Abstract
Clinical protocols utilize bone marrow to seed synthetic and decellularized allogeneic bone grafts for enhancement of scaffold remodeling and fusion. Marrow-derived cytokines induce host neovascularization at the graft surface, but hypoxic conditions cause cell death at the core. Addition of cellular components that generate an extensive primitive plexus-like vascular network that would perfuse the entire scaffold upon anastomosis could potentially yield significantly higher-quality grafts. We used a mouse model to develop a two-stage protocol for generating vascularized bone grafts using mesenchymal stem cells (hMSCs) from human bone marrow and umbilical cord-derived endothelial cells. The endothelial cells formed tube-like structures and subsequently networks throughout the bone scaffold 4–7 days after implantation. hMSCs were essential for stable vasculature both in vitro and in vivo; however, contrary to expectations, vasculature derived from hMSCs briefly cultured in medium designed to maintain a proliferative, nondifferentiated state was more extensive and stable than that with hMSCs with a TGF-β-induced smooth muscle cell phenotype. Anastomosis occurred by day 11, with most hMSCs associating closely with the network. Although initially immature and highly permeable, at 4 weeks the network was mature. Initiation of scaffold mineralization had also occurred by this period. Some human-derived vessels were still present at 5 months, but the majority of the graft vasculature had been functionally remodeled with host cells. In conclusion, clinically relevant progenitor sources for pericytes and endothelial cells can serve to generate highly functional microvascular networks for tissue engineered bone grafts.
Keywords: endothelial cell, mesenchymal stem cell, pericyte, vasculogenesis
Bone grafting is required to correct deficits arising from congenital defects, trauma, and disease. Autologous grafts are considered the gold standard with the best clinical outcome. They are vascularized and contain vital cells and growth factors that facilitate remodeling to form a strong unified structure with the host site, but significant limitations associated with autologous grafts include availability of donor tissue (restricting size and shape) and requirement of an additional surgical procedure entailing added patient suffering and increased operating time and cost.
Autologous bone marrow aspirate is used to seed synthetic and allogeneic grafts for spinal fusion and nonunion of long bone fractures (1, 2). The marrow is a rich source of cytokine-secreting cells that promotes both bone formation and angiogenesis; however, the neovasculature derives from the recipient tissue, with negligible cellular contribution from the transplanted marrow (1). Only the periphery of the graft is efficiently vascularized. Because the inner cell mass must rely upon mass transport for oxygen and metabolic requirements (3), a central zone of necrosis frequently occurs that results in a shell of ossification at the surface, with shallow penetration. The size, strength, and rate of remodeling of such constructs are directly limited by constraints to oxygen diffusion in supporting interior cell populations, restricting utility of nonautologous grafts primarily to small structures and bone extenders.
Endothelial cells will spontaneously form microcapillary-like networks with lumens in vitro when cultured in the presence of specific growth factors such as VEGF (4). Coculturing with an additional cell type to serve as mural cells and inclusion of ECM are necessary to maintain and mature the networks (5–11). The ability to precisely control in vitro experimental conditions directly led to identifying the mechanisms of many fundamental aspects of this process over the past several decades (5–7, 10–14). Several groups have recently shown that microvascular structures that are preformed in vitro by using endothelial and smooth muscle progenitor cells can anastomose with the host vasculature when implanted in a nude mouse model (15–20). In this study we demonstrate that human mesenchymal stem cells (hMSCs) coimplanted with endothelial cells (ECs) in a fibronectin-containing collagen gel act as a source of perivascular cells and generate a stable functional vasculature within a porous scaffold. The potential of MSCs both as a source for the osteogenic component (cells attached to the surface of the scaffold) and as perivascular and vessel stabilizing cells is explored. The osteoprogenitor nature of hMSCs and their potential to differentiate into the osteogenic lineage has been extensively exploited for bone regenerative therapy; thus, characterizing their role in vasculogenesis could provide unique solutions for successful bone tissue engineering. Efficient incorporation of such vascular networks in bone grafts in a clinical setting could potentially greatly increase their utility and reduce the reliance on autologous harvesting of bone.
Results
Vascularized Bone Graft Model.
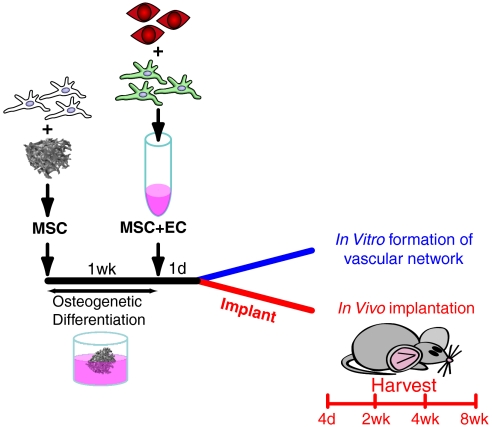
We developed a two-step scaffold seeding protocol (Fig. 1) in order to provide the hMSCs with a 3D porous scaffold that resembles trabecular bone structure on which to differentiate into osteoblasts. We first seeded the hMSCs intended for bone formation and allowed them to attach and grow on the surface of the scaffold for a week. During this period, an osteoinductive medium was used. The scaffolds were then seeded with collagen-fibronectin gel containing fluorescently labeled human umbilical vein endothelial cells (HUVECs) and hMSCs and incubated overnight in endothelial cell medium EGM-2. The cell/scaffold constructs were then either grown in vitro or implanted subcutaneously in mice for analysis. A detailed protocol and analysis of the resulting bone scaffolds is described below.
Fig. 1.
Protocol timeline. Unlabeled hMSCs were seeded and allowed to attach to the bone scaffold. Medium containing osteoinductive agents was used to commit these cells to the bone lineage, after which it was replaced with a hydrogel containing labeled ECs (red) and additional hMSCs (25% labeled green) to serve as pericytes. The scaffold was continued to be cultured in vitro or implanted the following day.
Formation of Vascular Network Within the 3D Porous Scaffold in Vitro.
Scaffolds were fabricated with interconnected porosity to replicate the structure of trabecular bone from the copolymer (85∶15 molar ratio) poly(DL-lactide-co-glycolide) (PLGA) (Fig. 2A). A light vacuum was used to ensure homogeneous seeding of suspended cells throughout the scaffold. The ability of the scaffold to support the formation of a 3D vascular network throughout the interconnected pores was then evaluated. tdTomato-labeled ECs and eGFP-labeled hMSCs were mixed at a ratio 4∶1 in the collagen-fibronectin gel and either cultured in endothelial (EGM-2) medium in a multiwell plate or applied to the PLGA scaffold. Fluorescent microscopy demonstrated the presence of an extensive vascular network within the collagen-fibronectin gel (Fig. 2B) that was stable for several weeks. A continuous three-dimensional network of HUVEC-hMSC engineered vessels formed throughout the porosity within the PLGA scaffold also remained stable for greater than a month in vitro (Fig. 2C). Furthermore, the eGFP-labeled hMSCs had migrated to line the tubular structures formed by the tdTomato-labeled HUVECs and acquired a flattened, elongated morphology with stellate processes (Movie S1).
Fig. 2.
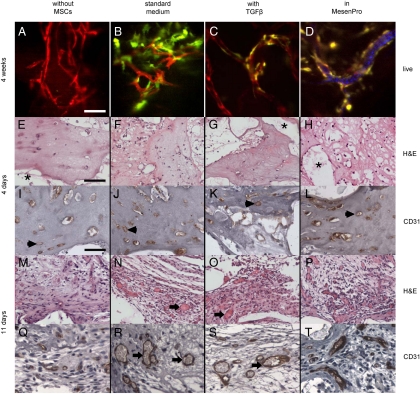
hMSCs contribute to in vitro formation of a 3D vascular network within a porous scaffold. (A) The bone scaffold was synthesized of the biocompatible polymer (85∶15) PLGA, by using sucrose leaching to generate interconnected pores averaging 1000 μm. (B) Stable network containing HUVECs and hMSCs in fibronectin-containing collagen gel at 21 days postseeding. (C) Physical interaction of hMSCs with EC network formed in the PLGA scaffold at 28 days postseeding. (D) Relative transcriptional response of the smooth muscle markers smooth muscle actin (ACTA2), calponin (CNN1), SM22 (TAGLN), and smoothelin (SMTN) to the 5-day preconditioning treatment. (E–H) In vitro formation of networks by HUVECs imaged with confocal microscopy at 10 days (E) without hMSCs, (F) with standard condition hMSCs, (G) with hMSCs cultured with TGF-β, and (H) hMSCs cultured in low serum MesenPro medium. (Scale bars: A = 1 mm;  ;
; 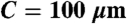 ,
,  . ∗P < 0.05.)
. ∗P < 0.05.)
Preinduction of hMSCs into Pericytes.
During vasculogenesis, mural wall cells are recruited to the newly formed endothelial cell network and direct its maturation through both soluble factors and direct contact (21). Multiple studies utilizing vasculogenesis and angiogenesis models have demonstrated that contact and communication between ECs and smooth muscle cells (SMCs)/pericytes are required for vascular maturation (22, 23). We reasoned that predifferentiation of hMSCs to smooth muscle prior to addition to the vascular component may accelerate formation and maturation of the network. In addition to hMSCs grown in standard culture conditions, we utilized hMSCs that had been grown for 5 days in standard medium supplemented with 1 ng/mL of TGF-β, a well-established inducer of smooth muscle phenotype (24). For comparison purposes, we simultaneously cultured hMSCs for 5 days in a commercial low serum medium (MesenPro) specifically formulated to maintain hMSCs in an undifferentiated proliferative state. As expected, TGF-β induced a SMC phenotype as evidenced by an increase in expression for several smooth muscle markers (Fig. 2D), whereas the low serum conditions caused a small decrease relative to standard hMSC medium.
The ability of each hMSC population to assist HUVECs in forming and maintaining a network when cocultured in a collagen-fibronectin gel was then evaluated in vitro (Fig. 2E–H). Endothelial cultures without hMSCs (Fig. 2E) formed short-lived multicellular cords that were much shorter and wider than those formed by hMSC-containing cultures; they were already regressing and falling apart by day 6 with the HUVECs subsequently dying. All hMSC-containing cultures formed extensive vascular networks, although with noticeable differences. Constructs containing the TGF-β-induced hMSCs were delayed in tube formation, with ECs only starting to coalesce at day 5. Both standard medium and MesenPro-precultured hMSCs, however, formed extensive networks rapidly. At day 5, most hMSCs were closely associated with ECs; however, the MesenPro-derived hMSCs were more numerous, indicating that they continued to rapidly proliferate even after being switched to medium optimized for EC culture. More interestingly, this population of hMSCs also made far more extensive contact with the ECs, often stretching between bifurcated branches. Tubes with extensive hMSC coverage were often quite large.
Formation of Engineered Vessels Within the 3D Porous Scaffold in Vivo.
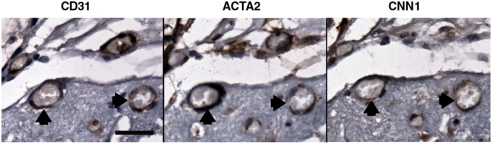
To determine whether cocultures of HUVECs-hMSCs are able to self-assemble in vivo in the scaffold and anastomose with the host vasculature to become functional blood vessels, constructs were implanted subcutaneously in immunodeficient mice. At various time periods between 4 days and 5 months after implant, the scaffolds were examined. Intravital microscopy confirmed the formation of vascular networks within the scaffolds (Fig. 3A–D). In agreement with previous observations (22, 25, 26), when constructs with HUVECs alone were implanted, circular structures formed by day 4 after implantation, but the engineered vessels appeared counterfeit, remained immature, and eventually regressed. Implanted constructs generally showed relative behavior similar to in vitro culture, with endothelial-only scaffolds showing poor survival and marginal lumen formation and no evidence of anastomosis. Here, we found that HUVECs coseeded with hMSCs were capable of vasculogenesis. However, the density and persistence of the engineered neovasculature were substantially different depending on hMSC pretreatment. hMSC-containing scaffolds had vascular structures forming by day 4 (Fig. 3E–L) and extensive association of hMSCs by week 4. Scaffolds utilizing MesenPro-treated hMSCs again produced the most extensive networks intimately surrounded by hMSCs. In addition, immunohistochemical staining demonstrated multiple vessels positive for the human-specific endothelial marker CD31 that were evenly distributed throughout the scaffold. Furthermore, by day 11 blood cells were found in the vessels that stained positively with antihuman CD31 indicating the connection of the engineered vessels with the host vasculature (Fig. 3M–T). Adjacent serial sections of an explanted scaffold were stained with CD31 to identify the human-derived vessels and the pericyte markers smooth muscle actin and calponin (Fig. 4). The hMSCs localized to the vessels were expressing the smooth muscle markers; although they made extensive contact with the ECs, coverage appeared incomplete as is typical of most microvasculature.
Fig. 3.
Formation of engineered vessels in vivo. (A–D) Networks formed by HUVECs imaged with confocal laser-scanning microscopy at 4 weeks (A) without hMSCs, (B) with standard condition hMSCs, (C) with hMSCs cultured with TGF-β, and (D) with hMSCs cultured in low serum MesenPro medium. For (D) only, maximum intensity projection of 30 consecutive frames (1 sec) was used to demonstrate flow of DiD-labeled blood cells. H&E and human-specific CD31 immunohistochemical staining of scaffolds explanted 4 (E–L) and 11 (M–T) days after implantation. Acellular regions (*) derive from scaffold. EC-derived tubular structures have formed by 4 days (arrowheads) but do not contain blood cells indicating that they are not yet functional. By 11 days, the structures were larger with more robust CD31 staining, and most lumens were filled with blood cells (arrows). The symbols mark only a representative sample of indicated structures. (Scale bars: 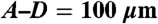 ;
; 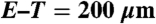 .)
.)
Fig. 4.
Perivascular localization of hMSCs. A seeded scaffold containing HUVECs and MesenPro-treated MSCs was explanted after 4 weeks and embedded in paraffin. Serial sections cut from a region containing several transverse vessels (two of which are marked by arrowheads) were immunohistologically stained to identify human-derived structures: a human-specific antibody for PE-CAM (CD31) stained graft-derived endothelium, vascular-associated MSCs stained positive for the mural markers smooth muscle actin (ACTA2) and calponin (CNN1). Numerous erythrocytes within lumens indicate that the vasculature has anastomosed and is functional. Positive signals are brown with hematoxylin counterstaining. (Scale bar: 25 μm.).
Functionality of the Engineered Vessels.
In order to test for permeability of anastomosed vessels, the fluorescent dye Evans blue was injected systemically through the tail vein of the mice. At 2 weeks postimplantation, graft EC-derived vessels with associated hMSCs were patent and functional, as the lumens of the networks were clearly delineated. However, after several minutes, significant accumulation of background fluorescence occurred in all graft conditions indicating that the vessels were still immature and leaky (Fig. 5A). The assay was repeated at 8 weeks postimplantation on additional implants, and only very minimal leakage was detectable even 5 hours post injection, indicating that the endothelium had further matured to develop functional tight junctions (Fig. 5B). Occasional small pools of blood resulting from minor trauma caused by in vivo imaging 2 weeks postimplantation indicated the fragility of the structures; after this period, there was no evidence of any hemorrhagic event during imaging, indicating that the maturing vessels more closely resemble normal rather than tumor pathology. Inosculation points were also visible where the engineered vessels had anastomosed with the host vasculature (Fig. 5C). Nascent, immature vessels often support only irregular blood flow; therefore, to further evaluate the functionality of the engineered vessels we injected DiD-labeled mouse blood cells systemically and subsequently visualized flow through both the graft and the nearby host vasculature. The rate of flow was indistinguishable (Fig. 5D and Movie S2). Confocal images taken at 4 weeks postimplantation were used to determine graft vessel density (Fig. 5E) and mean diameter (Fig. 5F). Without hMSCs, tubular networks were fragmentary and meager and did not appear patent. Vessels derived from TGF-β-treated hMSCs produced vessels of a slightly larger diameter relative to standard medium conditions but had a lower overall vessel density. hMSCs precultured in MesenPro produced vascular networks with both a higher vessel density and a larger diameter than those of all other tested conditions.
Fig. 5.
Assessment of functionality of the engineered vessels within the porous scaffold. At 2 weeks, tail vein injection of Evans blue was used to visualize flow through the grafts by intravital microscopy in order to verify anastomosis and assess vessel permeability (A). The assay was repeated at 8 weeks postimplantation on additional implants with a second vascular probe, Qtracker800. In this case only very minimal leakage was detected. (C) Points of anastomosis, where dye-labeled serum flowing through the graft (red) and adjacent host vasculature clearly indicate that the engineered vessels have anastomosed with the mouse vessels. (D) Vibrant DiD-labeled RBC flow rates through graft and neighboring host vessels were calculated. Vessel density (E) and average diameter (F) in grafts with indicated hMSCs 4 weeks after implanting. ∗P < 0.05, ∗∗P < 0.01 compared with standard medium conditions. (Scale bar: 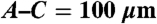 .)
.)
Induction of Osteogenic Lineage.
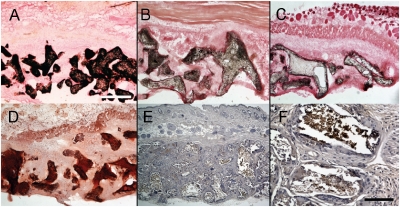
After 8 weeks, scaffolds were harvested and frozen sections were prepared and analyzed by von Kossa staining for evidence of calcium production and consequent bone formation. Extensive calcium phosphate deposition was detected that was restricted to the surface of the scaffold pores (Fig. 6A). No significant staining was apparent in the pore spaces that would indicate that the vascular component containing hMSCs contributed to bone formation. Coculture of ECs with hMSCs has been shown to have a close reciprocal relation and can induce bone formation in the absence of external osteogenic stimuli (27). We therefore repeated the scaffold seeding procedure, but during the 1-week culture period we omitted osteogenic inducers from the culture medium, and then the fibronectin-containing collagen gel with ECs and MSCs was added and implanted the following day. Explanted scaffolds had significant mineralization at 8 weeks (Fig. 6B), although less than the osteo-induced samples. Parallel scaffolds without the vascular component produced a very modest staining for calcium (Fig. 6C). As the von Kossa technique sometimes detects non-calcium-containing mineral deposits (e.g., urates), the presence of calcium was confirmed by Alizarin red staining (Fig. 6D). Scaffold surfaces were also positive for osteocalcin, a late marker of osteoblast differentiation (Fig. 6E, magnified in Fig. 6F).
Fig. 6.
Osteogenic induction. At 8 weeks, scaffolds that had been seeded with standard condition osteoinduced hMSCs were explanted and calcium deposits stained with von Kossa. Calcium deposition was extensive and restricted to the pore surfaces of the scaffolds. Vascularized scaffolds (B) in which the initial seeding for attached hMSCs had not undergone prior in vitro osteogenic induction had more extensive mineralization than avascular scaffolds (C) but less than (A). A section from a scaffold (A) also stained with Alizarin red (D). This scaffold was also immunopositive for the late marker of osteoblast differentiation, osteocalcin (brown with hematoxylin counterstaining) (E, magnified in F). (Scale bars:  ;
;  .)
.)
Discussion
New bone formation in bone grafts occurs only in the presence of a vascular network within the implant, with the greatest amounts of newly laid down bone occurring in the most vascularized areas (28). For metabolically active tissues such as newly formed bone to grow beyond the oxygen diffusion limit of a couple hundred microns, formation of new blood vessels is required (3). Numerous strategies for vascularization involve highly sophisticated scaffolds and local delivery of angiogenic factors to enhance ingrowth of vessels from the host (29). Because of the considerable amount of time required for angiogenesis, the use of larger bone grafts is not often feasible as necrosis develops in the core with only a shell of bone at the surface. Thus the ability to promote rapid vascularization of bone grafts in a clinically relevant manner could vastly expand the utility of synthetic bone grafts.
The development of a mature and functional vasculature, however, does not depend only on migration and proliferation of endothelial cells but requires cooperation and symbiosis between them and mural cells (pericytes in small and SMCs in larger-sized vessels) (23, 26, 30). In addition, ECM is required in order to provide both structural support for the cellular component and sites to sequester important growth factors and other signaling molecules (10–13). Many studies have demonstrated that formation of stable and functional vascular networks necessitates the coimplantation of ECs with perivascular cells, a role that has been undertaken by human saphenous vein SMCs or mesenchymal precursor cells such as the mouse embryonic cell line 10T1/2 (24, 31). Furthermore, it is suggested that 10T1/2 cells differentiate into SMCs or pericytes through heterotypic interactions with ECs (23, 24, 26). Recently, the perivascular/pericyte origin of bone marrow MSCs (32, 33) has been identified, and the ability of these cells to differentiate to SMCs and stabilize nascent blood vessels (22, 34, 35) is being characterized.
Here we demonstrated that hMSCs specifically assume the role of perivascular cells and act as a potent stabilizer of the engineered blood vessels formed in the porous bone scaffold. Their differentiation state prior to coimplantation with ECs, however, is an important parameter that supports the network, because the population that remained in an undifferentiated proliferated state formed stronger, more mature networks. The hMSCs fulfill at least two roles in supporting the vasculogenesis process, synthesis of trophic factors (22, 23) and contributing the mural component (9, 19, 21, 24, 25), neither of which is well understood at present. Both in vitro and in vivo, the tubular networks formed by monocultures of ECs such as HUVECs are not stable. Inclusion of mesenchymal-derived cells that can serve as pericytes such as the 10T1/2 cell line, embryonic and dermal fibroblasts, or hMSCs generates networks that are more robust and can anastomose to form functional vessels in vivo (5, 7, 9, 11, 28). Furthermore, it is suggested that 10T1/2 cells differentiate into SMCs or pericytes through heterotypic interactions with ECs (10, 11, 13). Treatment with TGF-β is a well-established protocol to generate a more mature smooth muscle phenotype (24). We found that transcription of several genes expressed in smooth muscle, including the late marker smoothelin, was up-regulated after a relatively brief culture period in medium containing additional TGF-βand expected that these cells would better support vasculogenesis. However, the resulting networks were inferior to those generated by cells that had not been treated with the cytokine. Although the resulting networks did anastomose and support blood flow, they were noticeably less complex. The TGF-β-treated hMSCs were tightly associated with the endothelium but were smaller and fewer in number and so generated vessels with relatively sparse pericytes. In contrast, MesenPro-treated hMSCs generated structures with very extensive mural coverage. MesenPro supports proliferation, whereas TGF-β is a potent growth inhibitor; the impact of this preconditioning persisted even after implantation. Both the phenotype and the relative number of pericytes influence native microvascular properties (e.g., leakiness, degree of branching, and vessel density). These parameters will undoubtedly be important in constructing functional vasculature in engineered tissue as well.
As previously reported (14), the hMSCs implanted with ECs differentiated to the osteoblasts lineage without prior induction, although mineralization occurred at reduced levels. It is not yet known whether this was because a smaller number of cells committed to the lineage or differentiation lagged. The osteogenic model we chose, ectopic bone formation in an immunodeficient mouse, uniquely allowed us to generate vascularized tissue by using clinically relevant human cell sources. Complementary studies for evaluating the effects of vasculogenesis on load-bearing bone grafts need to be performed in a large animal model but will first require developing autologous cell sources.
Characterization of the dynamics of graft vessel remodeling, particularly with respect to hMSC and EC proliferation and persistence, is ongoing. In our current model, the vasculature generated by the implanted cells was gradually replaced by host vasculature. For clinical purposes, long-term maintenance of the engineered vasculature may not be necessary or even desirable if it can be replaced by patient-supported angiogenesis. Osteoblasts and pericytes are readily generated from autologous bone marrow-derived cells; all potential autologous sources of EC progenitors are currently significantly more difficult to isolate and expand. This is particularly true for the older patient population. If vasculogenesis of the implanted engineered tissue were to function as a bridge to support initial metabolic needs until it is replaced by angiogenic ingrowth, any required immunosuppression resulting from the use of nonautologous endothelial cell sources could be temporary. In our model, network formation occurred rapidly, but anastomosis was first detected eleven days postimplantation for all hMSC-containing scaffolds. Although the vasculature at this stage was immature and leaky to the fluorescent indicators, it was functional and without cellular extravasation. Providing separate hMSC populations that have been individually optimized for specific functions (bone formation, trophic factor production, pericytes) could give a significant advantage in accelerating vasculogenesis.
Materials and Methods
Fabrication of PLGA 3D Porous Scaffolds.
Five-millimeter-diameter porous bone scaffolds were fabricated from PLGA (85/15) by using the sucrose leaching technique. For detailed information, see SI Methods.
Cell Isolation and Culture.
Bone marrow samples were obtained according to guidelines established by the Massachusetts General Hospital Institutional Review Board from patients undergoing hip replacement surgery. hMSC cultures were established as previously described (36) and maintained in mesenchymal stem cell growth medium (MSCGM) (Cambrex). All experiments were performed with hMSCs having less than six cell passages. For osteogenic induction, hMSC-seeded scaffolds were cultured in osteoinductive medium (MSCGM with 50 μg/mL L-ascorbic acid 2-phosphate, 10 mM β-glycerol phosphate, and 10-8 M dexamethasone). When indicated, 1 ng/mL of TGF-β was added to MSCGM; MesenPro RS Medium was obtained from Invitrogen. HUVECs were maintained in 0.1% gelatin-coated plates in EGM-2 medium (Cambrex). Lentivirus was used to fluorescently label hMSCs (eGFP) and HUVECs (tdTomato) in order to visualize vascular structures; details are provided in SI Methods.
Tissue Engineered Blood Vessel Model Within PLGA Scaffold.
The engineered blood vessels within the PLGA scaffold were prepared by mixing 1 × 106 EC and 2.5 × 105 MSCs and suspended in 1 mL of type-1 collagen (PureCol; Advanced BioMatrix) and fibronectin (Invitrogen) gel solution prepared as previously described (26). Then 100 μL of gel with cells was added over PLGA scaffolds applying a slight vacuum to ensure removal of any trapped air in the center of the scaffold. Constructs were maintained in culture in EGM-2 endothelial cell medium for the duration of the in vitro experiments or overnight prior to implantation in vivo.
In Vivo Imaging.
An immunodeficient mouse model was used in order to support in vivo growth of bone scaffolds with human cells. All procedures were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital and performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Constructs were implanted subcutaneously in the backs of severe combined immunodeficient mice (NOD.CB17; Jackson Laboratory). The custom-built video-rate laser-scanning confocal microscope (37) used for in vivo imaging is described in SI Methods.
Whole blood from a Balb/C mouse (Jackson Laboratory) was diluted 10× with RPMI 1640 cell culture media (Mediatech) plus 0.1% BSA (Sigma-Aldrich). The cells were stained with 100 μM Vibrant DiD for 30 minutes at 37 °C and then washed with PBS, and up to 1 × 108 cells were injected retroorbitally. Flow of DiD-labeled cells was captured in live video mode. Lengths of individual engineered and native vessels were measured by manual tracing of perfused vessels by using ImageJ software (38) and speed determined by tracking individual cells across isolated frames. For display purposes, color levels and contrast of individual images and movies were adjusted. Average vessel density and calculated average diameter were determined on images derived from maximum intensity projections of 4 consecutive z slices by using ImageJ.
Gene Expression and Histochemical Staining.
Detailed information is included in SI Methods.
Supplementary Material
Acknowledgments.
We thank Katelyn McGovern (Massachusetts General Hospital) for assistance with analyzing confocal images and Dr. Patrick Au (Massachusetts General Hospital) for assistance with preparing the engineered blood vessel model. This work was sponsored by a grant from the Stanley H. Durwood Foundation and the Harvard Stem Cell Institute. Imaging studies were supported by the Massachusetts General Hospital Advanced Microscopy Program start-up fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.F.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905445107/DCSupplemental.
References
- 1.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A(7):1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Muschler GF, et al. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res. 2003;407:102–118. doi: 10.1097/00003086-200302000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138(4):745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, et al. Development of capillary networks from rat microvascular fragments in vitro: The role of myofibroblastic cells. Microvasc Res. 1987;33(2):194–210. doi: 10.1016/0026-2862(87)90017-3. [DOI] [PubMed] [Google Scholar]
- 6.Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207(2):491–498. doi: 10.1002/jcp.20584. [DOI] [PubMed] [Google Scholar]
- 7.Supp DM, Wilson-Landy K, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002;16(8):797–804. doi: 10.1096/fj.01-0868com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernon RB, Sage EH. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res. 1999;57(2):118–133. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- 9.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12(13):1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 10.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97(5 Pt 1):1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci-Polym Ed. 1998;9(12):1267–1278. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 13.Hoying JB, Williams SK. Effects of basic fibroblast growth factor on human microvessel endothelial cell migration on collagen I correlates inversely with adhesion and is cell density dependent. J Cell Physiol. 1996;168(2):294–304. doi: 10.1002/(SICI)1097-4652(199608)168:2<294::AID-JCP8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: Role of extracellular matrix. J Cell Biol. 1989;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 16.Nor JE, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81(4):453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd BR, et al. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vasc Biol. 2004;24(5):898–904. doi: 10.1161/01.ATV.0000124103.86943.1e. [DOI] [PubMed] [Google Scholar]
- 18.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5(5):1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 19.Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA. The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Pt A. 2009;15(8):2015–2027. doi: 10.1089/ten.tea.2008.0318. [DOI] [PubMed] [Google Scholar]
- 20.Rouwkema J, de Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12(9):2685–2693. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 21.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZZ, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25(3):317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 24.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141(3):805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au P, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111(3):1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koike N, et al. Tissue engineering: Creation of long-lasting blood vessels. Nature. 2004;428(6979):138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 27.Kaigler D, et al. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19(6):665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 28.Deleu J, Trueta J. Vascularisation of bone grafts in the anterior chamber of the eye. J Bone Joint Surg Br. 1965;47:319–329. [PubMed] [Google Scholar]
- 29.Young S, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Pt A. 2009;15(9):2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen LL, D’Amore PA. Cellular interactions in vascular growth and differentiation. Int Rev Cytol. 2001;204:1–48. doi: 10.1016/s0074-7696(01)04002-5. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 32.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Gong Z, Calkins G, Cheng EC, Krause D, Niklason LE. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Pt A. 2009;15(2):319–330. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melero-Martin JM, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veilleux I, Spencer JA, Biss DP, Côté D, Lin CP. In vivo cell tracking with movie rate multimodality laser scanning microscopy. IEEE J Sel Top Quant. 2008;14:10–18. [Google Scholar]
- 38.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43(Suppl 1):25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]