
- Risk was shown to be modifiable in the operating room. A higher hematocrit (28% vs 26%) on cardiopulmonary bypass (CPB) was associated with a lower incidence of stroke. This corroborates previous findings that hematocrit on CPB is important [5]. Not all factors believed to be a risk in the operating room were actually a risk. Circulatory arrest, as performed and compared, did not impact neurodevelopment. Fruitful areas for further study will include hybrid stage I versus conventional stage I palliation for single ventricle and arch obstruction, further human studies on pCO2 management strategy for CPB, rate of CPB flow, and others.
- Postoperative measurements, such as systemic oxygen delivery [6], length of stay in the intensive care unit, and other factors are also important and potentially modifiable.
Survival with CHD has become an expectation. It is our duty to ensure that those unfortunate enough to have CHD not only survive, but live. Continued critical data analysis in the areas outlined, trials, and modification of practice will allow us to help our patients live—in the fullest sense.
Glen Van Arsdell, MD
Division of Cardiovascular Surgery
The Hospital for Sick Children
555 University Ave, Suite 1525
Toronto, Ontario, Canada M5G 1X8
glen.vanarsdell@sickkids.ca
References
1. Chen J, Zimmerman RA, Jarvik GP, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg 2009;88:823–9.
2. Shillingford AJ, Glanzman MM, Ittenbach RF, et al. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics 2008;4:e759–67.
3. Gaynor JW, Wernovskiy G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2007;133:1344–53.
4. Tabbutt S, Nord AS, Jarvik GP, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics 2008;121:476–83.
5. Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003;126:1765–74.
6. Hoffman GM, Mussatto KA, Brosig CL, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 2005;10:1094–100.
References
- 1.Bellinger DC, Wypij D, Kuban KCK, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 2.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16:92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 3.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. 1999;103:402–408. doi: 10.1542/peds.103.2.402. [DOI] [PubMed] [Google Scholar]
- 4.Jonas RA. Hypothermia, circulatory arrest, and the pediatric brain. J Cardiothorac Vasc Anesth. 1996;10:66–74. doi: 10.1016/s1053-0770(96)80180-7. [DOI] [PubMed] [Google Scholar]
- 5.Glauser TA, Rorke LB, Weinberg PM, Clancy RR. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991–1000. [PubMed] [Google Scholar]
- 6.van Houten JP, Rothman A, Bejar R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13:47–53. doi: 10.1055/s-2007-994202. [DOI] [PubMed] [Google Scholar]
- 7.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12) suppl 1:I109–I114. [PubMed] [Google Scholar]
- 8.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–1745. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 10.Clancy RR, McGaurn SA, Wernovsky G, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–357. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 11.Pellicer A, Cabanas F, Garciaalix A, Perezhigueras A, Quero J. Stroke in neonates with cardiac right-to-left shunt. Brain Dev. 1992;14:381–385. doi: 10.1016/s0387-7604(12)80344-5. [DOI] [PubMed] [Google Scholar]
- 12.Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64:715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 13.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein-e type-2 allele for late-onset Alzheimer-disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BA, Ades A. Delivery room and early postnatal management of neonates who have prenatally diagnosed congenital heart disease. Clin Perinatol. 2005;32:921–946. doi: 10.1016/j.clp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Bandla HPR, Hopkins RL, Beckerman RC, Gozal D. Pulmonary risk factors compromising postoperative recovery after surgical repair for congenital heart disease. Chest. 1999;116:740–747. doi: 10.1378/chest.116.3.740. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart-surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 17.Miura T, Sakamoto T, Kobayashi M, Shin’oka T, Kurosawa H. Hemodilutional anemia impairs neurologic outcome after cardiopulmonary bypass in a piglet model. J Thorac Cardiovasc Surg. 2007;133:29–36. doi: 10.1016/j.jtcvs.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: Results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–1774. doi: 10.1016/j.jtcvs.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:1523–1530. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 20.McQuillen PS, Barkovich AJ, Hamrick SEG, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]