Abstract
Background
Studies with L-arginine supplementation have shown inconsistent effects on endothelial function. The generation of guanidinoacetate (GAA) from L-arginine with subsequent formation of creatine and homocysteine and consumption of methionine may reduce the pool of L-arginine available for nitric oxide generation. Experimental studies suggest that creatine supplementation might block this pathway. We sought to determine the effects of L-arginine, creatine, or the combination on endothelium-dependent vasodilation and homocysteine metabolism in patients with coronary artery disease.
Methods
Patients with coronary artery disease were randomized to L-arginine (9gm/day), creatine (21gm/day), L-arginine plus creatine, or placebo for 4 days (n=26–29/group). Brachial artery flow-mediated dilation and plasma levels of L-arginine, creatine, homocysteine, methionine, and GAA were measured at baseline and follow up.
Results
L-arginine and creatine supplementation had no effects on vascular function. L-arginine alone increased GAA (P<0.01) and the ratio of homocysteine to methionine (P<0.01) suggesting increased methylation demand. The combination of creatinine and L-arginine did not suppress GAA production or prevent the increase in homocysteine-to-methionine ratio. Unexpectedly, creatine supplementation (alone or in combination with L-arginine) was associated with an 11 to 20% increase in homocysteine concentration (P<0.05), which was not attributable to worsened renal function, providing evidence against an effect of creatine on decreasing methylation demand.
Conclusion
The present study provides no evidence that L-arginine supplementation improves endothelial function and suggests that L-arginine may increase methylation demand. Creatine supplementation failed to alter the actions of L-arginine on vascular function or suppress methylation demand. The unexpected increase in homocysteine levels following creatine supplementation could have adverse effects and merits further study, since creatine is a commonly used dietary supplement.
Keywords: L-arginine, creatine, homocysteine, nitric oxide, methylation, endothelial function
Introduction
Nitric oxide (NO) generated by the endothelium serves as a critical regulator of vascular homeostasis by modulating vascular tone, platelet reactivity, and inflammation. NO is generated from its precursor amino acid L-arginine by the enzyme nitric oxide synthase (NOS).1 Impairment of the normal biologic activity of endothelium-derived NO contributes to the development of atherosclerosis and its ischemic manifestations.2–4
Supplying additional L-arginine in atherosclerotic disorders may augment NO production and improve endothelial function. Beneficial effects of L-arginine on endothelium-dependent vasodilation have been shown in animal models and human subjects.5–8 Clinical studies with L-arginine have also suggested beneficial actions in clinical ischemia.7 In contrast, other studies have failed to demonstrate beneficial effects of L-arginine on endothelial function.9–11 In fact, one group reported a paradoxical finding of L-arginine negating the protective effects of inducible NOS gene deficiency in apolipoprotein E knockout mice – raising the possibility that L-arginine contributed to the progression of atherosclerosis.12 Furthermore, concern for potential detrimental effects of L-arginine on mortality following myocardial infarction has been raised.13
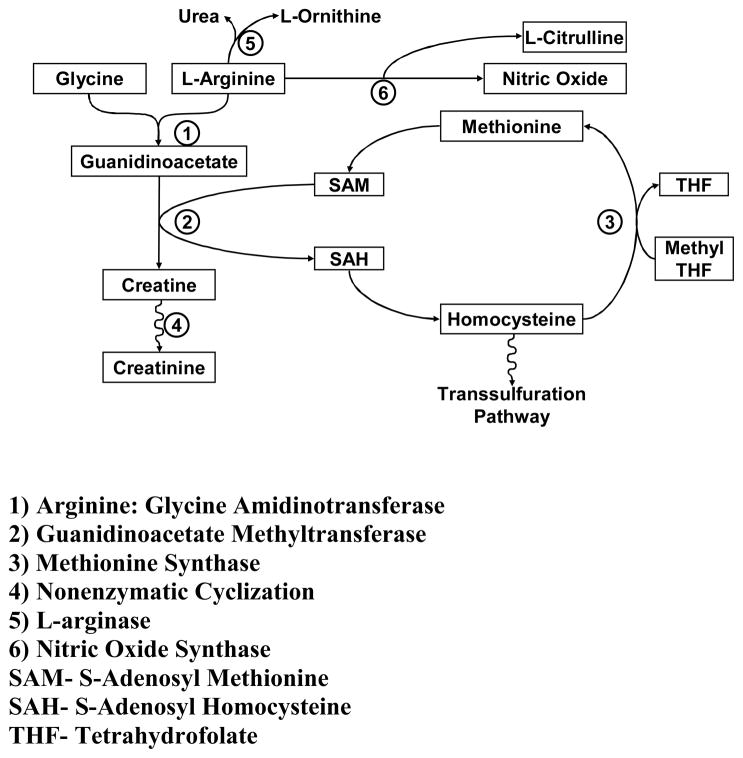
A possible explanation for the inconsistent results of L-arginine supplementation may reside in its numerous metabolic fates. NO synthesis has been shown to reflect only a minority (~1%) of L-arginine flux.14 In contrast, a much greater proportion of L-arginine flux is attributable to synthesis of creatine by the enzyme L-arginine:glycine amidinotransferase (AGAT).15 Generation of creatine is estimated to consume ~70% of labile methyl groups, with S-adenosyl methonine (SAM) serving as the methyl donor (Figure 1).16 This methylation demand may reduce the available methyl groups required in numerous biological reactions. In fact, hypomethylation and aberrant methylation have been shown to have adverse consequences for endothelial cell function and growth.17–19 In the process of donating methyl groups to guanidinoacetate (GAA), SAM is metabolized to S-adenosyl homocysteine (SAH) and eventually homocysteine. Given the known adverse effects of homocysteine on endothelial function, L-argininehas the potential to paradoxically impair endothelial function.20–22
Figure 1.
Relationship between L-arginine, creatine and homocysteine metabolism.
Since creatine reduces the activity of AGAT by feedback inhibition, augmenting creatine concentrations has the potential to decrease conversion of L-arginine to creatine.23, 24 This effect would be expected to increase the availability of L-arginine for NO synthesis, reduce methylation demand, and decrease homocysteine levels.25–27 Thus, we sought to determine the effects of short-term L-arginine and creatine supplementation on endothelial function and homocysteine metabolism. Our hypothesis was that the combination of L-arginine and creatine would enhance endothelium-dependent vasodilation and reduce homocysteine levels.
Methods
Study Population
Subjects with proven coronary artery disease were recruited from the patient population at Boston Medical Center. Coronary artery disease was confirmed by coronary angiography or clinical history of myocardial infarct. Subjects were excluded for the following reasons: creatine or L-arginine use within 1 month, myocardial infarction or intervention within 2 weeks, or change in medication within 1 week. Since the placebo supplement contained lactose, potential subjects with milk allergy or lactose intolerance were also excluded. The study was approved by the Institutional Review Board of Boston University Medical Center. All participants gave written informed consent.
Baseline demographic variables, biochemical assessment, and vascular function testing were assessed during the first visit. Repeat biochemical assessment and vascular function testing was performed on the 4th day of taking the study supplementation.
Vascular Function Assessment
All cardiovascular medications were continued during the study, but subjects were required to be on stable doses of these agents for at least four weeks prior to the study. Prior to each visit, all vasoactive medications, including nitrates, calcium channel blockers, angiotensin converting enzyme inhibitors, beta-adrenergic blockers, and other vasodilators, were withheld for 24 hours. Patients were asked to fast and refrain from smoking (if applicable) overnight.
Endothelium-dependent, flow-mediated and endothelium-independent, nitroglycerin-induced dilation were assessed using high-resolution ultrasonography of the brachial artery.28,29 Briefly, the brachial artery was imaged at baseline for assessment of resting arterial diameter and flow. After a 5-minute cuff occlusion, brachial artery flow was measured during the hyperemic phase. Images of the brachial artery were obtained 1 minute after cuff release to measure diameter and calculate flow-mediated dilation (FMD). After a 10 minute rest period, images were recorded before and 4 minutes after administration of a sublingual nitroglycerin tablet (0.4 mg). Nitroglycerin was omitted if the subject declined, had a previous adverse reaction to nitroglycerin, had a history of migraine headaches, had a systolic blood pressure less than 100 mmHg, or used a type 5 phosphodiesterase inhibitor within 1 week of the study. All images were digitized online and analyzed offline using customized software (Medical ImagingApplications, Inc) in a blinded manner.
L-Arginine and Creatine Supplementation
Subjects were randomized in a double-blind fashion to one of four treatment groups: L-arginine (9 gm/day), creatine (21 gm/day), L-arginine (9 gm/day) plus creatine (21 gm/day), or placebo. The materials used in the study included General Nutrition Centers (GNC) A-Z Arginine 1000 mg tablets, Twinlab Creatine Fuel Powder (Micronized Formula), and gelatin capsules and/or lactose powder for placebo controls. The supplementation protocol was given for 3 days as follows: 7 grams of creatine three times per day (with the powder mixed in juice), 3 grams of L-arginine three times a day, and placebo tablets and/or powder three times a day, based upon the treatment assignment. The 4th day of supplementation consisted of 7 gm of creatine once, 9 gm of arginine once, both creatine and L-arginine, or placebo in the morning 2–3 hours prior to undergoing repeat biochemical and vascular function evaluations.
Biochemical Measurements
Plasma homocysteine, L-arginine, and creatine concentrations were measured using chemiluminescence, radioimmunoassay extraction, and enzymatic assay, respectively, via standard techniques adapted for automated clinical chemistry laboratory analyzers. Guanidinoacetate (GAA) and methionine were measured using tandem mass spectroscopy.30 Cystatin C was measured by a particle enhanced immunonepholometric assay.31 Glomerular filtration rate was calculated at baseline using the Cockcroft-Gault formula and at both visits from the cystatin C values (using the formula y=77.24x−1.2623).32
Statistical Analysis
All data are expressed as mean ± standard deviation, except as otherwise noted. Baseline clinical, biochemical, and vascular imaging variables were compared either by chi squared testing for categorical variables or one-way analysis of variance (ANOVA) for continuous variables. Group differences in response to all supplementation regimens were compared using two-way repeated measures ANOVA with post hoc testing (using the Student Newman Keuls method). Repeated analyses were also performed for primary outcomes including baseline variables with a trend towards a difference, as well as calculated glomerular filtration rate (by the Cockcroft-Gault formula), as covariates in separate repeat measures ANOVA. Analysis was completed using SPSS for Windows version 12.0.1 (SPSS Inc, Chicago, Illinois). Statistical significance was defined as a P level <0.05.
The study was designed to test the hypothesis that FMD would be increased to a greater extent following treatment with L-arginine and creatine compared to L-arginine alone, creatine alone, or placebo. The study had 80% power (alpha = 0.05) to detect an improvement in FMD of 2.1 percent with a sample size of 25 subjects per group.
Results
Subject Characteristics
A total of 119 subjects were recruited and randomized in the study. Seven subjects withdrew from the study prior to their follow up assessment on the 4th day of supplementation, which left a total of 112 patients (26–29 per group) with repeat biochemical and vascular function assessment for analysis. Baseline characteristics, including demographics, medical conditions, medication usage, and laboratory data, in each of the four groups are shown in Table 1. There were no significant differences in these baseline characteristics among the groups. However, a possible trend towards a difference between groups was seen with tobacco use, previous CABG, previous stroke or carotid disease, and multivitamin or folate supplementation.
Table 1.
Baseline characteristics.
| Placebo | Arginine | Creatine | Arginine/Creatine | P value | |
|---|---|---|---|---|---|
| N=29 | n=28 | n=26 | n=29 | ||
| Age | 58 ± 12 | 60 ± 9 | 60 ± 11 | 57 ± 8 | 0.44 |
| Men, No. (%) | 22 (76) | 23 (89) | 18 (69) | 23 (82) | 0.36 |
| Caucasian, No. (%) | 24 (83) | 22 (85) | 17 (65) | 20 (71) | 0.46 |
| BMI | 30.8 ± 5.7 | 27.2 ± 9.6 | 29.9 ± 6.6 | 29.6 ± 7.7 | 0.35 |
| Systolic BP (mm Hg) | 139 ± 22 | 137 ± 19 | 134 ± 23 | 129 ± 19 | 0.25 |
| Diastolic BP (mm Hg) | 76 ± 10 | 74 ± 10 | 71 ± 11 | 72 ± 9 | 0.22 |
| Past Medical History, No. (%) | |||||
| Diabetes | 9 (31) | 6 (23) | 6 (23) | 10 (36) | 0.67 |
| Hypertension | 18 (62) | 17 (65) | 19 (73) | 17 (61) | 0.78 |
| Hypercholesterolemia | 24 (83) | 23 (89) | 22 (85) | 22 (78.6) | 0.8 |
| Tobacco use within last year | 14 (48) | 11 (42) | 6 (23) | 14 (50) | 0.17 |
| Family history of CAD | 17 (59) | 13 (50) | 14 (54) | 14 (50) | 0.9 |
| Myocardial infarction | 19 (66) | 20 (77) | 17 (65) | 17 (61) | 0.63 |
| Previous CABG | 6 (21) | 4 (15) | 11 (42) | 9 (32) | 0.13 |
| Previous PCI | 23 (79) | 19 (73) | 16 (62) | 18 (64) | 0.46 |
| Stroke or carotid disease | 4 (14) | 1 (4) | 1 (4) | 0 (0) | 0.13 |
| Medication, No. (%) | |||||
| ASA/Clopidogrel | 24 (82.8) | 22 (84.6) | 25 (96.2) | 25 (92.6) | 0.34 |
| ACE inhibitor | 12 (41.4) | 11 (42.3) | 10 (38.5) | 13 (48.1) | 0.91 |
| Beta blocker | 21 (72.4) | 22 (84.6) | 21 (80.8) | 20 (74.1) | 0.67 |
| Statin | 22 (75.9) | 21 (80.8) | 22 (84.6) | 23 (85.2) | 0.79 |
| MVI/Folate | 5 (17.2) | 7 (26.9) | 9 (34.6) | 2 (7.4) | 0.08 |
| Baseline Laboratory Values | |||||
| Total cholesterol (mg/dl) | 163.9 ± 29.8 | 169.8 ± 39.4 | 165.8 ± 56.1 | 160 ± 35.4 | 0.86 |
| HDL-cholesterol (mg/dl) | 41.9 ± 7.8 | 44.5 ± 10.1 | 45.5 ± 16.1 | 42 ± 9.7 | 0.6 |
| LDL-cholesterol (mg/dl) | 93 ± 24.7 | 93.2 ± 30.7 | 82.7 ± 23.5 | 87.6 ± 29.4 | 0.48 |
| Triglyceride (mg/dl) | 152.2 ± 86.5 | 156.2 ± 87.1 | 168.3 ± 174.4 | 149.3 ± 82.7 | 0.93 |
| Glucose (mg/dl) | 131.6 ± 62.3 | 120 ± 38.1 | 116.3 ± 43 | 111.9 ± 34.9 | 0.42 |
Supplementation
The current supplementation regimen with L-arginine and creatine was effective in increasing their respective levels (Table 2). There was a 2 to 2.5-fold increase in plasma L-arginine levels with L-arginine supplementation and 15 to 20-fold increase in plasma creatine levels with creatine supplementation, either used alone or in combination. Creatine supplementation had no significant effect on L-arginine levels and L-arginine supplementation had no significant effect on creatine levels. The increase in creatine and L-arginine remained significant after adjusting for each covariate (including tobacco use, previous CABG, previous stroke, and multivitamin or folate supplementation, as well as glomerular filtration rate).
Table 2.
Biochemical measurements before and after supplementation.
| Placebo | Arginine | Creatine | Arginine/Creatine | Overall P Value | |
|---|---|---|---|---|---|
| Arginine Plasma Concentration (umol/L) | |||||
| Pre-treatment | 71.7 ± 25.2 | 80.7 ± 30.4 | 60.9 ± 21.7 | 69 ± 21.7 | <0.001 |
| After intervention | 81.1 ± 21.9 | 173.9 ± 61.3 * | 74.6 ± 30.9 | 175.5 ± 93 * | |
| Creatine Plasma Concentration (mg/dl) | |||||
| Pre-treatment | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.8 ± 0.5 | 0.5 ± 0.3 | <0.001 |
| After intervention | 0.8 ± 0.7 | 0.8 ± 1.0 | 15.1 ± 7.5 * | 7.9 ± 6.8 * | |
| Homocysteine (umol/L) | |||||
| Pre-treatment | 10.7 ± 3.2 | 11.1 ± 3.3 | 11.1 ± 3.6 | 10.7 ± 3.1 | 0.006 |
| After intervention | 10.7 ± 3.5 | 11.2 ± 3.1 | 12.3 ± 3.5 * | 12.9 ± 4.2 * | |
| Methionine (umol/L) | |||||
| Pre-treatment | 18 ± 4 | 17.4 ± 3.4 | 20.1 ± 3.8 | 19.5 ± 5.4 | 0.05 |
| After intervention | 18 ± 3.3 | 15.4 ± 7.9 | 22.1 ± 4 | 16.1 ± 4.4 * | |
| Homocysteine/Methionine Ratio | |||||
| Pre-treatment | 0.66 ± 0.31 | 0.7 ± 0.34 | 0.61 ± 0.18 | 0.67 ± 0.42 | 0.002 |
| After intervention | 0.61 ± 0.24 | 0.9 ± 0.44 * | 0.64 ± 0.18 | 0.96 ± 0.52 † | |
| Guanidinoacetate (umol/L) | |||||
| Pre-treatment | 1.6 ± 0.9 | 1.6 ± 0.6 | 1.4 ± 0.7 | 1.5 ± 0.6 | 0.007 |
| After intervention | 1.6 ± 0.6 | 2.5 ± 1.2 † | 2.1 ± 1 * | 2.6 ± 1 † | |
| Creatinine (mg/dl) | |||||
| Pre-treatment | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.2 | <0.001 |
| After intervention | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.2 ± 0.3 † | 1.1 ± 0.3 † | |
| Cystatin C (mg/L) | |||||
| Pre-treatment | 1.074 ± 0.5 | 1.023 ± 0.2 | 1.137 ± 0.4 | 1.033 ± 0.2 | 0.003 |
| After intervention | 1.075 ± 0.4 | 1.024 ± 0.2 | 1.081 ± 0.3 * | 1.101 ± 0.3 * | |
| Calculated GFR (mL/min) | |||||
| Pre-treatment | 81.9 ± 29.6 | 78.0 ± 15.2 | 71.8 ± 17.4 | 77.5 ± 15.2 | 0.03 |
| After intervention | 80.6 ± 27.7 | 78.0 ± 15.0 | 75.6 ± 17.6 * | 73.2 ± 20.1 * |
Values are represented as mean ± standard deviation.
P<0.05.
P<0.001.
Vascular Function Assessment
The effects of treatment on vascular function are displayed in Table 3. As shown, brachial artery diameter, flow, reactive hyperemia, flow-mediated dilation, and nitroglycerin-mediated dilation were similar among groups. There were no significant effects of treatment on any measure of vascular function, even after adjusting for covariates (including tobacco use, previous CABG, previous stroke, and multivitamin or folate supplementation, as well as glomerular filtration rate).
Table 3.
Vascular function parameters before and after supplementation.
| Placebo | Arginine | Creatine | Arginine/Creatine | Overall P Value | |
|---|---|---|---|---|---|
| Baseline arterial diameter (mm) | |||||
| Pre-treatment | 4.75 ± 0.56 | 4.68 ± 0.53 | 4.52 ± 0.69 | 4.78 ± 0.81 | |
| After intervention | 4.76 ± 0.46 | 4.68 ± 0.54 | 4.55 ± 0.7 | 4.72 ± 0.74 | 0.77 |
| Baseline arterial flow (cm/sec) | |||||
| Pre-treatment | 287 ± 123 | 264 ± 89 | 210 ± 93 | 217 ± 88 | |
| After intervention | 569 ± 1558 | 242 ± 78 | 370 ± 587 | 219 ± 87 | 0.56 |
| Hyperemic flow (cm/sec) | |||||
| Pre-treatment | 1357 ± 519 | 1432 ± 439 | 1227 ± 547 | 1288 ± 348 | |
| After intervention | 1379 ± 482 | 1366 ± 496 | 1321 ± 595 | 1355 ± 5343 | 0.63 |
| Flow-Mediated Dilation (%) | |||||
| Pre-treament | 7.8 (3.8) | 6.9 (3.6) | 9.1 (5.8) | 7.4 (3.1) | |
| After treatment | 8.1 (4.3) | 8 (3.6) | 7.6 (4.3) | 7.9 (4.8) | 0.18 |
| Nitroglycerin-Mediated Dilation (%) | |||||
| Pre-treament | 11.2 (4.7) | 11.2 (4.5) | 13.2 (6.3) | 12.2 (5.2) | |
| After treatment | 9.2 (4.4) | 9.3 (4.2) | 11.0 (5.0) | 9.2 (3.8) | 0.88 |
Values are represented as mean ± standard deviation. The number of subjects undergoing testing with NMD were 18 in placebo, 17 in L-arginine, 9 in creatine, and 13 in L-arginine plus creatine.
Biochemical Assessment
Table 2 displays the effects of the four treatments on intermediates related to the metabolism of arginine. L-arginine supplementation alone was associated with increased plasma GAA concentration (P<0.01), due to L-arginine conversion to GAA by the enzyme AGAT. Homocysteine and methionine levels did not change following L-arginine supplementation; however, the ratio of homocysteine-to-methionine increased significantly (P=0.008). Taken together, increased GAA levels and an increase in the ratio of homocysteine-to-methionine suggest increased methylation demand and flux through this pathway (Figure 1).
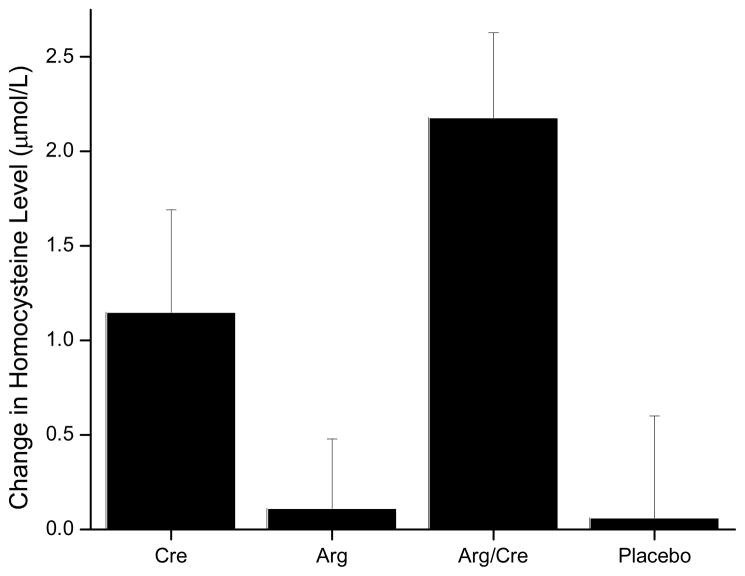
Creatine supplementation alone was associated with increased creatinine levels (P<0.001), which was expected since creatinine is formed by spontaneous cyclization of creatine. Unexpectedly, creatine supplementation was associated with an increase in homocysteine concentration (P=0.03), arguing against our hypothesis that creatine supplementation would lead to a decrease in methylation demand (Figure 2). The increase in homocysteine concentration was unlikely to reflect a worsening of renal function following creatine supplementation, since cystatin C concentrations did not increase, and in fact, were significantly decreased following creatine supplementation (P=0.03). This was indicative of an increase in the calculated glomerular filtration rate or improvement in renal function. We anticipated that creatine supplementation would also reduce plasma GAA levels by decreasing the activity of AGAT, but, instead, observed an increase (P=0.03).
Figure 2.
Change in plasma concentration of homocysteine with supplementation of creatine (Cre), L-arginine (Arg), L-arginine plus creatine (Arg/Cre) and placebo. Figure is represented as change from baseline +/− standard error.
Treatment with the combination of L-arginine and creatine, was associated with the expected increases in GAA (P<0.01) and creatinine (P<0.001). As with L-arginine alone, combination treatment was associated with an increase in the ratio of homocysteine-to-methionine (P<0.001). In addition, the combination of L-arginine and creatine increased homocysteine levels (P<0.05) and decreased methionine levels (P<0.05), providing evidence against our hypothesis that supplemental creatine would decrease flux through this pathway. There was an unexpected increase in cystatin C levels with a reduction in calculated glomerular filtration rate with the combination of L-arginine and creatine (P<0.05).
The effect of time and treatment on homocysteine, homocysteine-to-methionine ratio, and GAA and cystatin C levels remained significant after adjusting for each covariate (including tobacco use, previous CABG, previous stroke, and multivitamin or folate supplementation, as well as glomerular filtration rate) in separate analyses.
Discussion
The present study demonstrated no significant effect of L-arginine supplementation on brachial artery flow-mediated dilation in patients with coronary artery disease. There also were no significant effects on baseline brachial artery diameter, flow, or extent of reactive hyperemia. The study had good statistical power to detect an effect on flow-mediated dilation comparable to that produced by other risk reducing interventions.33 Creatine alone and creatine in combination with L-arginine also did not alter these measures of vascular function. Plasma analysis demonstrated the expected increases in arginine and creatine levels following supplementation. In addition, there was evidence of increased methylation demand following L-arginine supplementation with increased levels of GAA and an increase in the ratio of homocysteine-to-methionine that were not blunted by creatine supplementation. Unexpectedly, creatine supplementation was associated with an increase in plasma homocysteine levels that was not attributable to alterations in renal function.
The lack of an effect of L-arginine supplementation on endothelial function is consistent with the findings reported by some investigators,10, 11 but contrasts with others.5–9, 34 For example, hypercholesterolemic adults given L-arginine supplementation showed an improvement in endothelium dependent dilation.34 By contrast, L-arginine supplementation for six months in patients with peripheral arterial disease reduced nitric oxide availability and endothelium-dependent vascular function compared to placebo.11 Furthermore, a clinical trial found no improvement in vascular stiffness, and a possible association with higher post-infarction mortality.13
There are a number of potential explanations to account for the failure of L-arginine supplementation to improve NO-mediated vasodilation. The present study focused on the possibility that metabolism of L-arginine to GAA with subsequent formation of creatine and homocysteine might be playing a role in human subjects (Figure 1). Prior support for this possibility is provided by a study demonstrating that GAA supplementation increased homocysteine concentration.26 In the present study, we observed an increase in GAA levels with L-arginine supplementation, but did not find an increase in homocysteine levels. We did find, however, that homocysteine levels were elevated relative to its precursor substrate methionine, with an increase in the homocysteine to methionine ratio. This finding supports the concept that there is an increase in methylation demand (or a reduction in bioavailable methyl donors) with L-arginine supplementation.35 Our current observation is in contrast with a decrease in homocysteine levels (and a decreased homocysteine to methionine ratio) seen following L-arginine supplementation in hypercholesterolemic subjects.36
Consequences of increased methylation demand may have significant effects on vascular cell function.17–19 Several studies have proposed mechanisms through which hyperhomocysteinemia and a disturbance in methylation may contribute to cardiovascular disease.37 Increased concentrations of homocysteine were shown to have inhibitory effects on cyclin A transcription and endothelial cell growth through hypomethylation related mechanisms.19 It was suggested that hyperhomocysteinemia induced endothelial NO system dysfunction may occur through aberrant methylation in the dimethylarginine dimethylaminohydrolase gene.18 Protein hypomethylation has also been shown to increase rates of endothelial cell apoptosis and premature senescence.17
The failure to detect an increase in plasma homocysteine levels, despite an apparent effect of L-arginine on its metabolism, may reflect its multiple metabolic pathways. Homocysteine may undergo remethylation to methionine in a folate-dependent process, catabolism to cysteine in the transulfuration pathway, or export to the extracellular space (Figure 1).38 Given these multiple fates of homocysteine, it may be difficult to detect a change in homocysteine levels with intact pathways and replete folate status to handle an increased load. Thus, it remains possible that L-arginine metabolism imposes a methylation demand that counterbalances the effects of NO generated despite the absence of a change in measured plasma homocysteine.
There are a number of other possible explanations to account for the lack of an effect of L-arginine on endothelial function. For example, it is conceivable that the dose and duration of L-arginine treatment were inadequate. These possibilities seem unlikely, since the dose was similar to prior studies, and we observed a 2-fold increase in plasma L-arginine levels. Although the duration of supplementation in this study was less than some other protocols,13, 34 beneficial effects have been reported after very short-term L-arginine administration.7 Another possibility is hydrolysis of L-arginine to L-ornithine and urea by the enzyme L-arginase. An increase in the activity of L-arginase may deplete intracellular L-arginine levels, and decrease NO production.39 Similarly, an endogenous inhibitor of NOS, such as asymmetric dimethylarginine (ADMA), may prevent L-arginine augmentation in NO production.40 It is recognized that medical conditions including coronary artery disease and renal impairment have been shown to have high levels of endogenous NOS inhibitors, including ADMA.41,42
The subjects in our study were selected due to the presence of coronary artery disease; however, many of the subjects also had chronic kidney disease. This is evident by a mean glomerular filtration rate of 68.7 ± 27.7 mL/min (by the Cockcroft-Gault formula) -consistent with stage 2 disease by the National Kidney Foundation-Dialysis Outcomes Quality Initiative Clinical Practice Guidelines.43 This provides two major risk factors for elevated levels of endogenous inhibitors of NOS that might have contributed to the negative results of our study. It has previously been shown that supplementation with L-arginine does not affect endothelial function, or renal perfusion, in chronic kidney disease.44–46 Unfortunately, measurement of ADMA levels, or L-arginase activity, were beyond the scope of the present study.
To gain further insight into the contribution of increased methylation demand as an explanation for the lack of benefit of L-arginine, we measured vascular function and plasma markers before and after supplementation with creatine alone and in combination with L-arginine. We anticipated that creatine supplementation would reduce GAA and homocysteine production.24,27 We hypothesized that this effect of creatine would increase L-arginine levels and, thus, improve NO-dependent vasodilation. In contrast to prior observations that creatine supplementation reduce GAA levels,26 we did not find a reduction in GAA levels or a change in endothelial function.
A potential explanation for this failure to inhibit GAA production may be inadequate dose or duration of creatine supplementation. We however selected our creatine regimen based upon prior studies revealing creatine supplementation within this general dose and duration has a significant effect of the biochemical pathways of interest. It has been shown that GAA levels may be reduced by 50% following a creatine load of 20 grams for 1 week.24 Furthermore, much lower doses of creatine are required to provide twice the daily demand - about 2–5.5 gms daily.27 This results in a modest reduction in homocysteine levels (change of −0.9 μmol/L compared with +0.2 μmol/L) with 4 weeks of therapy. In addition, it is acknowledged that after ~ 2 days of a creatine load that maximal tissue accumulation occurs.47 Despite these effects it remains possible that the use of a longer duration of creatine or usage of alternative approaches to inhibit conversion of L-arginine to GAA might have allowed for the testing of our hypothesis.
Through inhibition of GAA production, we anticipated that creatine supplementation would have a favorable effect on homocysteine metabolism. This is supported by previous study findings that creatine supplementation may reduce homocysteine levels, although not supported by others studies finding no effect.26, 27, 48,49 In contrast, our study found an unanticipated increase in homocysteine of 11 to 20% following creatine supplementation. We sought to explain the mechanism to account for our observed increase in homocysteine levels with creatine supplementation. The kidney plays a significant role in homocysteine metabolism with homocysteine levels increasing as glomerular filtration rate (GFR) decreases.50 There is the potential for subtle alterations in renal function that occur during the acute phase of creatine supplementation due to water retention as creatine loads into muscles. This concept was supported by a reduction in GFR in rats receiving creatine.51 Our finding of an increase in creatinine with creatine supplementation is not reflective of alteration in renal function as creatine undergoes spontaneous cyclization to form creatinine. To elucidate this potential mechanism further we measured cystatin C levels since this is a sensitive marker of GFR that is independent of creatine.50,52 We, however, found no consistent effect of creatine on renal function, as reflected by changes in cystatin C levels (levels increased with creatine alone and decreased with L-arginine plus creatine supplementation). Thus, it does not appear that worsened renal function, as assessed by a decline in glomerular filtration rate, accounts for the increase in homocysteine levels seen with short-term creatine.
As mentioned above with L-arginine suplementation, the inclusion of subjects with chronic kidney disease may be relevant to the interpretation of our study results. Creatine supplementation does not appear to effect homocysteine in chronic hemodialysis, already replete with folate and B vitamins.49 We however found that the paradoxical effect of creatine on homocysteine remained significant after adjusting for differences in renal function as well as multivitamin (or folate) use. Thus, the mechanism to explain the increase in homocysteine levels with creatine supplementation in our study remains uncertain and may warrant further investigation. There are potential implications of this increase in homocysteine, including adverse vascular effects, such as activation of coagulation, stimulation of monocyte adhesion, oxidation of LDL, and impairment of NO-mediated vascular responses.20
In conclusion, our study showed no benefit of short-term L-arginine supplementation on vascular function in patients with stable coronary artery disease, providing further evidence against its clinically utility in this patient population, although chronic kidney disease was a common coexistent illness. Furthermore, our study provided evidence for increased methylation demand with an increase in GAA and homocysteine-to-methionine ratio with L-arginine supplementation. The clinical implications of this finding may merit further study. In addition, we administered creatine to inhibit L-arginine metabolism to GAA in an attempt to investigate further whether this mechanism played a role in the vascular responses to L-arginine supplementation. Despite a marked increase in plasma creatine concentrations, we observed no favorable effects on GAA levels or vascular function. There was an unexpected increase in homocysteine levels following creatine supplementation not attributed to alteration in renal function, which could have clinical implications since creatine is a commonly used as dietary supplement in athletes and body builders. The consequences of long-term creatine supplementation in patients with atherosclerotic disease remain unknown.
Acknowledgments
The authors thank Dr. John T. Brosnan for his willingness to review preliminary data and provide his insight. This study was supported by NIH grants HL75795, HL60886, HL55993, HL083269, HL083781 and HL15157-31.
References
- 1.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long- term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 3.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 4.Gokce N, Keaney JF, Jr, Menzoian JO, Watkins M, Hunter L, Duffy SJ, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 5.Girerd XJ, Hirsch AT, Cooke JP, Dzau VJ, Creager MA. L-Arginine augments endothelium-dependent vasodilation in cholesterol-fed rabbits. Circ Res. 1990;67:1301–1308. doi: 10.1161/01.res.67.6.1301. [DOI] [PubMed] [Google Scholar]
- 6.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-Arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceremuzynski L, Chamiec T, Herbaczynska-Cedro K. Effect of supplemental oral L-arginine on exercise capacity in patients with stable angina pectoris. Am J Cardiol. 1997;80:331–333. doi: 10.1016/s0002-9149(97)00354-8. [DOI] [PubMed] [Google Scholar]
- 8.Lerman A, Burnett JCJ, Higano ST, McKinley LJ, Holmes DRJ. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 9.Thorne S, Mullen MJ, Clarkson P, Donald AE, Deanfield JE. Early endothelial dysfunction in adults at risk from atherosclerosis: different responses to L-arginine. J Am Coll Cardiol. 1998;32:110–116. doi: 10.1016/s0735-1097(98)00211-3. [DOI] [PubMed] [Google Scholar]
- 10.Blum J, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, Waclawiw MA, Panza JA, Cannon RO. Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000;101:2126–2129. doi: 10.1161/01.cir.101.18.2160. [DOI] [PubMed] [Google Scholar]
- 11.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Kuhlencordt P, Urano F, Ichinose H, Astern J, Huang P. Effects of chronic treatment with L-arginine on atherosclerosis in ApoE knockout and ApoE/Inducible NO synthase double-knockout mice. Arterioscler Thromb Vasc Biol. 2003;23:97–103. doi: 10.1161/01.atv.0000040223.74255.5a. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Becker L, Kass D, Champion H, Terrin M, Forman S, Ernst K, Kelemen M, Townsend S, Capriotti A, Hare J, Gerstenblith G. L-Arginine therapy in acute myocardial infarction: VINTAGE MI (Vascular Interaction With Age in Myocardial Infarction Randomized Clinical) Trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 14.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA. 1996;93:11460–11465. doi: 10.1073/pnas.93.21.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53:161–176. [PubMed] [Google Scholar]
- 16.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 17.Polotskaia A, Wang M, Patschan S, Addabbo F, Chen J, Goligorsky MS. Regulation of arginine methylation in endothelial cells: role in premature senescence and apoptosis. Cell Cycle. 2007;20:2524–2530. doi: 10.4161/cc.6.20.4799. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JG, Liu ZH, Wang LZ, Jiang YD, Wang SR. Dysfunction of endothelial NO system originated from homocysteine-induced aberrant methylation pattern in promoter region of DDAH2 gene. Chin Med J. 2007;23:2132–2137. [PubMed] [Google Scholar]
- 19.Jamaluddin MS, Yang X, Wang H. Hyperhomocysteinemia, DNA methylation and vascular disease. Clin Chem Lab Med. 2007;12:1660–1666. doi: 10.1515/CCLM.2007.350. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo J. Adverse effects of supplemental L-arginine in atherosclerosis: Consequences of methylation stress in a complex catabolism. Arterioscler Thromb Vasc Biol. 2002;23:3–5. doi: 10.1161/01.atv.0000040860.71626.9d. [DOI] [PubMed] [Google Scholar]
- 21.Nappo F, De Rosa N, Marfella R, De Lucia D, Ingrosso D, Perna A, Farzati B, Giugliano D. Impairment of endothelial function by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA. 1999;281(22):2113–2118. doi: 10.1001/jama.281.22.2113. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt RT, Forgione MA, Cap A, Leopold J, Rudd MA, Trolliet M, Heydrick S, Klings E, Moldovan N, Yaghoubi M, Goldschmidt P, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:383–91. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire DM, Gross MD, Van Pilsum JF, Towle HC. Repression of rat kidney L- arginine:glycine amidinotransferase synthesis by creatine at a pretranslational level. J Biol Chem. 1984;259:12034–12038. [PubMed] [Google Scholar]
- 24.Derave W, Marescau B, Eade E, Eijnde B, De Deyn P, Hespel P. Plasma guanidine compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol. 2004;97:852–857. doi: 10.1152/japplphysiol.00206.2004. [DOI] [PubMed] [Google Scholar]
- 25.Taes Y, Delannghe J, De Vriese A, Rombaut R, Camp J, Lameire N. Creatine supplementation decreases homocysteine in an animal model of uremia. Kidney Int. 2003;64:1331–1337. doi: 10.1046/j.1523-1755.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 26.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effect of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab. 2001;281:E1095–E1100. doi: 10.1152/ajpendo.2001.281.5.E1095. [DOI] [PubMed] [Google Scholar]
- 27.Korzun W. Oral creatine supplementation lowers plasma homocysteine concentrations in humans. Clin Lab Sci. 2004;17:102–106. [PubMed] [Google Scholar]
- 28.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 29.Vita JA, Frei B, Holbrook M, Gokce N, Leaf C, Keaney JF., Jr L-2-oxothiazolidine-4-carboxylic acid reverses endothelial dysfunction in patients with coronary artery disease. J Clin Invest. 1998;101:1408–1414. doi: 10.1172/JCI1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodamer OA, Bloesch SM, Gregg AR, Stockler-Ipsiroglu S, O’Brien WE. Analysis of guanidinoacetate and creatine by isotope dilution electrospray tandem mass spectrometry. Clin Chim Acta. 2001;308:173–178. doi: 10.1016/s0009-8981(01)00480-6. [DOI] [PubMed] [Google Scholar]
- 31.Newman DJ, Thakkar H, Edwads RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin c measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:1848–1876. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 32.Larsson A, Malm J, Grubb A, Hansson L-O. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 33.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 34.Clarkson P, Adams M, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clinical Invest. 1996;97:1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- 36.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 37.Joseph J. Hyperhomocysteinemia and cardiovascular disease: new mechanisms beyond atherosclerosis. Metab Syndr Relat Disor. 2003;2:97–104. doi: 10.1089/154041903322294425. [DOI] [PubMed] [Google Scholar]
- 38.Loscalzo J. Homocysteine trials- Clear outcomes for complex reasons. N Engl J Med. 2006;354:1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 39.Katusic Z. Mechanisms of endothelial dysfunction induced by aging: Role of arginase I. Circ Res. 2007;101:640–641. doi: 10.1161/CIRCRESAHA.107.162701. [DOI] [PubMed] [Google Scholar]
- 40.Boger RH. Asymmetric dimethylarginine (ADMA): A novel risk marker in cardiovascular medicine and beyond. Ann Intern Med. 2006;38:126–136. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 41.Kielstein JT, Cooke JP. Should we measure asymmetric dimethylarginine in patients with coronary artery disease? Clin Chem. 2007;53:161–163. doi: 10.1373/clinchem.2006.078881. [DOI] [PubMed] [Google Scholar]
- 42.Zoccali CA, Kielstein JT. Asymmetric dimethylarginine: a new player in the pathogenesis of renal disease? Current Opinion Nephrol Hypertension. 2006;15:314–320. doi: 10.1097/01.mnh.0000222701.22583.e8. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg E, Eknoyan G, Levin N, Eschbach J, Golper T, Owen W, Schwab S. Methods used to evaluate the quality of evidence underlying the National Kidney Foundation-Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: Description, findings, and implications. Am J Kidney Dis. 2000;36:1–11. doi: 10.1053/ajkd.2000.8233. [DOI] [PubMed] [Google Scholar]
- 44.Bennett-Richards KJ, Kattenhorn M, Donald AE, Oakley GR, Varghese Z, Bruckdorfer KR, Deanfield JE, Rees L. Oral L-arginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int. 2002;62:1372–1378. doi: 10.1111/j.1523-1755.2002.kid555.x. [DOI] [PubMed] [Google Scholar]
- 45.Miller HI, Dascalu A, Rassin TA, Wollman Y, Chernichowsky T, Iaina A. Effects of an acute dose of L-arginine during coronary angiography in patients with chronic renal failure. Am J Nephrol. 2003;23:91–95. doi: 10.1159/000068036. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XZ, Ardissino G, Ghio L, Tirelli AS, Dacco V, Colombo D, Pace E, Testa S, Claris-Appiani A. L-arginine supplementation in young renal allograft recipients with chroin renal dysfunction. Clin Nephrol. 2001;55:453–459. [PubMed] [Google Scholar]
- 47.Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH. American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Steenge GR, Verhoef P, Greenhaff P. The effect of creatine and resistance training on plasma homocysteine concentration in healthy volunteers. Arch Intern Med. 2001;161:1455–1456. doi: 10.1001/archinte.161.11.1455. [DOI] [PubMed] [Google Scholar]
- 49.Taes Y, Delanghe J, DeBacquer D, Langlois M, Stevens L, Geerolf I, Lameire N, DeVriese A. Creatine supplementation does not decrease total plasma homocysteine in chronic hemodialysis patients. Kidney Int. 2004;66:2422–2428. doi: 10.1111/j.1523-1755.2004.66019.x. [DOI] [PubMed] [Google Scholar]
- 50.Bostom AG, Bausseman L, Jacques PF, Liaugaudas G, Selhub J, Rosenberg IH. Cystatin C as a determinant of fasting plasma total homocysteine levels in coronary artery disease patients with normal serum creatinine. Arterioscler Thromb Vasc Biol. 1999;19:2241–2244. doi: 10.1161/01.atv.19.9.2241. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira LF, Hueber DM, Lutjemeier BJ, Townsend DK, Barstow TJ. Effects of creatine supplementation on body composition and renal function in rats. Med Sci Sports Exerc. 2005;37:1525–1529. doi: 10.1249/01.mss.0000177555.94271.44. [DOI] [PubMed] [Google Scholar]
- 52.Bostom AG, Gohh RY, Busserman L, Hakas D, Jacques PF, Selhub J, Dworkin L, Rosenberg IH. Serum cystatin C as a determinant of fasting total homocysteine levels in renal transplant recipients with a normal serum creatinine. J Am Soc Nephrol. 1999;10:164–166. doi: 10.1681/ASN.V101164. [DOI] [PubMed] [Google Scholar]