Abstract
Theory suggests evolutionary change can significantly influence and act in tandem with ecological forces via ecological-evolutionary feedbacks. This theory assumes that significant evolutionary change occurs over ecologically relevant timescales and that phenotypes have differential effects on the environment. Here we test the hypothesis that local adaptation causes ecosystem structure and function to diverge. We demonstrate that populations of Trinidadian guppies (Poecilia reticulata), characterized by differences in phenotypic and population-level traits, differ in their impact on ecosystem properties. We report results from a replicated, common garden mesocosm experiment and show that differences between guppy phenotypes result in the divergence of ecosystem structure (algal, invertebrate, and detrital standing stocks) and function (gross primary productivity, leaf decomposition rates, and nutrient flux). These phenotypic effects are further modified by effects of guppy density. We evaluated the generality of these effects by replicating the experiment using guppies derived from two independent origins of the phenotype. Finally, we tested the ability of multiple guppy traits to explain observed differences in the mesocosms. Our findings demonstrate that evolution can significantly affect both ecosystem structure and function. The ecosystem differences reported here are consistent with patterns observed across natural streams and argue that guppies play a significant role in shaping these ecosystems.
Keywords: ecological–evolutionary feedbacks, intraspecific variation, ecosystem function
Ecosystem ecologists commonly view populations as homogeneous biomass pools in which individuals operate in identical ways to influence nutrient and energy flows (1). Individual organisms can influence ecosystem processes by altering their body size (material storage), changing their consumption and excretion characteristics (material flux) (2), modifying their internal stoichiometry (3), or physically altering their habitat (4, 5). Differences among individuals can, via natural selection, become converted into differences among populations and, hence, in the impact of a locally adapted population on the structure of its ecosystem. Furthermore, empirical evidence suggests the evolution of organismal traits that can affect habitat utilization happens on timescales similar to ecological processes (6). One possible consequence of rapid evolutionary change is that it can change ecological dynamics and set up feedbacks between ecological and evolutionary processes (7 –9). Central to this hypothesis is the assumption that phenotypic variation translates into variation in how individuals and populations impact their environment (10).
Prior research has already established the links between ecology and evolution. Laboratory studies focused on a model predator–prey interaction demonstrated that evolution of the prey population significantly altered the nature of predator–prey cycles (9). Evidence from natural or seminatural settings have shown that phenotypic differences in prey selectivity (11, 12) or nutrient recycling (13) can alter community and ecosystem structure (11 –13) and some aspects of ecosystem function (11). Studies of natural populations of landlocked and anadromous alewives (Alosa pseudoharengus) established differences in how these two forms influence the structure of the zooplankton community (12), then how the effects of landlocked alewives on the zooplankton community may have fed back on the subsequent evolution of the trophic morphology of alewives (12, 14, 15). The influence of the phenotype on ecosystem structure has also been documented in Trinidadian guppies (Poecilia reticulata) (13). Guppies from low predation (LP) localities co-occur with killifish (Rivulus hartii), an omnivore that may also prey upon juvenile guppies (16). Guppies from high predation (HP) localities co-occur with a diversity of predators, including the pike cichlid (Crenicichla alta) (16 –18). When LP and HP phenotypes were placed in mesocosms with killifish, mesocosms with HP guppies had higher algal accrual rates (13). HP guppies had less chlorophyll-a in their guts and, at the population-level, excreted NH4 at a higher rate, either of which could contribute to observed differences in algal accrual. However, such changes in the community structure do not always translate into changes in ecosystem function (19). Harmon et al. (11) tested the idea that three-spined sticklebacks (Gasterosteus aculeatus) adapted to foraging on different items could cause divergent effects on ecosystem function. They found that stickleback morphs did influence algae biomass and productivity by setting up a positive feedback between dissolved organic carbon and algae productivity. The mechanisms by which sticklebacks initiate this effect are unknown.
Here, we tested the ability of Trinidadian guppies from LP and HP population types with distinct genotypes and population characters to cause changes in ecosystem structure and function. Previous studies have documented that guppies from HP localities experience higher mortality rates and consequently exhibit phenotypic and genetic differences in their life history (18, 20), morphology (16), performance (21), and behavior (22) when compared to LP guppies. Experiments wherein guppies have been transplanted from HP sites to previously guppy-free (Rivulus only) sites demonstrate that these traits evolve on ecologically relevant time-scales (e.g., ref. 23). Combined, these results argue for a direct role of predators in shaping how guppies evolve. Variation in the mortality regime of guppy populations has indirect consequences that may also alter the way that guppies interact with their environment. Increased predation causes a decrease in the density and biomass of guppies and a shift to populations dominated by smaller individuals (24, 25). These changes are a direct consequence of predators eating larger guppies, the evolution of the life history toward earlier maturity, and the production of more offspring (25). All of these changes contribute to an increase in the per-capita food availability (26) and somatic growth rates in HP localities (24). Thus, both direct and indirect effects of predation may shape how guppies adapt to their local environment and alter the influence of the individual on the environment.
We report on a replicated common garden mesocosm experiment that tests the ability of multiple aspects of these two phenotypes and population characters to alter ecosystem structure and function. Mesocosms were stocked with either HP or LP fish at low or high densities (12 and 24 guppies, respectively). Four additional mesocosms per trial were set up with no guppies to evaluate the general effect of guppies on the ecosystem. The experiment was replicated with guppies from HP and LP sites on the Guanapo and Aripo Rivers. We quantified how guppies adapted to HP and LP environments differ in their impact on ecosystem structure (algal, invertebrate, and detrital standing stocks) and function (gross primary productivity, community respiration, leaf decomposition, and nutrient flux) after 4 weeks, and tested the effects using a priori contrasts. We first evaluated general effects of guppies on ecosystem variables by comparing fishless treatments with all treatments that contained guppies (C1). Second, we examined effects of evolved phenotypic differences on ecosystem processes by comparing effects of guppies from HP and LP treatments (C2). Third, we used density treatments to examine ecological consequences of guppy population density, which changes in response to predation (C3). Finally, we investigated the interaction between the phenotype and population density (C4). We also directly measured guppy interactions with their environment (feeding rate, resources consumed, nutrient excretion) in the mesocosms and resources consumed from wild-caught guppies to identify the specific components of the phenotype that may alter ecosystem structure and function.
Results
Guppies used in these experiments showed similar patterns of life-history differences between phenotypes observed in previous studies (18, 27). HP guppies carried more developing embryos (ANOVA: Phenotype, F 1,8 = 17.03, P = 0.003) (Tables S1 and S2) and allocated a higher proportion of their body mass to reproduction (ANOVA: Phenotype, F 1,5 = 24.62, P = 0.004) (Tables S1 and S2). These differences in life-history traits were consistent across the two experimental trials and support the validity of the phenotype treatments (Tables S1).
The presence of guppies in the mesocosms caused a significant reduction in algal standing stocks (Fig. 1, Table 1, and Tables S3) and a concomitant decline in area-specific gross primary productivity or GPP (Fig. 1, Table 1, and Tables S3) and community respiration (CR24) (Table 1 and Tables S3). However, guppies increased biomass-specific GPP and significantly depressed total invertebrate biomass (Fig. 1, Table 1, and Tables S3), especially chironomids, which were the dominant invertebrate taxon (Tables S3).
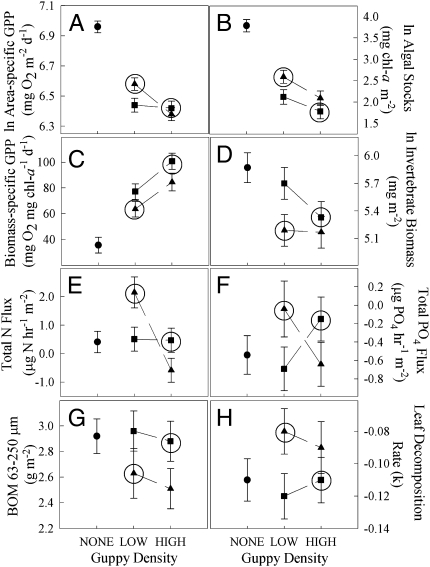
Fig. 1.
Estimated marginal means (±1 SE) of guppy effects on (A) area-specific GPP, (B) algal standing stocks, (C) biomass-specific GPP, (D) invertebrate biomass, (E) total N flux, (F) PO4 flux, (G) BOM, and (H) leaf decomposition rate. No guppy (circles), LP guppy (squares), and HP guppy (triangles). Circled values are LP and HP phenotypes at their natural relative densities.
Table 1.
Ecosystem effects
| Effect | Algae | A-GPP | CR24 | B-GPP | Leaf decomp | Total invert | Chirono invert | BOM 63–250 | BOM > 250 | PO4 flux | NH4 flux | NO3 flux | Total N flux |
| Fish (C1) | N* | N* | N‡ | F* | — | N‡ | N‡ | — | F | — | — | — | — |
| Phenotype (C2) | HP‡ | HP | HP | LP‡ | LP‡ | LP† | LP‡ | — | LP† | — | — | — | — |
| Density (C3) | LD‡ | LD‡ | — | HD§ | — | LD | — | HD‡ | — | — | HD | HD‡ | HD‡ |
| Phenotype x Density (C4) | — | Non† | — | — | — | Ord | Ord† | — | — | Non† | — | Non‡ | Non‡ |
Codes indicate treatment with the highest mean for the contrast (—, no trend; F, guppies; HD, high density; HP, high predation; LD, low density; LP, low predation; N, no guppies). Interactions are shown as ordinal (Ord) or nonordinal (Non). A-GPP, area-specific GPP; B-GPP biomass-specific GPP.
*, P < 0.001;†, P < 0.10;‡, P < 0.05;§, P < 0.01.
An increase in the guppy population density, regardless of phenotype, caused a further reduction in algal standing stocks and a decrease in area-specific GPP, but caused an increase in biomass-specific GPP (Fig. 1, Table 1, and Tables S3). Increased guppy density was also associated with an increase in the ash-free dry mass of benthic organic matter (BOM) between 63 and 250 μm (Fig. 1, Table 1, and Tables S3).
Guppy phenotype had an impact on standing stocks and ecosystem processes independent of density. Mesocosms with HP guppies had higher algal standing stocks but lower biomass-specific GPP (Fig. 1, Table 1, and Tables S3) than those with LP guppies. HP mesocosms had a marginally significant lower total invertebrate biomass (Fig. 1, Table 1, and Tables S3) and significantly lower chironomid biomass (Table 1 and Tables S3) than those with LP guppies. At the same time, HP guppies caused a significant decrease in the rate of leaf decomposition (Fig. 1, Table 1, and Tables S3) and a marginally significant decrease in standing stocks of BOM greater than 250 μm (Table 1 and Tables S3).
There were also significant interactions between guppy phenotype and population density for some ecosystem variables. Area-specific GPP of mesocosms with HP guppies was higher than those with LP guppies, but only at low population densities (Fig. 1, Table 1, and Tables S3). Flux of PO4 showed a marginally significant nonordinal interaction with higher net production in LP low-density and HP high-density treatments (Fig. 1, Table 1, and Tables S3). Total N (NH4 + NO3) flux showed a nonordinal interaction with little or no effect of density for LP, but decreased net production for HP low-density treatments and increased net production for HP high-density treatments (Table 1 and Tables S3).
There were significant river-of-origin (drainage) effects for 9 of the 13 ecosystem variables measured. However, there was never a significant interaction between treatment and drainage (Tables S3), so differences between guppies derived from HP versus LP environments were repeatable across rivers of origin.
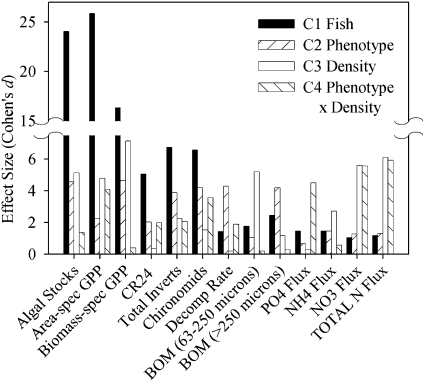
The presence of guppies in the mesocosms (C1) had the largest effect on 6 of the 13 measures of ecosystem structure and function (Fig. 2). Guppy phenotype (C2) had a larger impact than density (C3) on CR24, two measures of invertebrate biomass, leaf decomposition rates, BOM (> 250 μm), and PO4 flux. Density had a larger impact than phenotype on algal standing stocks, area-specific GPP, biomass-specific GPP, BOM (63–250 μm), and three measures of N flux (Fig. 2).
Fig. 2.
Effect sizes (Cohen’s d) from planned contrasts on ecosystem effects.
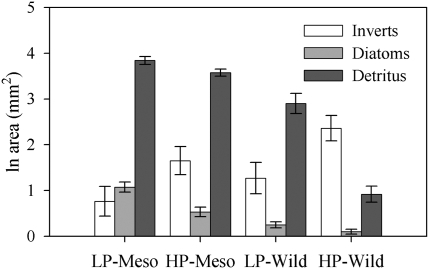
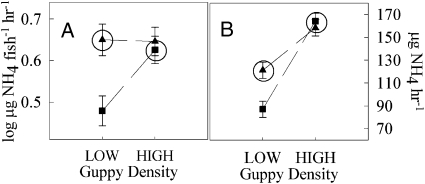
Analysis of gut contents showed that LP and HP fish from the experiment consumed different resources (Fig. 3, Table 2, and Tables S4). HP guppies had marginally more invertebrates and significantly less diatoms and detritus (Fig. 3, Table 2, and Tables S4) in their guts as compared to LP guppies. Identical analyses on guppies taken from natural streams show these same phenotype differences in resource consumption (MANOVA: Phenotype: F3,10 = 13.65, P = 0.001) (Fig. 3 and Tables S4), and confirm that patterns of resource consumption by guppies in the mesocosms reflect those in the wild. Differences in diet between the two guppy phenotypes were not associated with differences in feeding rates because both phenotypes pecked at the substrate at an equal rate (Table 2 and Tables S4). Individual female and juvenile guppies from HP environments excreted NH4 at higher rates than their LP counterparts (Fig. 4 Table 2, and Tables S4), but only in the low-density treatment. At the population-level, HP populations excreted NH4 at marginally significant higher rates than LP populations, but only in the low-density treatments (Fig. 4, Table 2, and Tables S4).
Fig. 3.
Estimated marginal means (±SE) of the area of a microscope slide covered by each of three food categories consumed by LP and HP guppies from the mesocosms and the wild.
Table 2.
Guppy mechanisms
| Feeding rates | Female excretion | Male excretion | Pop excretion | Diet | ||||||
| Effect | 14–18 mm | >18mm | NH4 | PO4 | NH4 | PO4 | NH4 | Invert | Diatom | Detritus |
| Phenotype | LP | — | HP‡ | — | LP | LP | HP† | HP† | LP§ | LP‡ |
| Density | LD | LD§ | — | HD‡ | — | HD | HD* | LD | HD‡ | HD |
| Phenotype × density | — | — | Ord‡ | — | Ord | Ord | Nonord* | — | — | — |
Codes indicate treatment with the highest mean for the contrast (—, no trend; F, guppies; HD, high density; HP, high predation; LD, low density; LP, low predation; N, no guppies). Interactions are shown as ordinal (Ord) or nonordinal (Non).
*, P < 0.001;†, P < 0.10;‡, P < 0.05;§, P < 0.01.
Fig. 4.
Estimated marginal means (±SE) of LP (squares) and HP (triangles) of (A) individual-level NH4 excretion rates and (B) population-level NH4 excretion rates. Circled values are LP and HP phenotypes at their natural relative densities.
Discussion
Mesocosms with HP guppies had higher algal standing stocks, higher area-specific GPP, lower biomass-specific primary production, lower invertebrate standing stocks, and lower leaf decomposition rates at the end of the 4-week period when compared to mesocosms with LP phenotypes. If we consider the effects of phenotype and density within the context of the natural densities in which these phenotypes are found (LP fish in higher densities), then differences in the ecosystem effects between populations often become more pronounced (Fig. 1). High-predation guppies display greater food selectivity, consuming more invertebrates and less diatoms and detritus. At the same time, individual and populations of HP guppies exhibited higher rates of NH4 excretion at low, but not high densities. These results thus argue that there are substantial differences among guppy phenotypes in their impact on ecosystem structure. We consider here three possible mechanisms for these differences: nitrogen excretion, dietary preference, and trophic cascades.
Palkovacs et al. (13) reported that individual HP guppies contained less chlorophyll-a in their guts and, at the population-level, excrete NH4 at a higher rates. They suggested that HP guppies may increase algal accrual rates by excreting NH4 at a higher rate, via differential consumption of algae or some combination of both. Increased nutrient excretion could cause changes in algal standing stocks by increasing primary production (measured as GPP). At the same time, decreased guppy grazing can reduce the rate of loss of algae. Thus, changes in nutrient excretion or grazing could act independently or synergistically to produce the observed changes in algal standing stocks. We found that individual HP guppies do have higher rates of NH4 excretion, but only at low densities. When scaled up to the population-level, the patterns of NH4 excretion mirrored those found at the individual level (Fig. 4). We found that HP guppies consumed less diatoms and detritus, but more benthic invertebrates compared to LP guppies in both the mesocosms and in samples of guts from wild-caught guppies (Fig. 3 and Table 2). These differences in gut contents act in opposition to the patterns of resource availability in the mesocosms; HP mesocosms have fewer invertebrates and more algae, but there are more invertebrates and less algae in their guts. The observed patterns in algal standing stocks (higher stocks in HP mesocosms at both densities) are consistent with decreased HP guppy grazing as the major factor driving the differences between HP and LP mesocosms. At the same time, the observed pattern of nutrient excretion between the phenotypes is consistent with differences in measurements of area-specific GPP: higher area-specific GPP in HP mesocosms at low, but not high density (Fig. 1A). However, when area-specific GPP is standardized by the amount of chlorophyll-a in each mesocosm (biomass-specific GPP—an index of individual production), LP mesocosms exhibit higher biomass-specific rates of production (Fig. 1C). Lower area-specific GPP coupled with higher biomass-specific GPP has been interpreted as evidence for grazers keeping producers in a rapid population growth phase (28).
Higher algal standing stocks in mesocosms with HP guppies could also arise from a trophic cascade, wherein HP guppies feed preferentially on invertebrates that graze on algae. Higher selectivity for invertebrates by HP phenotypes caused a larger reduction in invertebrate biomass, but these differences did not result in the expected pattern in algal stocks. Specifically, if HP guppies caused stronger trophic cascades then we should observe an increasing trend of algal standing stocks with guppy density and an interaction between phenotype and density with the largest differences between HP and LP at low densities (compare Figs. 1 B and D), but this was not the case.
We found a role for guppy phenotype in causing cascading effects in leaf decomposition rates, which would in turn cause differences in the rate nutrients in leaves are released into the aquatic ecosystem. The higher consumption rate of invertebrates by HP guppies was associated with lower rates of leaf decomposition. The previous mesocosm study with guppies found no association between guppy phenotype and invertebrate biomass or leaf decomposition rates (13). The lack of an observed effect in the previous study may have been caused by the use of mesh leaf-pack bags, which can exclude some invertebrate grazers (29). We employed a bagless leaf-pack design that allows full access to the leaf by stream biota. The dominant invertebrate taxa in mesocosms and in guppy guts were Chironomidae, which are typically classified as collector-gatherers (30). However, chironomids occupy diverse trophic niches and it is probable that taxa in our mesocosms feed on both autotrophs and microbial heterotrophs associated with leaves. Preferential predation on chironomids by guppies derived from HP environments could cause a reduction in the rate of nutrient input from terrestrial sources.
Ecosystem differences between HP and LP mesocosms often become more dramatic when phenotypes are viewed in the context of their natural population densities (Fig. 1); in natural populations, HP guppies are found at low population densities, while LP guppies are found at high population densities. For example, mesocosms with LP guppies had lower final algal standing stocks and algal standing stocks decreased with increasing guppy density for both phenotypes (Fig. 1B). In these cases, we see an additive role for both direct and indirect effects of guppy predators changing how guppies alter ecosystem structure and function. In other cases, the effect of guppy density acted to obscure the effect of the phenotypes. For example, invertebrate biomass decreased with increasing density of LP guppies, but remained nearly constant with increasing density of HP guppies (Fig. 1D). These cases highlight the need to consider the population-level context when testing the effects of the phenotype on the environment.
Diet preferences between LP and HP guppies could be driven by differences in the population density and resource availability in their natural environment. LP environments have higher guppy population densities (24, 25), which should depress per-capita food availability. In fact, LP habitats have consistently lower algal standing crops and lower primary productivity, and consequently guppies have lower growth rates relative to what is seen in HP environments (24, 25). Here we found that guppies from LP environments are less selective in their foraging, consuming invertebrates, diatoms, and organic matter. Under higher per-capita resource availability, typical of HP localities, guppies are instead more selective and consume a higher proportion of invertebrates. Diet differences may thus reflect the evolution of differences in selectivity under low versus high resource conditions.
Differences in the morphology of guppies from HP and LP environments have been interpreted as the result of varying selective pressures on swimming performance and escape from predation (31), yet differences in head shape are also suggestive of differences in trophic ecology reminiscent of those seen among benthic and limnetic sticklebacks that consume different resources (32). One consequence of these morphological differences is that benthic and limnetic sticklebacks differentially influence some measures of their ecosystem, as evaluated in replicate mesocosms (11). Our results lend support to the hypothesis that differences in the morphology of guppies are at least partially related to differences in trophic ecology.
Diet selectivity in alewives causes divergence of zooplankton communities, which can then subsequently feedback to alter the type of selection pressures experienced by the alewives (12, 14). We found similar effects of diet selectivity with guppies adapted to HP and LP environments and illuminate a potentially common theme in characterizing the effects of local adaptation on ecosystem structure and function. Because the differences in resource utilization of guppies affect multiple facets of ecosystem structure (i.e., algal, invertebrate, benthic organic material standing stocks) and function (i.e., GPP, leaf decomposition rates, nutrient flux) the results presented here expand the breadth of the possible effects of local adaptation beyond simple food-chain dynamics.
Conclusions
Ecology traditionally ignores any effect that evolution may have on ecological interactions because evolution is assumed to happen on a much slower time scale than ecology. Recent studies, including our experimental studies of evolution in guppies in natural streams, show that significant evolution can indeed occur on ecological time-scales (7, 33). We take a significant step in characterizing how the evolution of one member of a community can alter ecosystem structure and function by showing that guppies adapted to different environments differentially use resources and cause significant changes to their ecosystem in a matter of weeks. Our data suggest that the different impacts of LP and HP guppies are primarily a consequence of differences in diet between the phenotypes. We further demonstrate these effects are repeatable across two independent origins of the LP phenotype.
If the effects of phenotype and density are considered within the context of the natural densities in which these phenotypes are found (LP fish in higher densities), then differences in ecosystem effects between phenotype treatments often become more pronounced. For example, both phenotype and density contributed to differences in algal standing stocks. Surveys of HP and LP environments have shown higher algal standing stocks and higher productivity in HP localities (24, 26). These convergences between the mesocosms and natural streams suggest that ecosystem differences seen in natural streams are the combined product of local adaptation by guppies and the indirect effect of predators on guppy population density.
Materials and Methods
We constructed flow-through mesocosms by building eight rectangular block and cement structures (3 m × 1 m) adjacent to Ramdeem stream, a natural stream in Verdant Vale, Trinidad. These structures were subdivided to yield 16 mesocosms. We piped water from a nearby spring through three settling tanks. The final tank was fitted with 16, 50-foot 3/4-inch-diameter hoses to supply water to each mesocosm. We added a mixture of sand and gravel to a depth of ∼5 cm. Water was allowed to fill the mesocosms to a depth of 16 cm. Water inflow was adjusted using inline valves at the head of each mesocosm and water flowed out of the mesocosms through a fabric-covered drain at the foot of each channel. We inoculated the mesocosms with invertebrates from a nearby stream. We collected invertebrates from an area of the stream equivalent to the total benthic area of all of the mesocosms and added them in equal proportions to the mesocosms.
Guppies from the Aripo drainage and the HP location on the Guanapo have previously been shown to have evolved genetic differences in life history characters (18, 27). Low and high densities in the mesocosms reflected averages observed for LP and HP sites in prior studies of these communities (18, 24). If these values are translated into the mesocosms (1.5 m2), there should be 6.5 and 11.6 guppies with ranges from 0.6 to 24 and 3 to 37.5 guppies for low- and high-density treatments, respectively. Our density treatments represented a doubling of densities between high and low density, which is slightly larger than observed average differences between natural populations (Table 3). The absolute number of fish in each density treatment was higher than the means observed in HP and LP localities, but was well within the range of observed variation. We increased the absolute numbers above the means to reduce the effects of variation among individuals in each mesocosm replicate. In terms of biomass, natural LP populations exhibit a 4-fold increase in biomass compared to HP populations (24). By holding the size structure of the experimental populations equal, the change in biomass is approximately 2-fold in our experiment (Table 3), which results in an underestimate of the biomass effect between population types (24). The experiment was set up in a block design with one guppy density-times-phenotype combination per block. One “no fish” channel was set up per block, except for one block that received two. Treatments were randomly assigned to mesocosms in each trial. Mesocosms with fish contained guppy populations with size distributions and sex ratios intermediate between those observed between LP and HP populations (24, 25). Each experimental trial lasted for 28 days.
Table 3.
Number (n) and biomass (g) (mean ± SE) of guppies at the beginning, all guppies at the end, and offspring born in the mesocosms
| Beginning |
End |
|||||
| All guppies |
All guppies |
Offspring |
||||
| Treatment | n | g | n | g | n | g |
| LPLD | 12 | 1.46 (0.05) | 26.8 (1.9) | 2.61 (0.20) | 15.5 (2.0) | 0.40 (0.08) |
| LPHD | 24 | 2.88 (0.08) | 50.2 (4.5) | 4.04 (0.17) | 27.0 (4.2) | 0.36 (0.06) |
| HPLD | 12 | 1.49 (0.02) | 47.3 (5.2) | 2.61 (0.15) | 36.7 (5.0) | 0.67 (0.14) |
| HPHD | 24 | 2.93 (0.07) | 86.3 (7.9) | 3.80 (0.10) | 64.3 (8.0) | 0.71 (0.12) |
HPLD, high predation, low density; HPHD, high predation, high density; LPLD, low predation, low density; LPHD, low predation, high density.
Benthic algae stocks were measured by placing five, unglazed ceramic tiles (5 cm × 5 cm) in each mesocosm. Tiles were collected at weekly intervals during the experiment and measured for chlorophyll-a using standard fluorometric techniques (34). We collected a single tile per mesocosm for the first 3 weeks and two tiles on the final day, yielding a time-series of algae accrual in the mesocosms. We report standing stocks on the final day of sampling. Analyses of algal accrual can be found in SI Materials and Methods (Fig. S1 and Tables S5). We measured leaf decomposition by constructing bagless leaf packs (29) and measuring their mass loss as a function of time. The percent-dry mass remaining was natural-log transformed and regressed against the collection day for each mesocosm. The slope of this natural log-linear relationship was used as a measure of decomposition rate (k) (35). We estimated the biomass of invertebrates in the mesocosms by sampling a known area of the benthic area on the last day of the trials. Invertebrates were separated from other organic material under a dissecting microscope after staining with Rose Bengal dye for 24 h. Invertebrates were identified to the family level (30), counted, and measured for length. Biomass estimates were obtained using known length–mass relationships (36). The remaining BOM from these benthic samples were filtered through 63- and 250-μm sieves and processed to obtain ash-free dry mass.
We measured GPP and CR24 in the mesocosms near the end of the experiment (Gaunapo: day 24; Aripo: day 25). We took hourly measurements of the O2 concentration (mg O2 L−1), temperature (°C), and barometric pressure (mm Hg) in the final settling tank and at the foot of each mesocosm starting 1 h before sunrise and ending 2 h after sunset using a YSI Model 556. GPP and CR24 were calculated using a two-station method (37). Area-specific GPP was calculated as GPP divided by the area of the mesocosms and biomass-specific GPP was calculated as area-specific GPP divided by algal standing stocks. We measured PO4, NH4, and NO3 flux rates on the final day of the experiment (day 28) for the Aripo trial only. We collected water from just under the inflow and just inside the outflow in each mesocosm. Water was poured through a filter (Pall A/E glass fiber) and analyzed for ammonium using a fluorometric method (38), phosphate as soluble reactive phosphorus (SRP) colorimetrically by a molybdate-antimony analysis (39) and nitrate by ion chromatography.
One medium (14–18 mm) and one large (>18 mm) female was observed once per week to quantify the attack rates of guppies. Fish were given 5 min to acclimate to investigator presence before observations. Each fish was subsequently observed for at least 1 min. During this time we recorded the number of foraging attempts (pecks on the substrate or on drifting objects). We quantified guppy nitrogen and phosphorus excretion by removing fish from the mesocosm and placing each in a sealable plastic bag filled with 100 mL of filtered stream water. We sampled water from the bags before the introduction of the guppy and after 20 min of incubation. Water samples were analyzed for ammonium using fluorometry (38) and for phosphate, as SRP, colorimetrically by a molybdate-antimony method (39). SRP was measured for each trial, but samples from the Guanapo trial were contaminated and we subsequently present only data from the Aripo trial. We calculated the population-level excretion rates by using these observed data to derive the allometric relationship between excretion rates and body mass for each treatment and drainage of origin using the allometric equation: log10 excretion rate = log10(b) + m log10 (body mass). We then applied this formula to the observed mass distributions for each mesocosms on the final experimental day of the each trial.
Fish were removed from the mesocosms on day 28 and killed using an overdose of MS-222 according to UC Riverside IACUC AUP #A-20080008. Each fish was measured for standard length to the nearest hundredth of a millimeter and wet mass to the nearest thousandth of a gram. Offspring number was measured as the count of developing embryos carried by the female at the time of dissection. Reproductive allocation was calculated as the proportion of the total female dry mass attributed to developing embryo and reproductive tissue mass. Embryo stage of development was used as a covariate in analyses of reproductive allocation. Gastrointenstinal tissues were removed and preserved in 5% formalin.Gut contents in the esophagus, stomach, and proximal portion of the intestine were measured from four fish per mesocosm per trial. Food categories were quantified as invertebrates, detritus, and diatoms. Wild guppies were removed from the streams and immediately killed using an overdose of MS-222 and preserved in 5% formalin. Laboratory processing and analyses of the data were identical to those for the guppies from the mesocosm experiments. All fish were taken from the Aripo and Naranjo rivers in the same locations as the fish used in the mesocosm experiment.
We tested for significant differences using a priori contrasts in an ANOVA framework with one factor (“treatment”) and five levels: no guppies (None); low predation, low density (LPLD); low predation, high density (LPHD); high predation, low density (HPLD); high predation, high density (HPHD). Contrasts were designed to test specific hypotheses in the appropriate subsets of the data [C1 (Fish): None vs. LPLD, LPHD, HPLD, HPHD; C2 (Phenotype): LPLD, LPHD vs. HPLD, HPHD; C3 (Density): LPLD, HPLD vs. LPHD, HPHD; C4 (Phenotype × Density) LPLD, HPHD vs. LPHD, HPLD]. In this and all subsequent analyses, treatment, drainage, and block were entered as fixed effects. We included ambient light levels as a covariate when it explained a significant amount of variation in the dependent variable. We removed covariates, block, drainage, and interactions from the main model when the F-ratio ≤ 1 for each effect. We interpreted main effects when the interaction did not influence the rank order of the main effects (i.e., ordinal). Complete statistical results for ecosystem effects and estimated marginal means can be found in the supplemental materials (Tables S3, S6).
We used a linear mixed model to test for differences in guppy feeding and excretion rates. We treated the mesocosm as our unit of replication, but measured traits on multiple fish per mesocosm. Therefore, we included mesocosm as a random effect and modeled the variance to remove any statistical nonindependence within mesocosms and to avoid overinflating our degrees of freedom. The models were fit by restricted maximum likelihood to avoid biases in the estimates of within- and between-mesocosm variances. In summary, the full model included block, drainage, phenotype, density, their interactions as fixed effects, and mesocosm as a random effect. Males and females were analyzed separately because they were known a priori to differ in their traits. Gut contents were analyzed using MANOVA and subsequent univariate ANOVAs. Life-history variables were analyzed using an ANOVA framework. For these analyses, trait values were the average across guppies within a mesocosm. Block, drainage, and interactions with phenotype and density that had an F-ratio ≤ 1 were removed from the models. Body size was included as a covariate when appropriate. Dependent variables were log or arcsin square-root transformed when appropriate to conform to the model assumptions. Data from the low-density treatments only were analyzed for confirmation of the phenotypes. Complete statistical analyses and estimated marginal means for guppy traits can be found in the supplemental materials (Tables S1, S2, S4, S7, and S8).
Supplementary Material
Acknowledgments
We thank the Ramdeen family for the use of their land and water supply, Ronnie Hernandez and the Asa Wright Nature Center for providing housing and logistical support, Mary Alkins-Koo and the University of the West Indies for logistical support, and Yuridia Reynoso and the many technicians that helped to process samples. This research was funded by a National Science Foundation Frontiers Integrative Biological Research grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908023107/DCSupplemental.
References
- 1.Hairston NG, Jr, Hairston NG., Sr Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am Nat. 1993;142:379–411. [Google Scholar]
- 2.Vanni MJ. Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst. 2002;33:341–370. [Google Scholar]
- 3.Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. New Jersey: Princeton Univ Press; 2002. [Google Scholar]
- 4.Flecker AS. Ecosystem engineering by a dominant detritivore in a diverse tropical ecosystem. Ecology. 1996;77:1845–1854. [Google Scholar]
- 5.Pringle CM, et al. Effects of omnivorous shrimp in a montane tropical stream: sediment removal, disturbance of sessile invertebrates and enhancement of understory algal biomass. Oecologia. 1993;93:1–11. doi: 10.1007/BF00321183. [DOI] [PubMed] [Google Scholar]
- 6.Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- 7.Hairston NG, Jr, et al. Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett. 2005;8:1114–1127. [Google Scholar]
- 8.Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theater and the evolutionary play. Philos Trans R Soc Lond B Biol Sci. 2009;364:1629–1640. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, et al. Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- 10.Dieckmann U, Ferriere R. In: Evolutionary Conservation Biology. Ferriere R, Dieckmann U, Couvet D, editors. United Kingdom: Cambridge Univ Press; 2004. pp. 188–224. [Google Scholar]
- 11.Harmon LJ, et al. Evolutionary diversification in stickleback affects ecosystem functioning. Nature. 2009;458:1167–1170. doi: 10.1038/nature07974. [DOI] [PubMed] [Google Scholar]
- 12.Post DM, Palkovacs EP, Schielke EG, Dodson SI. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology. 2008;89:2019–2032. doi: 10.1890/07-1216.1. [DOI] [PubMed] [Google Scholar]
- 13.Palkovacs EP, et al. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philos Trans R Soc Lond Ser B Biol Sci. 2009;364:1617–1628. doi: 10.1098/rstb.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palkovacs EP, Post DM. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol Ecol Res. 2008;10:699–720. [Google Scholar]
- 15.Palkovacs EP, Post DM. Experimental evidence that phenotypic divergence in predators drives community divergence in prey. Ecology. 2009;90:300–305. doi: 10.1890/08-1673.1. [DOI] [PubMed] [Google Scholar]
- 16.Liley NR, Seghers BH. In: Function and Evolution in Behavior. Baerends GP, Beer C, Manning A, editors. United Kingdom: Oxford Univ Press; 1975. pp. 92–118. [Google Scholar]
- 17.Gilliam JF, Fraser DF, Alkins-Koo M. Structure of a tropical stream fish community: a role for biotic interactions. Ecology. 1993;74:1856–1870. [Google Scholar]
- 18.Reznick D, Endler JA. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata) Evolution. 1982;36:160–177. doi: 10.1111/j.1558-5646.1982.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 19.Sandin L, Solimini AG. Freshwater ecosystem structure-function relationships: from theory to application. Freshw Biol. 2009;54:2017–2024. [Google Scholar]
- 20.Reznick DN, Butler MJ, Rodd FH, Ross P. Life-history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution. 1996;50:1651–1660. doi: 10.1111/j.1558-5646.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghalambor CK, Reznick DN, Walker JA. Constraints on adaptive evolution: The functional trade-off between reproduction and fast-start swimming performance in the Trinidadian guppy (Poecilia reticulata) Am Nat. 2004;164:38–50. doi: 10.1086/421412. [DOI] [PubMed] [Google Scholar]
- 22.Magurran AE, Seghers BH, Shaw PW, Carvalho GR. The behavioural diversity and evolution of guppy, Poecilia reticulata, populations in Trinidad. Adv Stud Behav. 1995;24:155–202. [Google Scholar]
- 23.Reznick DN, Bryga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. [Google Scholar]
- 24.Reznick D, Butler MJ, Rodd H. Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am Nat. 2001;157:126–140. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- 25.Rodd FH, Reznick DN. Variation in the demography of guppy populations: The importance of predation and life histories. Ecology. 1997;78:405–418. [Google Scholar]
- 26.Grether GF, et al. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology. 2001;82:1546–1559. [Google Scholar]
- 27.Reznick DN. The impact of predation on life history evolution in Trinidadian guppies: genetic basis of observed life history patterns. Evolution. 1982;36:1236–1250. doi: 10.1111/j.1558-5646.1982.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre PB, Michel E, Olsgard M. Top-down and bottom-up controls on periphyton biomass and productivity in Lake Tanganyika. Limnol Oceanogr. 2006;51:1514–1523. [Google Scholar]
- 29.Rosemond AD, Pringle CM, Ramirez A. Macroconsumer effects on insect detritivores and detritus processing in a tropical stream. Freshw Biol. 1998;39:515–523. [Google Scholar]
- 30.Merritt RT, Cummins KW. An Introduction to the Aquatic Insects of North America. Dubuque, IA: Kendall/Hunt Pub Co; 1996. [Google Scholar]
- 31.Langerhans RB, DeWitt TJ. Shared and unique features of evolutionary diversification. Am Nat. 2004;164:335–349. doi: 10.1086/422857. [DOI] [PubMed] [Google Scholar]
- 32.McPhail JD. Ecology and evolution of sympatric sticklebacks (Gasterosteus): origin of the species pairs. Can J Zool. 1993;71:515–523. [Google Scholar]
- 33.Reznick DN, Shaw FH, Rodd FH, Shaw RG. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- 34.Steinman AD, Lamberti GA, Leavitt PR. In: Methods in Stream Ecology. Hauer FR, editor. Lamberti, GA: Academic Press; 2006. pp. 357–380. [Google Scholar]
- 35.Benfield EF. In: Methods in Stream Ecology. Hauer FR, editor. Lamberti, GA: Academic Press; 2006. pp. 711–720. [Google Scholar]
- 36.Benke AC, Huryn AD, Smock LA, Wallace JB. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc. 1999;18:308–343. [Google Scholar]
- 37.Bott TL. In: Methods in Stream Ecology. Hauer FR, editor. Lamberti, GA: Academic Press; 2006. pp. 663–690. [Google Scholar]
- 38.Holmes RM, et al. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci. 1999;56:1801–1808. [Google Scholar]
- 39.Wetzel RG, Likens GE. Limnological Analyses. Springer Science; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.