Abstract
Multiple observations in preclinical and clinical studies support a role for the immune system in controlling tumor growth and progression. Various components of the innate and adaptive immune response are able to mediate tumor cell destruction; however, certain immune cell populations can also induce a protumor environment that favors tumor growth and the development of metastasis. Moreover, tumor cells themselves are equipped with various mechanisms that allow them to evade surveillance by the immune system. The goal of cancer vaccines is to induce a tumor-specific immune response that ultimately will reduce tumor burden by tipping the balance from a protumor to an antitumor immune environment. This review discusses common mechanisms that govern immune cell activation and tumor immune escape, and some of the current strategies employed in the field of cancer vaccines aimed at enhancing activation of tumor-specific T-cells with concurrent reduction of immunosuppression.
1. Introduction
The role of the immune system in limiting tumor growth, designated as cancer immunosurveillance [1], has been first elucidated in mouse models of immune deficiency characterized by a high incidence of spontaneous and chemically induced tumors [2]. Those studies have identified several components of the innate and adaptive immune response being responsible for tumor elimination, including αβ and γδ T-cells [3], and NK cells [4]. Reinforcing the role of cytotoxic T lymphocytes (CTLs) in the eradication of malignant cells, transgenic mice deficient in perforin, a component of the cytolytic granules of T and NK cells, are more susceptible to spontaneous and chemically induced tumors than their wild type counterparts [5]. In humans, evidence on the role of the immune system in limiting tumor growth and progression is linked to observations indicating a positive correlation between the presence of tumor infiltrating CD8+ T-cells and good prognosis in various types of cancer. In colorectal cancer, for example, significantly higher levels of early memory and effector memory CD8+ T-cell infiltrates positively correlate with good clinical outcome, defined as absence of metastatic invasion, less advanced pathological stage, and increased survival [6, 7]. Similarly, the presence of intraepithelial tumor infiltrating CD8+ T-cells has been associated with the lack of tumor metastases in the draining lymph nodes of cervical cancer patients [8]. In non-small cell lung carcinoma patients, increasing numbers of tumor infiltrating CD8+, CD20+, and CD4+ T lymphocytes have also been shown to significantly correlate with improved disease-specific survival [9]. Altogether, these observations support a role for the immune system in controlling tumor burden and form the rationale for the development of vaccine-based interventions against cancer that rely on the stimulation of an effective antitumor immune response in the host.
The immune system, however, has two paradoxical roles in cancer. While various components of the innate and adaptive immune response are able to mediate tumor cell destruction, specific types of immune cells can also induce a protumor environment that favors tumor growth and the development of metastasis [10]. Among the latter are, for example, regulatory T (Treg) cells [11, 12], tumor associated macrophages (TAM) [13, 14], and type 2 helper CD4+ (Th2) T-cells [15, 16]. These various immune cells have been shown to accumulate at the site of the tumor, negatively impacting the establishment of antitumor T-cell responses, that is, creating an immunosuppressive tumor environment.
Cancer cells themselves are also equipped with mechanisms that allow them to evade recognition by the immune system or to negatively affect the functionality of effector T-cells. In order to avoid immune recognition, tumor cells have been shown to downregulate antigen expression, components of the antigen-processing and presentation machinery, and expression of major histocompatibility complex (MHC) molecules [17]. Decreased expression of costimulatory molecules of crucial importance to T-cell activation, and enhanced surface expression of molecules that negatively regulate activation of T-cells, such as PD-L1/B7-H1 and B7-H4, have also been demonstrated in various types of tumors [18–20]. Cancer cells can also restrain the function of the immune system by secreting a milieu of soluble factors that ultimately inhibit the activation, proliferation, and differentiation of the various components of the immune response. Among these molecules are TGF-β [21], IL-10 [22], IL-13 [23], and VEGF [24].
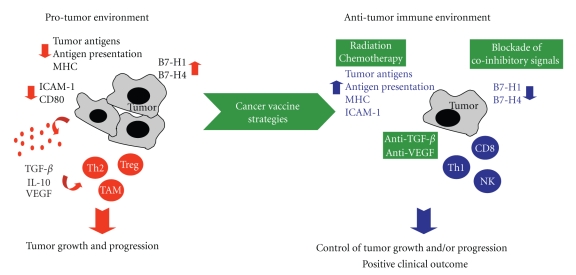
The goal of vaccine-based cancer immunotherapy approaches is to induce a tumor-specific immune response that ultimately will reduce tumor burden by tipping the balance from a protumor to an antitumor immune environment (Figure 1). This review discusses strategies employed in the field of cancer vaccines aimed at enhancing activation of tumor-specific T-cells with concurrent reduction of immunosuppression. Specifically, vaccine design, immune adjuvants, and multimodal approaches using vaccines in combination with other treatment modalities will be discussed here, with a particular emphasis on studies conducted at the National Cancer Institute, NIH.
Figure 1.
Cancer vaccine strategies aimed at shifting the immune environment of a tumor from protumorigenic to antitumorigenic.
2. Vaccine Design: Choice of Vaccine-Delivery System(s)
Depending on the vaccine-delivery system of choice, cancer vaccines can elicit an immune response against an individual or multiple tumor antigens. A list of the various types of vaccine-delivery systems under investigation in the field, either at the preclinical or clinical stages, is presented in Table 1. Multiple studies have demonstrated that combinations of some of the strategies in the form of diversified prime/boost regimens may enhance the outcome of the intervention against the tumor [25]. Moreover, it has also been shown that concurrent vaccination with two distinct vaccine platforms targeting the same antigen can elicit a more diverse population of antigen-specific T-cells thus resulting in higher antitumor immunity [26].
Table 1.
Vaccine-delivery systems.
| Vaccine-delivery systems | |
|---|---|
| Immunization against multiple antigens* | Immunization against specified antigen(s) |
| Cell-based | Cell-based |
| Autologous whole-tumor cells | DCs pulsed with peptide |
| Allogeneic whole-tumor cells | Genetically-modified DCs |
| Genetically-modified tumor cells | |
| DCs-tumor fusion | Protein/Peptide based |
| DCs loaded with tumor lysate | Protein Peptide Agonist peptide Antiidiotype MAb Mab fusion proteins |
| DCs transfected with tumor-derived RNA | |
| Protein/Peptide based | |
| Tumor lysates | Vector-based |
| Heat shock proteins-tumor peptides | Plasmid DNA |
| Bacterial vectors | |
| Listeria | |
| Salmonella | |
| Yeast vectors | |
| Viral vectors | |
| Adenovirus | |
| Vaccinia | |
| Avipox (fowlpox) | |
| MVA | |
*Vaccine formulation includes known and unknown antigens.
Among the various types of vaccine delivery systems, there are strategies based on the use of whole tumor cells, dendritic cells-(DCs-) tumor cell fusions, or preparations of DCs loaded with tumor protein lysates or tumor-derived RNA (Table 1, left column). These vaccine platforms induce an immune response against multiple tumor targets, either known or unknown. Other vaccine modalities (Table 1, right column) are based on the previous characterization of tumor antigens to be used as “specified” targets in the vaccine formulation.
3. Tumor Antigens
Tumor antigens are molecules either exclusively expressed in the tumor cells, designated as “tumor-specific antigens”, or molecules that are overexpressed in cancerous versus normal tissues, designated as “tumor-associated antigens”. A comprehensive list of tumor antigens and their corresponding T-cell epitopes can be found at [27, 28]. Table 2 shows a brief list of selected examples for either type of antigen. Tumor-specific antigens appear de novo after cancer cells acquire mutations within the coding regions of certain genes, for example the oncogene ras [29] and the tumor suppressor p53 [30], or novel fusion proteins are generated as in the case of the Bcr-Abl fusion in chronic myeloid leukemia [31]. Moreover, in tumors driven by infectious agents like human papillomavirus (HPV) or Epstein-Barr virus (EBV), virally-derived products become de novo targets of T-cell immune responses directed against the tumor. The majority of tumor-associated antigens so far identified have a certain level of expression in normal tissues and thus tolerance to these antigens often exists.
Table 2.
Human carcinoma antigens.
| Carcinoma antigens* | |
|---|---|
| Tumor-specific antigens | Tumor-associated antigens |
| Mutated molecules | Carcinoembryonic antigen (CEA) |
| K-RAS | Mucin 1 (MUC-1) |
| p53 | Prostate-specific antigen (PSA) |
| Fusion molecules | Prostate acid phosphatase (PAP) |
| BCR-ABL | Prostate stem-cell antigen (PSCA) |
| Brachyury | |
| Virally-derived molecules | TERT |
| HPV-16 E6, E7 | Wilm's tumor 1 (WT1) |
| EBNA1, LMP1 and LMP2 | Her-2/neu |
| Sox-2 | |
| NY-ESO-1 | |
| Cyclin D1 | |
| Mesothelin | |
| Survivin | |
*Included is only a partial list of antigens for human carcinomas.
A strategy used by tumors to escape immune recognition and destruction is the complete or partial loss of an antigen(s) [17]. Both in experimental animal models [32, 33], and in human cancer [34], it has been shown that “antigen-negative tumor variants”, characterized by the loss of the targeted antigen, can emerge subsequently to an immune intervention. An approach to overcome this problem is the targeting of “functionally relevant antigens”, which are proteins with an essential role during tumor initiation, growth, survival, or metastasis [35]. It can be hypothesized that an immune intervention directed against a functioning tumor antigen would greatly reduce the emergence of antigen-negative variants, since cells that lose the antigen will fail to grow, survive, or metastasize.
4. Targeting of Molecules that Control Metastatic Dissemination
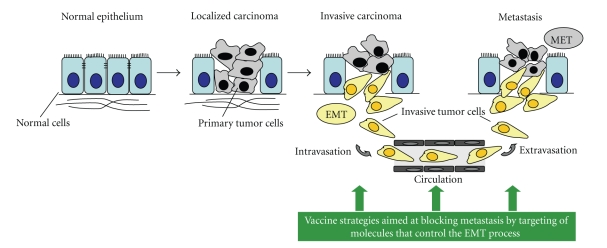
In order to achieve the various steps along the metastatic cascade, epithelial tumor cells may need to undergo a phenotypic conversion into mesenchymal cells via a process designated as epithelial-to-mesenchymal transition (EMT) [36]. The EMT program involves the downregulation of epithelial proteins such as E-cadherin and cytokeratins, and the upregulation of mesenchymal proteins including Fibronectin, N-cadherin, and Vimentin. Various genes normally expressed in the early embryo have been implicated in the control of the EMT triggered during tumor progression, including Twist, Snail, Slug, Goosecoid, and SIP1 [36, 37]. The transcription factors encoded by these genes can impart to tumor cells the traits of mesenchymal cells, including motility and the ability to invade the extracellular matrix (ECM). The expression of Twist, for example, has been found to be elevated in various types of cancer, including breast, prostate, and cervical cancer, with higher levels of Twist protein being detected in prostate cancer tissues of high Gleason score [38, 39]. Since the EMT process appears to be a necessary step for tumor cells to initiate the metastatic cascade [37, 40], interfering with EMT in early stages of the disease is likely to prevent tumor cell spreading and might also be effective in treating established metastatic lesions (Figure 2). An example of a tumor antigen with a functionally relevant role in the EMT program is the T-box transcription factor Brachyury, highly expressed in various human tumors of epithelial origin, but not in most human normal adult tissues [41]. It has been recently demonstrated that Brachyury overexpression in epithelial tumor cells induces an EMT, promoting the expression of mesenchymal markers and downregulation of epithelial markers, with concomitant increase in tumor cell migration and invasion [42]. Additionally, stable silencing of Brachyury expression in Brachyury-positive human carcinoma cells has been shown to downregulate mesenchymal markers and upregulate epithelial markers with simultaneous loss of cell migration and ECM invasion. In vivo, Brachyury-inhibited human tumor cells had a decreased ability to form experimental lung metastases after intravenous injection, as well as to disseminate from the primary, subcutaneous tumor to the site of metastases [42]. A CD8 T-cell epitope of Brachyury capable of expanding Brachyury-specific T-cells from the peripheral blood of cancer patients was recently identified [41]; Brachyury-specific T-cells have been used to efficiently lyse Brachyury-positive tumor cells in vitro. The successful expansion of T-cells directed against the transcription factor Brachyury exemplifies the ability of T-cell mediated immunotherapies to target (a) highly conserved tumor proteins, and (b) tumor proteins irrespective of their cellular localization. Because of its relevant role during tumor progression, Brachyury is an appealing tumor antigen for interventions aimed at interfering with the metastatic spreading of tumor cells (Figure 2). Additionally, the transcription factors Twist, Snail, and Slug, among others, which are critically involved in the control of EMT during tumor progression, could also be explored as novel tumor antigens for the targeting of metastatic disease.
Figure 2.
The epithelial-to-mesenchymal transition (EMT) in tumor progression: an opportunity to target metastatic tumor cells. The epithelial-to-mesenchymal transition (EMT) and its reverse process, designated as mesenchymal-to-epithelial transition (MET), are involved in the progression of epithelial tumors towards metastasis. Vaccine strategies targeting molecules that control the EMT process, for example, the transcription factor Brachyury, could be used to block tumor spreading.
5. Antigen Cascade
A phenomenon observed with cancer vaccines is the induction of immune responses to tumor antigens that are not present in the vaccine formulation. For example, it has been shown that CEA-transgenic mice cured of CEA-positive tumors by a CEA/TRICOM vaccine regimen were able to subsequently reject CEA-positive as well as CEA-negative tumors, and that this effect was mediated by the generation of specific T-cell immune responses directed against gp70, an antigen expressed by the tumor but not present in the vaccine [43]. The same phenomenon has also been reported in clinical studies [44, 45]. Altogether these studies showed that the immune response initiated against a tumor antigen included in the vaccine formulation is then followed by cross-priming and initiation of an “antigen cascade” that expands the immune response to additional antigens expressed on the tumor, thus potentiating antitumor immunity.
6. Enhancing Activation of Tumor-Specific T-Cells: The Use of Costimulation in the Vaccine Formulation
Optimal activation of T-cells is known to require at least two signals. The first signal is mediated by the interaction between the peptide/MHC complex on the surface of antigen presenting cells (APC) and the T-cell receptor (TCR) on the surface of T-cells. The second signal, also designated as costimulation, is mediated by the interaction between accessory molecules located on the surface of the APC and their corresponding ligand(s) on the T-cells [46]. Activation via the TCR in the presence of adequate costimulatory signals results in the clonal expansion, differentiation, and expression of effector functions by antigen-specific T-cells. Optimization of the mechanism of T-cell activation is critical to achieve a successful immune response to an antigen included in a vaccine formulation. A list of various strategies being explored in the field of cancer vaccines to achieve enhanced antitumor immune responses is presented in Table 3. One of them is the delivery of a single or multiple costimulatory molecules along with the tumor antigen as part of the vaccine formulation. One of the most studied T-cell costimulatory molecules is B7-1 [47, 48]. In preclinical studies it has been shown that mice immunized with an admixture of recombinant vaccinia (rV-) viruses encoding for a tumor antigen (rV-CEA or rV-MUC-1) and the costimulatory molecule B7-1 (rV-B7-1) generate effective antigen-specific T-cell immune responses that translate into successful antitumor immunity [49]. Furthermore, it was demonstrated that combinations of various costimulatory molecules act synergistically to further enhance antigen-specific T-cell responses. Using recombinant vaccinia and fowlpox viruses encoding for the tumor antigen CEA and three different costimulatory molecules (B7-1, ICAM-1, LFA-3, designated as TRICOM), T-cell responses against the tumor antigen were further enhanced above the level observed when only one costimulatory molecule was used [50]. In preclinical studies with human T-cells in vitro, enhanced activation of antigen-specific T-cells was observed against DCs modified by infection with rF-TRICOM [51]. Moreover, the same vector was successfully used to enhance the antigen-presentation potency of freshly isolated B cells, resulting in enhanced activation of antigen-specific T-cell responses in vitro [52]. Results from a Phase II randomized clinical trial in 125 metastatic prostate cancer patients were recently reported [53]; patients were randomized to receive either a vaccine regimen consisting of two recombinant viral vectors each encoding for prostate specific antigen (PSA) and the TRICOM molecules (rV-PSA/TRICOM and rF-PSA/TRICOM), or control empty vector (control arm). The results from this trial demonstrated a 44% reduction in the death rate and an 8.5 month improvement in median overall survival (OS) in men in the vaccine compared to the control arm [53]. Additionally, NCI also reported the results from a randomized Phase II trial in 32 patients with metastatic castrate-resistant prostate cancer, who received a prime with rV-PSA/TRICOM and booster vaccinations with rF-PSA/TRICOM. Twelve of 32 patients showed declines in serum PSA post-vaccination; patients with greater PSA-specific T-cell responses showed a trend toward enhanced survival. In general, there was evidence of enhanced median overall survival, particularly among patients with more indolent type of disease [54]. Overall, cancer vaccine strategies incorporating costimulatory molecules have demonstrated the generation of antitumor immunity and evidence of clinical benefit in cancer patients.
Table 3.
Strategies to enhance antitumor T-cell responses*.
| Strategy |
|---|
| Use of costimulation in vaccine formulation |
| Cytokines |
| Effect on APC (GM-CSF) |
| Effect on T-cells (IL-2, IL-7, IL-15, IL-12) |
| Radiation |
| Chemotherapy |
| Small molecule targeted therapies |
| Inhibition of coinhibitory signals |
| At the tumor site (B7-H1, B7-H4) |
| Directly on T-cells (CTLA-4) |
| Depletion/inhibition of Treg cells |
| Inhibition of immunosuppressive cytokines |
| AntiTGF-β |
| AntiVEGF |
* Only a partial list is included here.
7. The Use of Cytokines as Vaccine Adjuvants
7.1. Cytokines that Affect the APCs
Biological adjuvants are agents generally used for improving the immunogenicity of an antigen in a vaccine formulation. Several cytokines have the ability to enhance immune responses by either (a) promoting the differentiation, activation, or recruitment of APC, therefore enhancing antigen presentation and activation of antigen-specific T-cell responses, or (b) by directly acting on various subsets of T-cells. Within the first group, one of the most studied cytokines is the granulocyte-macrophage colony-stimulating factor (GM-CSF). It has been demonstrated in preclinical studies that subcutaneous injections of GM-CSF at the vaccination site can significantly increase the infiltration of DCs in regional lymph nodes that drain the site of vaccination [55, 56]. In several preclinical studies, tumor cells or DCs genetically engineered to secrete biologically active GM-CSF have been used to generate a systemic antitumor immune response [57, 58]. The use of GM-CSF at high doses, however, could be detrimental in the context of vaccines since it may result in immune suppression via the activation and expansion of myeloid-derived suppressor cells [59]. In the clinical setting, two placebo-controlled Phase III trials in patients with hormone-refractory prostate cancer, for example, have been performed with an autologous DC-based vaccine, designated Sipuleucel-T, which is genetically modified to express prostatic acid phosphatase (PAP) as a tumor antigen, and GM-CSF. Results from the initial trial with 127 patients [60] demonstrated a 4.5-month improvement in median survival in the vaccine versus placebo group, though without meeting the primary endpoint of time to progression. Results from a subsequent Phase III, placebo-controlled trial measuring overall survival as the primary endpoint have been recently reported [61], indicating a statistically significant survival advantage in patients in the vaccine versus placebo group.
7.2. Cytokines that Affect the T-Cell Compartment
The second group includes cytokines that directly affect the T-cell compartment by promoting T-cell proliferation, activation, and effector function. Among these, the cytokines IL-2 [62, 63], IL-7 [64], IL-15 [65, 66], and IL-12 [67] are currently under investigation to enhance antitumor immune responses elicited by a vaccine. The most used of these cytokines is IL-2, a T-cell growth factor which, as a single agent, has demonstrated clinical responses in patients with metastatic renal cell carcinoma [68] and metastatic melanoma [69]. A disadvantage, however, in the use of IL-2 therapy in vivo, particularly with high-dose IL-2, is the rate of associated toxicities [70]. Moreover, IL-2 mediates not only the proliferation of activated, effector T-cells and NK cells, but also the development and homeostasis of regulatory T-cells (Tregs), which constitutively express elevated levels of the IL-2R alpha (CD25) [71]. In vitro, IL-2 has also been used to expand tumor-specific T-cells to be used for adoptive immunotherapy [72].
The cytokines IL-15 and IL-7 are also T-cell growth factors; their function, however, is different to that of IL-2 in vivo. IL-15 is necessary for the development and homeostasis of memory CD8 T-cells and NK cells [65]. It has also been demonstrated a role for IL-15 in the induction of long-lived, high avidity CD8 T-cells [73, 74] and, unlike IL-2, there is no role for IL-15 on the proliferation of Tregs. Up to date, studies with IL-15 as an adjuvant for cancer vaccine strategies have only been conducted in preclinical models, with encouraging results [67]. The cytokine IL-7, another T-cell growth factor, targets a different population of T-cells, promoting the expansion of naïve T-cells and thus increasing the diversity of T-cell repertoire after lymphopenia [64, 65]. IL-7 has also demonstrated positive results as an adjuvant cytokine for cancer vaccine interventions in preclinical studies [67].
Another cytokine that is under investigation as a vaccine adjuvant is IL-12, which promotes Th1 polarization, proliferation of activated T-cells and NK cells, and cell-mediated immunity. IL-12 has been shown to have potent antitumor effects in preclinical models [75]. In humans, however, the systemic delivery of IL-12 has resulted in elevated toxicities [76], hence leading towards the investigation of alternative modes for local delivery of IL-12. For example, in preclinical studies, a coformulation of IL-12 with chitosan intravesically delivered was well tolerated and very efficient at curing mice with superficial bladder cancer. A durable antitumor immune response was also generated in mice receiving IL-12/chitosan, providing them with complete protection from intravesical tumor rechallenge [77]. Overall, the use of cytokines as vaccine adjuvants to enhance the immune response to a tumor is a very promising and active field of investigation. Current research is focused on understanding the proper ways of delivery for each particular cytokine in order to maximize the immune adjuvant effects while reducing potential toxicities, when used in combination with various types of vaccine platforms.
8. Vaccine Plus Radiation
As it was mentioned above, a mechanism by which tumor cells escape immune recognition and attack is through the downregulation of tumor antigens, MHC expression, or various components of the antigen processing/presentation machinery. A strategy to overcome these obstacles is the use of radiation on tumor cells. Radiation is the standard of care for many types of cancer because of its direct cytotoxic effect on the tumor or its palliative effects on the patient. It has been recently reported that local irradiation of tumors with doses insufficient to induce tumor cell death could result in changes on the phenotype of the tumor cells that include the upregulation of MHC, Fas, ICAM-1, and various tumor associated antigens [78–80]. As a result of these changes, irradiated tumor cells are more susceptible to T-cell mediated immune attack. In preclinical studies with a murine colon carcinoma tumor model, sublethal, local tumor irradiation significantly improved the therapeutic efficacy of a recombinant rV-/rF-CEA/TRICOM vaccine regimen against CEA-positive tumors in CEA-transgenic mice [81], while radiation alone or vaccine alone had no effect on tumor growth. In the clinical setting, the approach has been investigated in a phase II clinical trial in patients with localized prostate cancer, randomized to receive a PSA-based poxviral vaccine plus radiotherapy versus radiotherapy alone [82]. The results from this trial indicated increases in PSA-specific T-cell responses of at least 3-fold in patients in the combination arm; the authors also reported evidence of de novo generation of T-cells to well-described prostate-associated antigens not found in the vaccine, providing indirect evidence of immune-mediated tumor killing. These studies thus demonstrated a new paradigm for the use of local tumor irradiation in combination with active vaccine therapy to elicit an effective antitumor immune response.
9. Vaccines Plus Cytotoxic Drugs
Because of the widespread use of chemotherapy for the treatment of most malignancies, it is rational to design combinatorial approaches using vaccines plus standard chemotherapeutic agents. Like radiotherapy, the use of various types of chemotherapy in combination with vaccines has resulted in enhanced antitumor immune responses. Although the mechanisms involved vary among the various types of cytotoxic drugs employed, in general, drugs can: (a) induce “immunogenic death” of tumor cells, leading to activation of DCs followed by antigen presentation to T-cells [83, 84], or (b) modulate the phenotype of the tumor cells making them more susceptible to immune-mediated killing. For example, it has been shown that treatment of human colon carcinoma cell lines with 5-fluorouracil or cisplatin enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes, by inducing expression of ICAM-1 and Fas [85]. Similarly, treatment of renal cell carcinoma cells with subtoxic concentrations of adriamycin has been shown to upregulate the expression of ICAM-1 and LFA-3, as well as to enhance T-cell mediated killing, Fas-mediated, and TRAIL-mediated killing of tumor cells [86]. Taxanes, on the other hand, are a widely used type of chemotherapeutic agents known to have various effects on the immune system, promoting macrophage activation and release of inflammatory cytokines at the tumor site, thus enhancing tumor lysis [87]. In preclinical studies with CEA-transgenic mice transplanted with CEA-positive tumor cells, enhanced antitumor effect was achieved by using a combination of a rV-/rF-CEA/TRICOM vaccine regimen plus docetaxel, compared to that of vaccine or docetaxel alone [88]. In preclinical models as well, cyclophosphamide, doxorubicin, and paclitaxel have all been shown to enhance the antitumor immune response elicited with a GM-CSF-secreting, HER2/neu-expressing whole-tumor cell vaccine in tumor bearing neu-transgenic mice [89]. The authors were able to demonstrate that the increased antitumor effects in the combination group (vaccine plus drugs) were due to enhanced vaccine efficacy rather than a direct cytolytic effect of the drugs on cancer cells [89].
Altogether, these studies demonstrated that, if used in appropriate schedule and at the correct doses, chemotherapeutic agents could enhance antitumor responses when used in combination with cancer vaccine modalities. Therefore, further studies investigating optimum schedules and dosing for various chemotherapeutic agents are needed in order to optimize the use of different cytotoxic agents in combination with cancer vaccines.
10. Vaccine Plus Small Molecule Targeted Therapies
As the molecular pathways involved in the various steps of carcinogenesis and tumor progression are being elucidated with the advent of sophisticated genetic and molecular techniques, a novel group of therapeutic cancer drugs aimed at inhibiting specific molecular pathways is emerging, designated as small molecule targeted therapies. These drugs are also now being investigated for their immune-modulatory functions to be potentially used in combination with cancer vaccines. For example, the anticancer agent lenalidomide (Revlimid, Celgene Corp., NJ, USA), which is FDA approved for the treatment of patients with multiple myeloma, has been shown to have several immune-modulatory effects that include costimulatory effects on CD3-activated T-cells, augmentation of NK cell cytotoxicity, and suppression of Treg cells proliferation and function [90, 91]. Results from a study combining a small molecule BCL-2 inhibitor and a rV-/rF-CEA/TRICOM vaccine regimen were recently reported [92]. It has been shown that, when administered after the vaccine, the BCL-2 inhibitor GX15-070 was able to increase the intratumoral ratio of activated, CD8+ T effector to Treg cells, thus resulting in significant reduction of pulmonary tumor nodules in a mouse model of experimental lung tumors [92]. As with chemotherapeutic agents, the use of small molecule targeted therapies could also be associated with potential toxicities and, in particular, with negative effects on the immune compartment. Thus, further studies investigating optimum schedules and dosing for various small molecule targeted drugs are needed in order to optimize their use in combination with cancer vaccines.
11. Inhibition of Coinhibitory Signals
An alternative strategy to enhance the outcome of an antitumor immune approach is to eliminate negative signals imparted to T-cells by coinhibitory molecules such as B7-H1, B7-H4, and cytotoxic T-lymphocyte antigen-4 (CTLA-4), among others. B7-H1 is constitutively expressed in many types of human tumors and has been shown to promote evasion of tumor immunity by promoting apoptosis of activated effector T-cells [18] and tumor resistance to T-cell mediated lysis [93]. In preclinical studies, blockade of B7-H1 with a specific monoclonal antibody has resulted in enhanced antitumor immune responses [93, 94]. B7-H4 is another member of the B7 family that has been implicated as a negative regulator of T-cell immunity [95]. It has been demonstrated that B7-H4 can inhibit T-cell proliferation and IL-2 production, and that blockade of B7-H4 in preclinical animal models results in enhanced cytotoxic T-cell responses against an alloantigen [19]. The expression of B7-H4 has been observed in many types of human cancer, such as breast [96], ovary [97], and lung [98]. In renal cell carcinoma, its expression has been correlated with more aggressive tumors, particularly in those cases where both B7-H1 and B7-H4 are aberrantly overexpressed [99]. Therefore, blocking of molecules such as B7-H1 and B7-H4 expressed on tumor cells can reduce coinhibitory signals directly at the site of the tumor (Table 3), resulting in enhanced antitumor immune responses.
A different strategy involves blocking of inhibitory molecules directly expressed on T-cells. An example is CTLA-4, a negative regulator of T-cell activation, which is expressed on the T-cells and, like its homolog CD28, binds to B7 on the surface of the APC. Binding of CTLA-4 to B7 initiates a negative signal cascade that leads to downregulation of the T-cell response [100, 101]. The blockade of CTLA-4 with specific, monoclonal antibodies (mAb) has been explored as a monotherapy or in combination with vaccine therapy in preclinical and clinical studies. In preclinical studies, the use of antiCTLA-4 mAb as a monotherapy has shown antitumor activity with immunogenic tumors [100, 101], but not with poorly immunogenic tumors, such as MC38 [102]. In combination with vaccines, antiCTLA-4 mAb has been used to enhance antitumor T-cell responses elicited by the vaccine, thus resulting in effective antitumor effects [102]. In the clinic, antiCTLA-4 has been used also as a monotherapy or in combination with other immune-mediated anticancer modalities [103]. Results from a phase I clinical trial with antiCTLA-4 mAb (ipilimumab) in patients with metastatic melanoma or renal cell cancer recently reported a 14% response rate and multiple, and at times severe, immune-mediated toxicities such as nephritis, panhypophysitis, and enterocolitis, among others [104, 105], this last point constituting a potential disadvantage of the approach.
12. Depletion/Inhibition of Regulatory T-Cells
Naturally occurring regulatory T (Treg) cells, characterized by expression of IL-2R alpha, the transcription factor Foxp3, CTLA-4, and glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), constitute 5% to 10% of peripheral CD4+ T-cells [11, 106]. Treg cells represent an important mechanism of peripheral T-cell tolerance through their inhibition of self-reactive effector T-cells [107], and have also been implicated in the lack of effective antitumor immunity as the number of Treg cells are increased in the tumors and peripheral blood of cancer patients [108, 109]. Previous studies in several models of mouse tumors have demonstrated that deletion of Treg cells by using an antiCD25 mAb enhanced the development of antitumor immunity leading to tumor rejection [110, 111]. Furthermore, it has been previously shown that antigen-specific T-cell responses induced by poxviral vaccines can be augmented by simultaneous administration of antiCD25 mAb in mice [112]. Among alternative modalities that can be used in humans to delete Treg cells is denileukin diftitox, (DAB389IL-2), a fusion protein of IL-2 and diphtheria toxin, previously shown in mouse preclinical studies to reduce Treg cells and to enhance antigen-specific immune responses induced by poxviral vaccines [113]. A potential drawback of both approaches is that activated, effector T-cells also transiently upregulate the expression of CD25 on their cell surface, therefore being at risk of depletion by strategies that target CD25. In the clinic, denileukin diftitox was previously shown to reduce Treg cells and to lead to objective clinical responses in patients with ovarian cancer [12, 114]. Moreover, antitumor immunotherapy approaches combining denileukin diftitox-mediated deletion of Treg cells followed by vaccination with RNA-transfected DCs, tumor antigen peptides, or DCs modified by infection with rF-CEA(6D)/TRICOM, have been shown to improve tumor-specific T-cell responses in patients with renal cell cancer, melanoma, or CEA-positive malignancies, respectively, [115–117].
13. Inhibition of Immunosuppressive Cytokines
Tumors can also evade immunosurveillance by directly secreting a number of inhibitory cytokines or by inducing various types of immune cells to secrete cytokines associated with reduced immune responses. One of these cytokines is the transforming growth factor beta (TGF-β), which can be directly secreted by many types of tumor cells, including breast, prostate, colon, liver, lung, and melanomas, among others [118, 119]. TGF-β exerts its negative effects on T-cells, NK cells, macrophages and DCs [120]. Several preclinical studies have demonstrated that blockade of TGF-β can reverse the immunosuppressive effects of the tumor microenvironment. For example, a small molecule TGF-β inhibitor was used in a mouse tumor model to rescue the functionality of infiltrating CD8+ T-cells (TILs), which are usually hyporesponsive [121]. Recently, it was also reported a synergistic improvement of a peptide vaccine modality in combination with a monoclonal antibody against TGF-β, in a murine tumor model [122]. Although no adverse effects have been observed in studies so far conducted, the dual role of TGF-β on normal versus tumor cells, where it can function as a suppressor or promoter of tumor development, respectively, may constitute a potential problem for this approach and indicates the necessity for detailed studies aimed at optimizing the use of antiTGF-β reagents while minimizing the potential for adverse effects.
Another cytokine that has a negative impact on the development of antitumor T-cell responses is the vascular-endothelial growth factor (VEGF). A major role of VEGF is to induce the process designated as tumor angiogenesis, which involves the development of an adequate tumor vasculature that will support a blood supply to the growing tumor [123, 124]. Additionally, VEGF contributes to tumor immune escape by inducing the development of immune cell populations with immunosuppressive functions, like immature DCs [24] and the recruitment of tumor-associated macrophages (TAM) to the tumor stroma [13, 14, 125]. Strategies aimed at inhibiting VEGF or its receptors, therefore, will not only disrupt the tumor vasculature thus impairing tumor growth, but will also improve antitumor immunity by eliminating inhibitory cell populations, resulting in enhanced responses to cancer vaccines. In preclinical studies, for example, an antiVEGF antibody enhanced the efficacy of a peptide-pulsed DC-based vaccine that resulted in prolonged and pronounced antitumor effect [126]. Therefore, inhibition of VEGF may be a valuable adjuvant in the immunotherapy of cancer. A disadvantage of antiVEGF therapies in the clinic has been the emergence of toxicities that included wound healing complications as well as adverse vascular effects.
14. Conclusions
Progress in understanding the molecular mechanisms that govern immune activation as well as the mechanisms used by tumor cells to evade surveillance by the immune system are advancing the development of immune-mediated therapies that could be effectively used against a range of human cancers. The combination of cancer vaccines with other therapeutical modalities, in particular established therapies such as radiation and chemotherapy, as well as small molecule targeted therapies, provides an opportunity to further improve the outcome of vaccine interventions against cancer. Moreover, several studies also indicated that patients who receive a cancer vaccine have an enhanced outcome to subsequent therapies, thus providing another possible approach for the use of cancer vaccines prior to other cancer interventions. A prospective randomized trial is being initiated to substantiate these findings. Unlike other modalities, cancer vaccines have so far demonstrated no associated toxicities and therefore their use could not only result in improved patient survival but also in improvements in quality of life.
Acknowledgment
The authors thank Debra Weingarten for editorial assistance in the preparation of this manuscript.
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V, Ikeda H, Bruce AT, et al. IFNγ, and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Girardi M, Glusac E, Filler RB, et al. The distinct contributions of murine T cell receptor (TCR)γδ+ and TCRαβ+ T cells to different stages of chemically induced skin cancer. Journal of Experimental Medicine. 2003;198(5):747–755. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth MJ, Thia KYT, Street SEA, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. Journal of Experimental Medicine. 2000;191(4):661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Broek ME, Kagi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. Journal of Experimental Medicine. 1996;184(5):1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England Journal of Medicine. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 7.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Piersma SJ, Jordanova ES, van Poelgeest MIE, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Research. 2007;67(1):354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 9.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clinical Cancer Research. 2008;14(16):5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer and Metastasis Reviews. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Seminars in Cancer Biology. 2006;16(2):115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. Journal of Clinical Investigation. 2006;116(8):2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeNardo DG, Barreto JB, Andreu P, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler A, Heidenreich R, Braumuller H, et al. EpCAM, a human tumor-associated antigen promotes Th2 development and tumor immune evasion. Blood. 2009;113(15):3494–3502. doi: 10.1182/blood-2008-08-175109. [DOI] [PubMed] [Google Scholar]
- 17.Marincola FM, Jaffee EM, Hickljn DJ, Ferrone S. Escape of human solid tumors from t-cell recognition: molecular mechanisms and functional significance. Advances in Immunology. 2000;(74):181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Medicine. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 19.Sica GL, Choi I-H, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 20.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunological Reviews. 2009;229(1):126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas DA, Massague J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Kurte M, Lopez M, Aguirre A, et al. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and transporter associated with antigen processing 1/2 in human melanoma cells. Journal of Immunology. 2004;173(3):1731–1737. doi: 10.4049/jimmunol.173.3.1731. [DOI] [PubMed] [Google Scholar]
- 23.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunology. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Medicine. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. Journal of Clinical Oncology. 2000;18(23):3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 26.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunology, Immunotherapy. 2010;59(3):397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clinical Cancer Research. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Bruggen P, Stroobant V, van Pel A, van den Eynde B. T-cell defined tumor antigens. http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm. [DOI] [PubMed]
- 29.Abrams SI, Stanziale SF, Lunin SD, Zaremba S, Schlom J. Identification of overlapping epitopes in mutant ras oncogene peptides that activate CD4+ and CD8+ T cell responses. European Journal of Immunology. 1996;26(2):435–443. doi: 10.1002/eji.1830260225. [DOI] [PubMed] [Google Scholar]
- 30.Offringa R, Vierboom MPM, van der Burg SH, Erdile L, Melief CJM. p53: a potential target antigen for immunotherapy of cancer. Annals of the New York Academy of Sciences. 2000;910:223–236. doi: 10.1111/j.1749-6632.2000.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 31.Kessler JH, Bres-Vloemans SA, van Veelen PA, et al. BCR-ABL fusion regions as a source of multiple leukemia-specific CD8+ T-cell epitopes. Leukemia. 2006;20(10):1738–1750. doi: 10.1038/sj.leu.2404354. [DOI] [PubMed] [Google Scholar]
- 32.Knutson KL, Lu H, Stone B, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. Journal of Immunology. 2006;177(3):1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 33.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. European Journal of Immunology. 2007;37(3):675–685. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. International Journal of Cancer. 1996;66(4):470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Hirohashi Y, Torigoe T, Inoda S, et al. The functioning antigens: beyond just as the immunological targets. Cancer Science. 2009;100(5):798–806. doi: 10.1111/j.1349-7006.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Reviews Molecular Cell Biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 37.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Kwok WK, Ling M-T, Lee T-W, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Research. 2005;65(12):5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 40.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell - mediated cancer immunotherapy. Clinical Cancer Research. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 42.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. Journal of Clinical Investigation. 2010;120(2):533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clinical Cancer Research. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield LH, Ribas A, Dissette VB, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clinical Cancer Research. 2003;9(3):998–1008. [PubMed] [Google Scholar]
- 45.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clinical Cancer Research. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 47.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 48.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clinical Cancer Research. 2007;13(18, part 1):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 49.Hodge JW, McLaughlin JP, Abrams SI, Shupert WL, Schlom J, Kantor JA. Admixture of a recombinant vaccinia virus containing the gene for the costimulatory molecule B7 and a recombinant vaccinia virus containing a tumor-associated antigen gene results in enhanced specific T-Cell responses and antitumor immunity. Cancer Research. 1995;55(16):3598–3603. [PubMed] [Google Scholar]
- 50.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MGO, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Research. 1999;59(22):5800–5807. [PubMed] [Google Scholar]
- 51.Zhu M, Terasawa H, Gulley J, et al. Enhanced activation of human T cells via avipox vector-mediated hyperexpression of a triad of costimulatory molecules in human dendritic cells. Cancer Research. 2001;61(9):3725–3734. [PubMed] [Google Scholar]
- 52.Palena C, Zhu M, Schlom J, Tsang K-Y. Human B cells that hyperexpress a triad of costimulatory molecules via avipox-vector infection: an alternative source of efficient antigen-presenting cells. Blood. 2004;104(1):192–199. doi: 10.1182/blood-2003-09-3211. [DOI] [PubMed] [Google Scholar]
- 53.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival (OS) analysis of a phase II randomized controlled trial (RCT) of a poxviral-based PSA targeted immunotherapy in metastatic castration-resistant prostate cancer (mCRPC) Journal of Clinical Oncology. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunology, Immunotherapy. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kass E, Panicali DL, Mazzara G, Seldom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Research. 2001;61(1):206–214. [PubMed] [Google Scholar]
- 56.Kass E, Parker J, Schlom J, Greiner JW. Comparative studies of the effects of recombinant GM-CSF and GM-CSF administered via a poxvirus to enhance the concentration of antigen- presenting cells in regional lymph nodes. Cytokine. 2000;12(7):960–971. doi: 10.1006/cyto.2000.0684. [DOI] [PubMed] [Google Scholar]
- 57.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22(20):3188–3192. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- 59.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Annals of Oncology. 2007;18(2):226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 60.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. Journal of Clinical Oncology. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 61.Schellhammer P, Higano C, Berger E, et al. A randomized, double-blind, placebo-controlled multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC). In: Proceedings of the American Urological Association Annual Meeting; April 2009; Chicago, Ill, USA. [Google Scholar]
- 62.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using Interleukin-2 alone or in conjunction with vaccines. Clinical Cancer Research. 2008;14(17):5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heemskerk B, Liu K, Dudley ME, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Human Gene Therapy. 2008;19(5):496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. Journal of Clinical Investigation. 2005;115(5):1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Reviews Immunology. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 66.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunological Reviews. 2008;222(1):357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 68.McDermott DF, Atkins MB. Immunotherapy of metastatic renal cell carcinoma. Cancer Journal. 2008;14(5):320–324. doi: 10.1097/PPO.0b013e31818675c4. [DOI] [PubMed] [Google Scholar]
- 69.Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treatment Reviews. 2007;33(5):484–496. doi: 10.1016/j.ctrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. Journal of Immunology. 1986;137(5):1735–1742. [PubMed] [Google Scholar]
- 71.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. Journal of Immunotherapy. 2003;26(4):332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villinger F, Miller R, Mori K, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22(25-26):3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 74.Kutzler MA, Robinson TM, Chattergoon MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. Journal of Immunology. 2005;175(1):112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 75.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine and Growth Factor Reviews. 2002;13(2):155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 76.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12 associated toxicity and interferon-γ production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 77.Zaharoff DA, Hoffman BS, Hooper HB, et al. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Research. 2009;69(15):6192–6199. doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. Journal of Immunology. 2003;170(12):6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 79.Garnett CT, Palena C, Chakarborty M, Tsang K-Y, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Research. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 80.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. Journal of Experimental Medicine. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Research. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 82.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clinical Cancer Research. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 83.Lake RA, van der Most RG. A better way for a cancer cell to die. The New England Journal of Medicine. 2006;354(23):2503–2504. doi: 10.1056/NEJMcibr061443. [DOI] [PubMed] [Google Scholar]
- 84.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 85.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunology, Immunotherapy. 2001;50(9):445–455. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu XX, Zeng Y, Jin XH, Kakehi Y. Enhanced susceptibility of adriamycin-treated human renal cell carcinoma cells to lysis by peripheral blood lymphocytes and tumor infiltrating lymphocytes. Oncology Reports. 2007;18(2):353–359. doi: 10.3892/or.18.2.353. [DOI] [PubMed] [Google Scholar]
- 87.Chan OTM, Yang L-X. The immunological effects of taxanes. Cancer Immunology, Immunotherapy. 2000;49(4-5):181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clinical Cancer Research. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Machiels J-PH, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Research. 2001;61(9):3689–3697. [PubMed] [Google Scholar]
- 90.Galustian C, Labarthe M-C, Bartlett JB, Dalgleish AG. Thalidomide-derived immunomodulatory drugs as therapeutic agents. Expert Opinion on Biological Therapy. 2004;4(12):1963–1970. doi: 10.1517/14712598.4.12.1963. [DOI] [PubMed] [Google Scholar]
- 91.Galustian C, Meyer B, Labarthe M-C, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunology, Immunotherapy. 2009;58(7):1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farsaci B, Sabzevari H, Di Bari MG, Takai S, Schlom J, Hodge JW. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. doi: 10.1002/ijc.25177. International Journal of Cancer, 2010 Jan 20. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Research. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 95.Prasad DVR, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 96.Tringler B, Zhuo S, Pilkington G, et al. B7-H4 is highly expressed in ductal and lobular breast cancer. Clinical Cancer Research. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 97.Tringler B, Liu W, Corral L, et al. B7-H4 overexpression in ovarian tumors. Gynecologic Oncology. 2006;100(1):44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y, Wang Y, Zhao J, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Krambeck AE, Thompson RH, Dong H, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16(1):23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 101.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunology. 2002;3(7):611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 102.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. Journal of Immunology. 2005;174(10):5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer Journal. 2009;15(3):169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 104.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. Journal of Clinical Oncology. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. Journal of Immunotherapy. 2005;28(6):593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Current Opinion in Immunology. 2003;15(6):690–696. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 107.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunology. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 108.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Research. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 109.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clinical Cancer Research. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 110.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Research. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 111.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. Journal of Immunology. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 112.Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clinical Cancer Research. 2005;11(12):4533–4544. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- 113.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. American Journal of Reproductive Immunology. 2005;54(6):369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 115.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. International Journal of Cancer. 2007;120(12):2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 116.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112(3):610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. Journal of Clinical Investigation. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. The New England Journal of Medicine. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 119.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer and Metastasis Reviews. 2001;20(1-2):133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 120.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clinical Cancer Research. 2007;13(18, part 1):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 121.di Bari MG, Lutsiak ME, Takai S, et al. TGF-beta modulates the functionality of tumor-infiltrating CD8+ T cells through effects on TCR signaling and Spred1 expression. Cancer Immunology, Immunotherapy. 2009;58(11):1809–1818. doi: 10.1007/s00262-009-0692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Terabe M, Ambrosino E, Takaku S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clinical Cancer Research. 2009;15(21):6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 124.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 125.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Reviews. 2008;222(1):155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 126.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clinical Cancer Research. 1999;5(10):2963–2970. [PubMed] [Google Scholar]