Abstract
We tested healthy preterm (born near 28 ± 2 weeks of gestational age) and full-term infants at various different ages. We compared the two populations on the development of a language acquisition landmark, namely, the ability to distinguish the native language from a rhythmically similar one. This ability is attained 4 months after birth in healthy full-term infants. We measured the induced gamma-band power associated with passive listening to (i) the infants’ native language (Spanish), (ii) a rhythmically close language (Italian), and (iii) a rhythmically distant language (Japanese) as a marker of gains in language discrimination. Preterm and full-term infants were matched for neural maturation and duration of exposure to broadcast speech. We found that both full-term and preterm infants only display a response to native speech near 6 months after their term age. Neural maturation seems to constrain advances in speech discrimination at early stages of language acquisition.
Keywords: gamma-band oscillations, preterm infant, speech, rhythm, development
How much do healthy immature brains gain from exposure to broadcast speech? Unfortunately, very few studies have investigated the interaction between experience and brain maturation in healthy premature infants during the early stages of cognitive development (1). Highly premature infants whose nervous system is immature are confronted with a wide range of external stimulation with unknown consequences on cognitive development. Here, we explore the neural correlate of a landmark of language acquisition, namely, the discrimination of the maternal language from rhythmically similar languages (2).
A fair number of behavioral studies have demonstrated that fetuses and neonates distinguish utterances of languages belonging to different rhythmic classes. Indeed, in a recent study, speech perception was examined in 104 low-risk fetuses at 33–41 weeks of gestational age using a familiarization/novelty paradigm (3). One of the experiments shows that fetuses of English-speaking mothers discriminate English from Mandarin, two languages that are very different in rhythm. Moreover, other studies with 2- to 4-day-old neonates and a larger set of language pairs show different responses depending on whether the utterances belong to the same or two different rhythmic classes (2, 4, 5). In other words, once neonates become habituated to utterances of a given language, they show an increase in their nonnutritive sucking rate only when they are exposed to utterances of languages that belong to a different rhythmic class. However, at this age, neonates do not distinguish a switch that occurs between two languages that belong to the same rhythmic class. Other studies have established that it is only after 4 months that full-term infants are able to distinguish utterances in their native language from those of a language that belongs to the same rhythmic class (6, 7). How does this ability develop in healthy highly premature infants? Would preterm infants exposed to their native language for near 6 months after birth perform like full-term infants who had a comparable amount of exposure to broadcast speech? Does postnatal exposure to the native language boost the development of the ability to distinguish rhythmically similar languages regardless of the learners’ stage of neural maturation? To explore this issue, in a cross-sectional study, we compared the patterns of oscillatory brain activity to native and foreign languages in a group of healthy full-term infants and a group of healthy premature infants matched by either maturational age or duration of exposure to broadcast speech (Fig. 1; Materials and Methods).
Fig. 1.
Maturational age and duration of exposure to broadcast speech. Horizontal bars indicate the duration of intra- and extrauterine life (green and yellow, respectively) for the four groups. The age in months for the evaluation of full-term and preterm infants is indicated at the top of the first horizontal bar (i.e., 3 and 6 months after birth for full-term infants, 6 months after birth and after term age for preterm infants). The term age corresponds to the date at which infants should have been born after 9 months of gestation.
All full-term infants were born at 39.6 ± 1.5 weeks after conception and tested either 3 (FT3) or 6 (FT6) months after birth. All premature infants were born 28.6 ± 1.6 weeks after conception, nearly 3 months before the expected term age (i.e., 40 ± 2 weeks). The preterm infants were tested either near 6 months (PT6) or 9 months (PT9) after birth. At the time of evaluation, PT6 and PT9 infants had been systematically exposed to broadcast speech for more than 4.5 months (Table S1), corresponding to the amount of broadcast speech that full-term infants require to discriminate their native language from other rhythmically similar languages (6, 7). The exposure to broadcast speech took place outside the incubator, because the speech signal is distorted inside the incubator (8). Exposure began very soon after birth thanks to the Kangaroo protocol, which promotes skin-to-skin contact between mother and infant. Broadcast speech exposure increased near 31 weeks of gestational age when infants were transferred from the incubator to open cradles. Here, they received broadcast speech from their parents, other relatives, and the medical staff. Finally, near 34 weeks of gestational age, all preterm infants were discharged and sent home, where they received as much broadcast speech as full-term infants (Table S1; Materials and Methods).
If neural maturation constrains the development of the ability to discriminate a switch between languages from the same rhythmic class, we can expect that the brain response in PT9 and FT6 infants will be similar, because both groups are tested when their neurological age is equivalent. However, PT6 infants ought to display brain responses similar to FT6 infants if postnatal exposure to the maternal language is the main parameter responsible for enhancing the ability to distinguish it from rhythmically similar languages. Although the PT6 and FT6 infants had equal exposure to speech, the neural maturation of FT6 infants is more advanced than that of PT6 infants.
Brain activity was measured using electroencephalographic recordings during passive listening to utterances in Spanish (the maternal language), Italian (a rhythmically close language), and Japanese (a rhythmically distant language) in the previously described four groups of infants (Fig. 2).
Fig. 2.
Stimulation protocol. All infants listened passively to a series of 54 utterances in Spanish (red squares), Italian (blue squares), and Japanese (green squares), obtained by adding 18 utterances provided by three different speakers per language.
Analysis of the oscillatory activity of the brain focuses on the induced gamma-band power, which corresponds to the nontime-locked oscillations occurring at frequencies greater than 20 Hz (Materials and Methods). The induced gamma-band power “provide(s) a specific neural marker for the perceptual binding of the spatially separate elements in the infant brain” (9; for a review, ref. 10). To the best of our knowledge, there are very few studies on the role of the gamma band during language acquisition. Recent studies in adults have shown an increase in oscillatory activity from 40 to 200 Hz during speech perception and/or production (11, 12).
In our study, we explored whether the power of the induced gamma-band oscillations can track the gains in language discrimination during the first stages of language acquisition in healthy full-term and premature infants. To estimate gains in neural maturation, we carried out periodical clinical assessments and also evaluated the early components of the event-related potentials (ERPs) that are associated with age-related changes in auditory processing. The latency and amplitude of the early speech ERPs change dramatically from 3 to 6 months of life in full-term infants (13, 14). These changes are mainly correlated with changes in myelinization, synaptic efficacy, neural connectivity, and the anatomy of the infant’s head (15, 16). In this study, we measure the early ERP response as a marker of the age-related changes in neural maturation. If speech exposure induces improvement in native language discrimination even when the brain is highly immature, the gamma-band response of PT6 infants should reveal discrimination of the rhythmically similar languages even when their ERP patterns are similar to those of the more immature full-term infants (i.e., FT3 infants).
Results
Fifteen healthy infants per group were analyzed (Materials and Methods). All infants evolved with normal pediatric and neural development after a 2-year outcome (Table S2).
Gamma-Band Power.
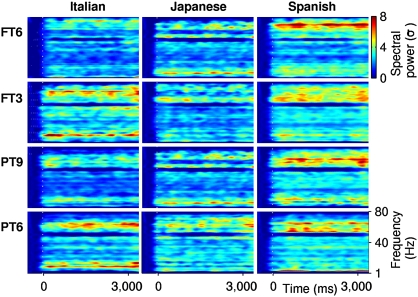
The computation of the time-resolved spectral power is detailed in Materials and Methods. The time course of the mean spectral power from 1 to 100 Hz for all languages and groups is displayed in Fig. 3.
Fig. 3.
Mean induced gamma-band power. The time-frequency maps illustrate the mean of the spectral power per language and group from 1–100 Hz. The groups and languages are indicated, respectively, on the left and at the top of the figure. The color bar to the upper right of the figure shows the spectral power scale (in SD units) for all maps. The frequency range is indicated in the y axis of the lower right map. Time is plotted in the x axis of the lower maps. The statistical analysis was focused on the high gamma frequency range (55–75 Hz) and from 300 to 2,800 ms after the onset of the sentences. Dotted white lines indicate the onset of the utterances.
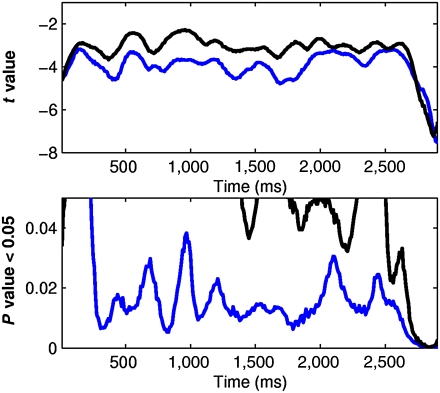
The statistical analysis of the spectral power was focused on the time range from 300 to 2,800 ms after the onset of the utterances and on the frequency range from 55 to 75 Hz. Our motivation arose from the observation that the oscillatory activity in this time-frequency range, but not in other time-frequency windows, displays significant differences with respect to the baseline when all languages and groups are pooled (Fig. 4; see Materials and Methods). We submitted the mean induced gamma-band power of this time-frequency window to repeated ANOVA with language (Spanish, Italian, and Japanese) as a within-subjects factor and group (FT3, FT6, PT6, and PT9) as a between-subjects factor.
Fig. 4.
Time-frequency windows for statistical comparisons. The time course of the t value and P value obtained from the comparison of the mean activity at the gamma range observed during the baseline and during listening to the utterances across all languages and all participants is plotted. The blue line shows the time course of the mean t value and P value under 0.05 for the middle gamma-band power from 55–75 Hz. The black line shows the same statistical values for the low gamma-band power from 21–40 Hz only for visual control comparison.
We found a main effect of language [F(1.02, 55.8) = 5.7, P < 0.01] with greater gamma-band power for Spanish and Italian than for Japanese (P < 0.01 and P < 0.04, respectively). Moreover, a significant interaction of language × group [F(3.02, 55.8) = 3.8, P < 0.02] was observed, because the mean gamma-band power for Spanish (the maternal language) was significantly greater than that observed for Italian utterances in FT6 and PT9 infants only. In fact, the gamma-band power for Spanish was significantly greater than that for Italian in FT6 (P < 0.01) and PT9 (P < 0.01) infants regardless of the fact that Spanish and Italian belong to the same rhythmic class. In contrast, FT3 and PT6 infants displayed similar gamma-band power for Spanish and Italian, suggesting that this neural marker for distinguishing between native and rhythmically similar languages arises only in more mature infants.
ERP Response.
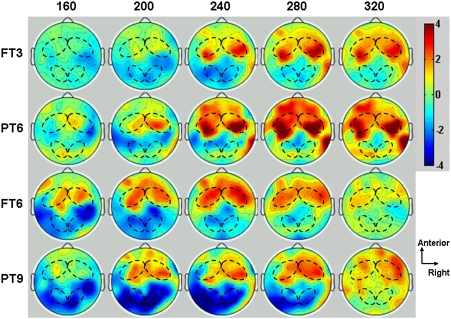
The ERP computation is detailed in Materials and Methods. The early ERP includes a positive component, peaking near 200 ms, observed in all groups of infants over the central anterior electrodes and a negative component, peaking at about 240 ms, observed only in older infants over the central posterior electrodes (Fig. 5). The mean latency peak and the mean zero-to-peak amplitude of the positive component were submitted to the corresponding one-way ANOVA with group (FT3, FT6, PT6, and PT9) as a between-subjects factor. Scheffé’s test was used for post hoc multiple comparisons.
Fig. 5.
Early ERP components per group and language. The topographic maps illustrate the time course of the mean amplitude of the early ERPs from 160–320 ms after onset of the utterances. The mean activity over a group of eight central/anterior electrodes (on the left and right sides, delimited by a black dotted line in the voltage maps) and over eight central posterior electrodes (on the left and right sides, indicated by a black dotted line in each voltage map) were used to estimate the latency and amplitude of the early ERP response. The activity plotted in each subjacent map corresponds to the grand average of the ERPs at the time indicated by the numerical label displayed at the top of the figure ± 20 ms. The groups are indicated on the left side of the figure. The color bar indicates the amplitude of the ERP response in microvolts.
A significant effect of group [F(3, 56) = 24.3, P < 0.005] was observed in the mean latency, because the mean latency of the positive peak was significantly shorter for older as compared with younger groups [i.e., latency was shorter for FT6 as compared with FT3 infants (P < 0.001) and for PT9 as compared with PT6 infants (P < 0.01)]. No significant differences in the mean zero-to-peak amplitude were observed for the positive component. We did not compare the negative component because it was not identified in several infants in the younger groups (i.e., FT3, PT6). The absence of a negative component around 250 ms has been previously reported in 3-month-old infants (14) as an age-related change in ERP topography. This does not mean that this component does not exist, but it could mean that the corresponding dipoles are oriented out of the recorded area in the scalp.
Clinical Assessments.
Development of all participants in our study was normal, as assessed through periodic evaluations that took place every 3 months until the infants were 24 months old (after the term age for premature infants). Preterm infants were also evaluated neurologically every 3 months and given a minimum of two ophthalmological and audiometric tests at the end of their second year of life. No neurological or developmental anomalies were detected in these clinical assessments. Clinical assessments of the full-term and preterm groups were not significantly different. The amount of word/sentence comprehension and the number of words produced evaluated using the McArthur Communicative Development Inventories (CDI) (17) at 12 months (after the term age in premature infants) showed that all infants were within the norm of the regional and international standards. Likewise, the Mental and Psychomotor Developmental Indexes of the Bayley test (18) applied at 24 months (after the term age in preterm infants) were within the regional and international standards (Table S2). Critically, there were no significant differences between full-term and preterm groups in the CDI or Bayley test scores.
Correlations Between the Clinical and Electrophysiological Evaluations.
No significant correlations were observed between the Mental and Psychomotor Developmental Indexes of the Bayley test given at 24 months and the gamma-band power amplitude (for any language) or latency of the early ERP measurements in any group of infants.
Discussion
The gamma-band results (Fig. 3) for the more mature infants (i.e., FT6 and PT9) are the only ones that show greater activity for Spanish as compared with Italian. These results mesh well with behavioral data showing that full-term infants distinguish the maternal language from a rhythmically similar language only 4 months after birth (6, 7). Clearly, FT6 and PT9 infants have had more than 4 months of exposure to broadcast speech. Crucially, the induced gamma-band response of PT6 and FT3 infants to Spanish and Italian is not significantly different between the two groups. In contrast, the FT6 infants respond significantly only to Spanish and not to Italian. The induced gamma-band response of the PT6 infants is thus more similar to that of the most immature infants (i.e., FT3), even though PT6 infants had received more than 4.5 months of broadcast speech exposure. We suggest that PT6 and FT3 infants still respond to Italian because they have not attained the level of neural maturation that might allow them to recognize that the maternal language is separate from other rhythmically similar languages. Although, we cannot discard the influence of other unknown factors, our results are consistent with numerous behavioral studies that have previously documented this language acquisition landmark.
Moreover, our early ERP results agree with previous studies showing that changes in latency, amplitude, and spatial distribution reflect age-related gains in neural maturation (14, 15). First, we found that the latency of the P200 was significantly longer in FT3 and PT6 infants as compared with F6 and PT9 infants, respectively, suggesting similar improvements in neural development for full-term and preterm infants. The previously mentioned studies showed that the latency of the P150 decreases significantly between 3 and 6 months of age in full-term infants. We conjecture that the P150 latency reported in previous studies and the P200 latency observed in the current study reflect the same neural and cognitive correlates. Previous studies used a few syllables and tones, whereas our study used a larger set of spoken sentences. Second, we showed that a clear N250 is mostly absent in FT3 and PT6 infants but becomes stronger in FT6 and PT9 infants. These observations are also consistent with previous studies showing that full-term infants demonstrate a substantial increase in N250 from 3 to 6 months of age (13). Taken together, these results confirm that the neural substrate for auditory processing is less mature in the younger groups of both full-term and preterm infants. Our results show that to attain the aforementioned early landmark of language acquisition, the highly premature infants must reach the required level of brain maturation. Indeed, the premature infants, who, like the full-term infants, had near 6 months of broadcast speech experience, failed to display a discriminatory neural signature to two rhythmically similar languages. In contrast, when the premature infants were given 9 months of exposure to clear speech, and thus reached a comparable level of neural maturation as the full-term infants, they displayed a discrimination response to the two rhythmically similar languages. Neural maturation and plasticity are the best potential candidates to explain the impact of experience on language acquisition. Our results suggest that neural maturation plays a more central role for language acquisition at this early stage of life.
Studies on gamma-band activity during early cognitive development are rare. However, a recent study has shown that 8-month-old but not 6-month-old infants display an increase in gamma-band power when they manifest behavioral evidence about the notion of object permanence (9). Further studies are needed to determine the precise nature of the changes in the gamma-band activity associated with speech processing. However, we can conjecture that the increase in gamma-band activity observed in our study reflects an improvement in the infants’ ability to focus on specific features of their native language, for instance, native phonemes and syllabic structures among others. Indeed, studies with adult participants have shown an increase in gamma-band activity during visual (19) and auditory (20) selective attention. Learning to distinguish the native language from other rhythmically similar languages requires the recognition of segmental properties that apply to one language but not to the other. Exposure to speech may allow for the characterization of vowels and consonants in Spanish that do not arise in Italian. Individuating such segmental properties requires a certain level of brain maturation as well as 4.5 months of exposure. However, as we pointed out previously, 4 months of exposure to the maternal language does not suffice to boost learning the linguistic elements that are unique to the maternal language in an immature brain.
From a methodological point of view, our results further support the functional role of gamma-band oscillations during the first steps of language acquisition. Contrary to a recent study that attributes the gamma activity recorded on the scalp to muscle activity alone (21), our results are congruent with the view that movements are not the only possible source for observed gamma-band oscillation activity. Indeed, our gamma-band response involves a narrow range of frequencies (55–75 Hz) and only appears for the native language exclusively in mature infants.
Our study presents two main findings. First, our gamma-band power-based study shows that brain maturation constrains the impact of experience on language acquisition during the first months of life. Second, gamma-band power is a sensitive marker for tracking the early stages of language learning and is congruent with a large number of behavioral studies. Nevertheless, other studies relying on different methodologies have found that fetuses can acquire some speech properties at earlier stages of neural maturation. Hopefully, the recent interest in studies of neonates and fetuses will help us to define when speech exposure most effectively triggers the earliest acquisition of language properties at different stages of maturation. Although our results are robust and significant, we agree that no single experiment can definitively rule out the intervention of unsuspected exposure factors. We hope that this warning will trigger future experiments using speech landmarks as a yardstick, leading to further investigations on the interplay of exposure and neural maturation.
Conclusion
The results of this study expand our understanding of the interaction of biological constraints and the experience that is needed to attain the early stages of language acquisition. These results provide important information for models of cognitive and language development for mature and immature infants. Moreover, they suggest that tracking brain oscillation can play an important role in the study of early language acquisition.
Materials and Methods
Participants.
We excluded seven FT3, eight FT6, five PT9, and seven PT6 infants because they failed to complete the experiment. At birth, all infants presented: (i) Apgar scores greater than 6 and 8 at 1 and 5 minutes, respectively; (ii) weight, size, and head circumference within the norm for gestational age; and (iii) normal otoacoustic emissions. Pediatric assessments for full-term and auditory brainstem-evoked responses, cranial ultrasound, and neuropediatric evaluation in premature infants were within the norm for gestational age and continued to be normal in all the periodical tests, which were continued until the end of the 2-year follow-up. Infants were only exposed to Spanish in a lower-middle class environment. In the 6 to 9 months after conception, full-term infants perceived muffled speech inside the uterus. Because the auditory system is functional near the 25th week of gestational age (for a recent review, ref. 22), preterm infants could perceive broadcast speech.
All preterm infants were periodically stimulated after birth, outside the incubator with broadcast speech mainly under the following circumstances (Table S1):
During the Kangaroo protocol, a medical procedure widely used in the neonatal intensive care unit, consisting of skin-to-skin contact between the mother and the newborn (23). The Kangaroo protocol starts as soon as possible after birth and lasts from 15 minutes to 4 hours per day while infants are in the incubator. On average, PT6 and PT9 infants were evaluated 6 and 8.5 months, respectively, after they started to be exposed to broadcast speech during the Kangaroo protocol.
As soon as the preterm newborns were physiologically stable and their temperature was regulated, they were placed in open cradles. The open cradles were located in a room shared by six to eight infants, where they were cared every 2 hours for feeding, cleaning, and clinical control. In this room, premature infants were regularly exposed to broadcast speech from their mothers, but they also could hear speech from other mothers, medical staff, relatives, and other persons who talked to or near to them. PT6 and PT9 infants were evaluated at 5.7 and 8.2 months, respectively, after they started to hear broadcast speech in open cradles.
Based on international recommendations (24), preterm infants were discharged to home when they were physiologically stable, had reached a weight of 1,800 g, and exhibited nutritive sucking that was mature enough to ensure proper feeding. On average, these criteria were reached at near 34 weeks of gestational age.
At the time of testing, the mean duration of exposure to broadcast speech at home was 4.8 and 7.4 months for PT6 and PT9 infants, respectively. The duration of the exposure to broadcast speech in FT3 and FT6 infants is shown in Table S3. The present study received approval from the Ethics Board for Biomedical Research of the Hospital Sótero del Río, Santiago de Chile. Parents signed a written informed consent form.
Stimuli.
We recorded speech samples from three female monolingual native speakers per language (i.e., three Spanish, three Italian, and three Japanese speakers) while they uttered a series of 54 sentences using adult-directed speech. We obtained stimuli from nine speakers to avoid a voice habituation response. From the set of the recorded utterances, we selected 18 per speaker, matched as much as possible for their acoustic properties (SI Sound Sample). This set of utterances was similar in mean energy (rms = 0.18, 0.19, and 0.18 Pa for Italian, Japanese, and Spanish, respectively), syllable number (15–18 syllables), and duration (2,800–3,000 ms). A native speaker of each language verified that the selected utterances were correct.
Procedure.
Infants were tested in a soundproof Faraday cage. While holding the infant during testing, the parent heard masking sounds. Utterances were pseudorandomly presented in groups of four consecutive utterances per language, separated by 800-ms silent pauses. Inside each group of utterances, no consecutive repetition of the same utterance or same speaker was allowed. Utterances from different languages were separated by 2,000–2,200-ms pauses. Repetition of groups of utterances from the same language was not permitted. All utterances were delivered at 60-dB sound pressure level. Randomly ordered attention-grabbing attracting images were centrally displayed on a monitor. The order of language presentation was counterbalanced across participants. The study was immediately stopped when the infants manifested discomfort (crying or fuzziness), which was very rare for the infants in this study. The experiment was stopped for less than 60 seconds for only four infants.
EEG Data Acquisition.
EEG data were collected using a 64-electrode geodesic-sensor-net (Electrical Geodesic, Inc. system) referenced to the vertex. The EEG was digitalized at 500 Hz.
Analysis of the Brain Oscillations.
The brain oscillatory activity analysis was focused on passive listening to each utterance. The raw EEG signal was low band-passed at 100 Hz with a 50-Hz notch filter (Fig. S1 shows an example of the signal with and without the 50-Hz notch filter) and then segmented into a series of 3,800-ms long epochs starting at 800 ms before the stimulus onset. To reject contaminated epochs, we applied an automatic process followed by visual verification. First, epochs containing more than 15 electrodes with voltage fluctuations exceeding ±130 μV, transients exceeding ±100 μV, or electrooculogram activity exceeding ±100 μV were rejected. All electrodes were taken into account for this procedure. Next, epochs contaminated by visually detected artifacts were also rejected from the analysis (the epochs rejected by visual criteria concerned, at most, one trial by an infant). Finally, the contaminated channels of the remaining epochs were excluded from the analysis. Each infant contributed 30 to 54 utterances per language to the computation of the spectral activity. For each nonrejected epoch, for each participant and at every frequency bin, we applied a sliding-window fast Fourier transform for amplitude values (window length = 232 ms, step = 10 ms, window overlap = 90% for the >10-Hz frequency range; window length = 500 ms, step = 10 ms, window overlap = 95% for the 1–10-Hz frequency range). For all infants and samples, the amplitude at every frequency bin was computed as follows (25). Signal windows (232 or 500 points) were zero-padded and fast Fourier-transformed to get an interpolated frequency resolution of ∼1 Hz per frequency bin. Instantaneous amplitude was then computed by taking the real and imaginary Fourier coefficients [C(f,t)r and C(f,t)i], squaring and adding them, and taking the square root (sqrt) as follows:
This amplitude is equivalent to the magnitude of the observed oscillation at a given time and frequency point, and it was used to construct a time-frequency map by epoch. Each time-frequency map was normalized against an 800-ms prestimulus baseline and averaged across the nonrejected epochs and 48 nonperipheral electrodes. Peripheral electrodes were frequently noisy across infants and were excluded from the analysis (Fig. S2). Normalization involves subtracting the baseline average and dividing it by the baseline SD on a frequency-by-frequency basis, wherein S is a signal, μ is the average of the signal during the baseline period, and σ is the SD of the same baseline period. The normalized signal, SN, is then computed by
To identify relevant time and frequency ranges for the analysis, we applied the following procedure. For each infant, we computed an average of the time-frequency responses for all three languages. This average comprises a 100 × 1,900 matrix of signal amplitude values: 100 frequency bins from 1–100 Hz and 1,900 time samples from 800 ms before the utterance onset until 3,000 ms thereafter (the sampling period was 2 ms). The first 400 time samples of this average response matrix correspond to the silent period right before utterance onset that we take as baseline. To obtain a single baseline value for each infant, we averaged the first 400 time samples of the response matrix at each frequency bin. We were thus left with a 100 × 1,501 matrix of response amplitudes per baby, wherein the first time sample corresponds to baseline. Each of the 100 × 1,500 nonbaseline values of these response matrices was submitted to a paired t test comparison (α = 0.5, two-tailed) with all infants pooled irrespective of group. At each frequency bin, we compared each of the 1,500 time samples of the infant group’s spectral power with the single point of the corresponding baseline. Using this procedure, we identified a time-frequency range from 55 to 75 Hz and from 300 to 2,800 ms after the onset of the utterances, wherein the spectral power of the brain activity was significantly different from baseline (Fig. 4). Because we pooled all groups and languages together, the obtained time-frequency range is not a priori biased toward any one of them.
ERP Analysis.
For the ERP analysis, the continuous EEG signal was low-pass-filtered (20 Hz) with a finite impulse response filter (Kaiser), which has a linear phase response [passband gain = 99% (50–99.9%, −0.1 dB), stopband gain = 1% (1–49.9%, −40.0 dB)], and was then segmented into a series of 3,200-ms long epochs starting 200 ms before the utterance onset. Epochs containing more than 15 electrodes with voltage fluctuations exceeding ±130 μV, transients exceeding ±100 μV, or electrooculogram activity exceeding ±100 μV were rejected. From the pool of artifact-free epochs (n = 162), we selected a subset of those for which all infants had contributed at least 1 epoch. This selection allowed us to compare the ERP responses across groups using the identical set of acoustically matched utterances. This set of utterances was averaged by infant, digitally transformed to an average reference, and baseline-corrected over a 200-ms prestimulus window. Each infant contributed 50 utterances to the computation of the ERP. We visualized the mean ERP using EEGLAB (26). The ERP comprised two early components: a central/anterior bilateral positivity observed in all groups and a central/posterior bilateral negativity observed almost exclusively in older infants (i.e., FT6, PT9) (Fig. 5). The mean latency of the peak and the mean zero-to-peak amplitude of the positive and negative components of the ERP were computed across the anterior and posterior groups of electrodes, where the maximum activity was observed across all groups (Fig. 5). The mean latency and amplitude of the early ERP components were then submitted to statistical comparisons using one-way ANOVA.
Supplementary Material
Acknowledgments
We thank the infants and parents who participated in our study. We also thank the personnel at the Hospital Sótero del Río y Consultorio Recreo, Santiago de Chile, who helped us to recruit the infants. We thank nurses Margarita Luna, Paula Soto, and Patricia Fernández for their help in many phases of the study; we also thank Lucía Melloni for her help in testing infants and for her contribution to the analysis of data. We express our gratitude to Susana Franck, who helped us with the editing of this manuscript. M.P. was supported by a grant from Fondecyt (Grant 1060767) and Conicyt-PBCT (Grant CIAE-05). J.M. was supported by a grant from the James S. McDonnell Foundation.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914326107/DCSupplemental.
References
- 1.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 3.Kisilevsky BS, et al. Fetal sensitivity to properties of maternal speech and language. Infant Behav Dev. 2009;32:59–71. doi: 10.1016/j.infbeh.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Nazzi T, Bertoncini J, Mehler J. Language discrimination by newborns: Toward an understanding of the role of rhythm. J Exp Psychol Hum Percept Perform. 1998;24:756–766. doi: 10.1037//0096-1523.24.3.756. [DOI] [PubMed] [Google Scholar]
- 5.Ramus F, Nespor M, Mehler J. Correlates of linguistic rhythm in the speech signal. Cognition. 1999;73:265–292. doi: 10.1016/s0010-0277(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 6.Nazzi T, Jusczyk P, Johnson EK. Language discrimination by English-learning 5-month-olds: Effects of rhythm and familiarity. J Mem Lang. 2000;43:1–19. [Google Scholar]
- 7.Bosch L, Sebastián-Gallés N. Native-language recognition abilities in 4-month-old infants from monolingual and bilingual environments. Cognition. 1997;65:33–69. doi: 10.1016/s0010-0277(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 8.Robertson A, Stuart A, Walker L. Transmission loss of sound into incubators: Implications for voice perception by infants. J Perinatol. 2001;21:236–241. doi: 10.1038/sj.jp.7210531. [DOI] [PubMed] [Google Scholar]
- 9.Csibra G, Davis G, Spratling MW, Johnson MH. Gamma oscillations and object processing in the infant brain. Science. 2000;290:1582–1585. doi: 10.1126/science.290.5496.1582. [DOI] [PubMed] [Google Scholar]
- 10.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 11.Canolty RT, et al. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci. 2007;1:185–196. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nir Y, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 13.Jing H, Benasich AA. Brain responses to tonal changes in the first two years of life. Brain Dev. 2006;28:247–256. doi: 10.1016/j.braindev.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushnerenko E, et al. Maturation of the auditory event-related potentials during the first year of life. NeuroReport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan HG, Jr, Kurtzberg D. Electrophysiologic indices of human brain maturation and cognitive development. In: Gunnar MR, Nelson CA, editors. Minnesota Symposia on Child Psychology. Hillsdale, NJ: Erlbaum; 1992. pp. 1–36. [Google Scholar]
- 16.Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. Nature and nurture in language acquisition: Anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006;29:367–373. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Jackson-Maldonado D, et al. Communicative Development Inventory: User’s Guide and Technical Manual. Baltimore: Brookes; 2003. (in Spanish) [Google Scholar]
- 18.Bayley N. Bayley Scales of Infant Development. 2nd Ed. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 19.Tallon-Baudry C. The roles of gamma-band oscillatory synchrony in human visual cognition. Front Biosci. 2009;14:321–332. doi: 10.2741/3246. [DOI] [PubMed] [Google Scholar]
- 20.Skosnik PD, Krishnan GP, O’Donnell BF. The effect of selective attention on the gamma-band auditory steady-state response. Neurosci Lett. 2007;420:223–228. doi: 10.1016/j.neulet.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 21.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 22.deRegnier RA. Neurophysiologic evaluation of brain function in extremely premature newborn infants. Semin Perinatol. 2008;32:2–10. doi: 10.1053/j.semperi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics. 2002;110:16–26. doi: 10.1542/peds.110.1.16. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate—Proposed guidelines. Pediatrics. 1998;102:411–417. [PubMed] [Google Scholar]
- 25.Melloni L, et al. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.